Jasmonates Coordinate Secondary with Primary Metabolism
Abstract
:1. Introduction
2. Lineage-/Species-Dependent Induction of Diverse Secondary Metabolites by JA-Ile Signaling
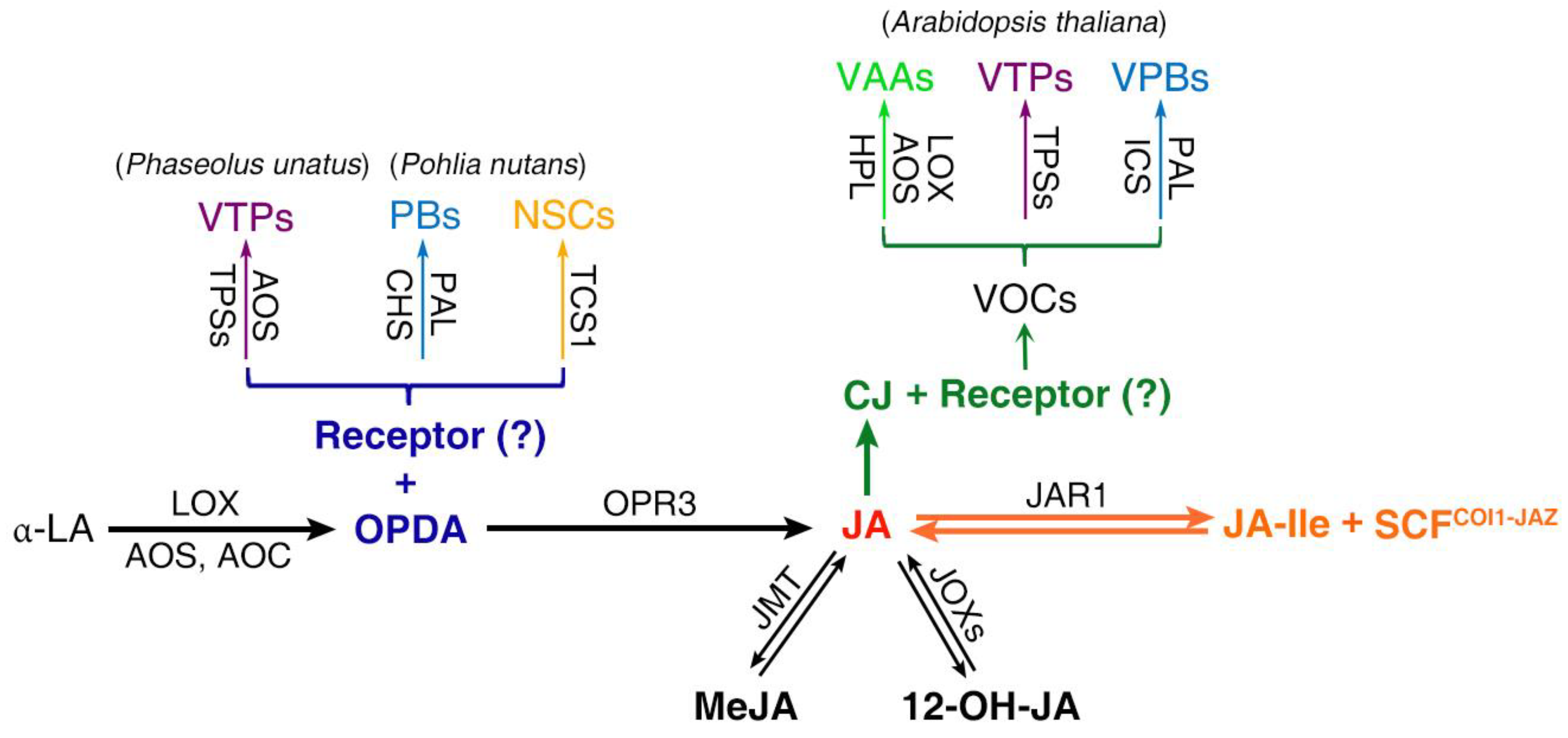
3. Secondary Metabolism Induced by OPDA and CJ
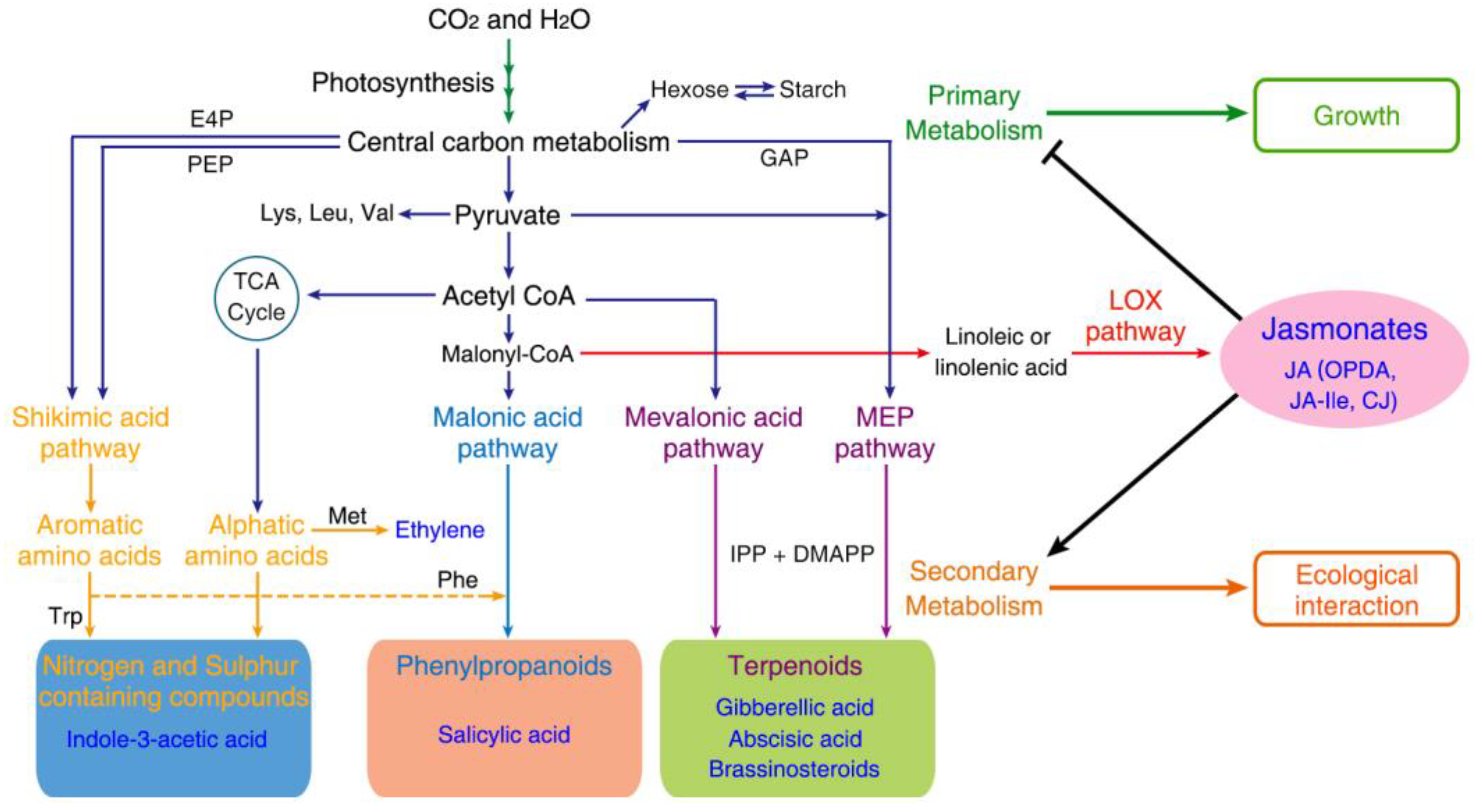
4. JAs Are Involved in Integrating and Orchestrating Primary and Secondary Metabolism
5. Conclusions, Perspectives and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wasternack, C.; Forner, S.; Strnad, M.; Hause, B. Jasmonates in flower and seed development. Biochimie 2013, 95, 79–85. [Google Scholar] [CrossRef]
- Browse, J. Jasmonate passes muster: A receptor and targets for the defense hormone. Annu. Rev. Plant Biol. 2009, 60, 183–205. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Song, S. Jasmonates: Biosynthesis, metabolism, and signaling by proteins activating and repressing transcription. J. Exp. Bot. 2017, 68, 1303–1321. [Google Scholar] [CrossRef]
- Li, M.; Yu, G.; Cao, C.; Liu, P. Metabolism, signaling, and transport of jasmonates. Plant Commun. 2021, 2, 100231. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C. Jasmonates: An update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. 2007, 100, 681–697. [Google Scholar] [CrossRef]
- Shan, X.; Yan, J.; Xie, D. Comparison of phytohormone signaling mechanisms. Curr. Opin. Plant Biol. 2012, 15, 84–91. [Google Scholar] [CrossRef]
- Koo, A.J. Metabolism of the plant hormone jasmonate: A sentinel for tissue damage and master regulator of stress response. Phytochem. Rev. 2018, 17, 51–80. [Google Scholar] [CrossRef]
- Howe, G.A.; Major, I.T.; Koo, A.J. Modularity in jasmonate signaling for multistress resilience. Annu. Rev. Plant Biol. 2018, 69, 387–415. [Google Scholar] [CrossRef]
- Wasternack, C.; Feussner, I. The oxylipin pathways: Biochemistry and function. Annu. Rev. Plant Biol. 2018, 69, 363–386. [Google Scholar] [CrossRef]
- Zhai, Q.; Deng, L.; Li, C. Mediator subunit MED25: At the nexus of jasmonate signaling. Curr. Opin. Plant Biol. 2020, 57, 78–86. [Google Scholar] [CrossRef]
- Sheard, L.B.; Tan, X.; Mao, H.; Withers, J.; Ben-Nissan, G.; Hinds, T.R.; Kobayashi, Y.; Hsu, F.F.; Sharon, M.; Browse, J.; et al. Jasmonate perception by inositol-phosphate-potentiated COI1-JAZ co-receptor. Nature 2010, 468, 400–405. [Google Scholar] [CrossRef]
- Chini, A.; Fonseca, S.; Fernandez, G.; Adie, B.; Chico, J.M.; Lorenzo, O.; Garcia-Casado, G.; Lopez-Vidriero, I.; Lozano, F.M.; Ponce, M.R.; et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature 2007, 448, 666–671. [Google Scholar] [CrossRef]
- Thines, B.; Katsir, L.; Melotto, M.; Niu, Y.; Mandaokar, A.; Liu, G.; Nomura, K.; He, S.Y.; Howe, G.A.; Browse, J. JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 2007, 448, 661–665. [Google Scholar] [CrossRef]
- Xie, D.X.; Feys, B.F.; James, S.; Nieto-Rostro, M.; Turner, J.G. COI1: An Arabidopsis gene required for jasmonate-regulated defense and fertility. Science 1998, 280, 1091–1094. [Google Scholar] [CrossRef] [PubMed]
- Taki, N.; Sasaki-Sekimoto, Y.; Obayashi, T.; Kikuta, A.; Kobayashi, K.; Ainai, T.; Yagi, K.; Sakurai, N.; Suzuki, H.; Masuda, T.; et al. 12-Oxo-phytodienoic acid triggers expression of a distinct set of genes and plays a role in wound-induced gene expression in Arabidopsis. Plant Physiol. 2005, 139, 1268–1283. [Google Scholar] [CrossRef] [PubMed]
- Monte, I.; Kneeshaw, S.; Franco-Zorrilla, J.M.; Chini, A.; Zamarreno, A.M.; Garcia-Mina, J.M.; Solano, R. An ancient COI1-independent function for reactive electrophilic oxylipins in thermotolerance. Curr. Biol. 2020, 30, 962–971. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Xue, J.Y.; Liu, L.W.; Sun, X.Q.; Zhou, G.C.; Chen, M.; Shao, Z.Q.; Hang, Y.Y. Divergence and conservative evolution of XTNX genes in land plants. Front. Plant Sci. 2017, 8, 1844. [Google Scholar] [CrossRef]
- Wang, F.; Yu, G.; Liu, P. Transporter-mediated subcellular distribution in the metabolism and signaling of jasmonates. Front. Plant Sci. 2019, 10, 390. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, M.; Zeng, R.; Groten, K.; Baldwin, I.T. Priming and filtering of antiherbivore defences among Nicotiana attenuata plants connected by mycorrhizal networks. Plant Cell Environ. 2019, 42, 2945–2961. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, C.; Yang, J.; Yu, N.; Wang, E. Hormone modulation of legume-rhizobial symbiosis. J. Integr. Plant Biol. 2018, 60, 632–648. [Google Scholar] [CrossRef]
- Shigeyama, T.; Tominaga, A.; Arima, S.; Sakai, T.; Inada, S.; Jikumaru, Y.; Kamiya, Y.; Uchiumi, T.; Abe, M.; Hashiguchi, M.; et al. Additional cause for reduced JA-Ile in the root of a Lotus japonicus phyB mutant. Plant Signal. Behav. 2012, 7, 746–748. [Google Scholar] [CrossRef]
- Basso, V.; Veneault-Fourrey, C. Role of jasmonates in beneficial microbe-root interactions. Methods Mol. Biol. 2020, 2085, 43–67. [Google Scholar] [PubMed]
- Huang, H.; Liu, B.; Liu, L.; Song, S. Jasmonate action in plant growth and development. J. Exp. Bot. 2017, 68, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, D.D.; Fang, X.; Chen, X.Y.; Mao, Y.B. Plant specialized metabolism regulated by jasmonate signaling. Plant Cell Physiol. 2019, 60, 2638–2647. [Google Scholar] [CrossRef]
- De Geyter, N.; Gholami, A.; Goormachtig, S.; Goossens, A. Transcriptional machineries in jasmonate-elicited plant secondary metabolism. Trends Plant Sci. 2012, 17, 349–359. [Google Scholar] [CrossRef]
- Memelink, J.; Verpoorte, R.; Kijne, J.W. ORCAnization of jasmonate-responsive gene expression in alkaloid metabolism. Trends Plant Sci. 2001, 6, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Strnad, M. Jasmonates are signals in the biosynthesis of secondary metabolites-pathways, transcription factors and applied aspects—A brief review. New Biotechnol. 2019, 48, 1–11. [Google Scholar] [CrossRef]
- Erb, M.; Kliebenstein, D.J. Plant secondary metabolites as defenses, regulators, and primary metabolites: The blurred functional trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef]
- Banks, J.A.; Nishiyama, T.; Hasebe, M.; Bowman, J.L.; Gribskov, M.; dePamphilis, C.; Albert, V.A.; Aono, N.; Aoyama, T.; Ambrose, B.A.; et al. The Selaginella genome identifies genetic changes associated with the evolution of vascular plants. Science 2011, 332, 960–963. [Google Scholar] [CrossRef]
- Goiris, K.; Muylaert, K.; Voorspoels, S.; Noten, B.; De Paepe, D.; Baart, G.J.E.; De Cooman, L. Detection of flavonoids in microalgae from different evolutionary lineages. J. Phycol. 2014, 50, 483–492. [Google Scholar] [CrossRef]
- Weng, J.K.; Philippe, R.N.; Noel, J.P. The rise of chemodiversity in plants. Science 2012, 336, 1667–1670. [Google Scholar] [CrossRef] [PubMed]
- Lei, L. Lignin evolution: Invasion of land. Nat. Plants 2017, 3, 17042. [Google Scholar] [CrossRef]
- Renault, H.; Alber, A.; Horst, N.A.; Basilio Lopes, A.; Fich, E.A.; Kriegshauser, L.; Wiedemann, G.; Ullmann, P.; Herrgott, L.; Erhardt, M.; et al. A phenol-enriched cuticle is ancestral to lignin evolution in land plants. Nat. Commun. 2017, 8, 14713. [Google Scholar] [CrossRef]
- Zust, T.; Agrawal, A.A. Trade-offs between plant growth and defense against insect herbivory: An emerging mechanistic synthesis. Annu. Rev. Plant Biol. 2017, 68, 513–534. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.W.; Guo, Z.Y.; Wang, K.M.; Wang, R.; Fang, C.Y. Comparative metabolomic analysis reveals the role of OsHPL1 in the cold-induced metabolic changes in rice. Plants 2023, 12, 2032. [Google Scholar] [CrossRef] [PubMed]
- Perez-Llorca, M.; Pollmann, S.; Muller, M. Ethylene and jasmonates signaling network mediating secondary metabolites under abiotic stress. Int. J. Mol. Sci. 2023, 24, 5990. [Google Scholar] [CrossRef]
- Yang, Z.; Li, Y.; Gao, F.; Jin, W.; Li, S.; Kimani, S.; Yang, S.; Bao, T.; Gao, X.; Wang, L. MYB21 interacts with MYC2 to control the expression of terpene synthase genes in flowers of Freesia hybrida and Arabidopsis thaliana. J. Exp. Bot. 2020, 71, 4140–4158. [Google Scholar] [CrossRef]
- Li, L.; Hao, X.; Liu, H.; Wang, W.; Fu, X.; Ma, Y.; Shen, Q.; Chen, M.; Tang, K. Jasmonic acid-responsive AabHLH1 positively regulates artemisinin biosynthesis in Artemisia annua. Biotechnol. Appl. Biochem. 2019, 66, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.N.; Xu, D.B.; Yan, X.; Wu, Z.K.; Kayani, S.I.; Shen, Q.; Fu, X.Q.; Xie, L.H.; Hao, X.L.; Hassani, D.; et al. Jasmonate-and abscisic acid-activated AaGSW1-AaTCP15/AaORA transcriptional cascade promotes artemisinin biosynthesis in Artemisia annua. Plant Biotechnol. J. 2021, 19, 1412–1428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Jin, X.; Chen, Y.; Wei, M.; Liao, W.; Zhao, S.; Fu, C.; Yu, L. TcMYC2a, a Basic helix-loop-helix transcription factor, transduces JA-signals and regulates taxol biosynthesis in Taxus chinensis. Front. Plant Sci. 2018, 9, 863. [Google Scholar]
- Zhou, Y.; Sun, W.; Chen, J.; Tan, H.; Xiao, Y.; Li, Q.; Ji, Q.; Gao, S.; Chen, L.; Chen, S.; et al. SmMYC2a and SmMYC2b played similar but irreplaceable roles in regulating the biosynthesis of tanshinones and phenolic acids in Salvia miltiorrhiza. Sci. Rep. 2016, 6, 22852. [Google Scholar]
- Xie, Y.; Tan, H.; Ma, Z.; Huang, J. DELLA proteins promote anthocyanin biosynthesis via sequestering MYBL2 and JAZ suppressors of the MYB/bHLH/WD40 complex in Arabidopsis thaliana. Mol. Plant 2016, 9, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Hashimoto, T. Tobacco MYC2 regulates jasmonate-inducible nicotine biosynthesis genes directly and by way of the NIC2-locus ERF genes. Plant Cell Physiol. 2011, 52, 1117–1130. [Google Scholar]
- Zhang, H.; Hedhili, S.; Montiel, G.; Zhang, Y.; Chatel, G.; Pré, M.; Gantet, P.; Memelink, J. The basic helix-loop-helix transcription factor CrMYC2 controls the jasmonate-responsive expression of the ORCA genes that regulate alkaloid biosynthesis in Catharanthus roseus. Plant J. 2011, 67, 61–71. [Google Scholar]
- Chen, C.; Liu, F.; Zhang, K.; Niu, X.; Zhao, H.; Liu, Q.; Georgiev, M.I.; Xu, X.; Zhang, X.; Zhou, M. MeJA-responsive bHLH transcription factor LjbHLH7 regulates cyanogenic glucoside biosynthesis in Lotus japonicus. J. Exp. Bot. 2022, 73, 2650–2665. [Google Scholar] [PubMed]
- Afrin, S.; Huang, J.J.; Luo, Z.Y. JA-mediated transcriptional regulation of secondary metabolism in medicinal plants. Sci. Bull. 2015, 60, 1062–1072. [Google Scholar]
- Nguyen, T.H.; Goossens, A.; Lacchini, E. Jasmonate: A hormone of primary importance for plant metabolism. Curr. Opin. Plant Biol. 2022, 67, 102197. [Google Scholar] [PubMed]
- Sohn, S.I.; Pandian, S.; Rakkammal, K.; Largia, M.J.V.; Thamilarasan, S.K.; Balaji, S.; Zoclanclounon, Y.A.B.; Shilpha, J.; Ramesh, M. Jasmonates in plant growth and development and elicitation of secondary metabolites: An updated overview. Front. Plant Sci. 2022, 13, 942789. [Google Scholar]
- Zhang, F.; Yao, J.; Ke, J.; Zhang, L.; Lam, V.Q.; Xin, X.F.; Zhou, X.E.; Chen, J.; Brunzelle, J.; Griffin, P.R.; et al. Structural basis of JAZ repression of MYC transcription factors in jasmonate signalling. Nature 2015, 525, 269–273. [Google Scholar]
- An, C.; Li, L.; Zhai, Q.; You, Y.; Deng, L.; Wu, F.; Chen, R.; Jiang, H.; Wang, H.; Chen, Q.; et al. Mediator subunit MED25 links the jasmonate receptor to transcriptionally active chromatin. Proc. Natl. Acad. Sci. USA 2017, 114, 8930–8939. [Google Scholar]
- Zhai, Q.; Li, C. The plant mediator complex and its role in jasmonate signaling. J. Exp. Bot. 2019, 70, 3415–3424. [Google Scholar] [PubMed]
- You, Y.; Zhai, Q.; An, C.; Li, C. LEUNIG_HOMOLOG mediates MYC2-dependent transcriptional activation in cooperation with the coactivators HAC1 and MED25. Plant Cell 2019, 31, 2187–2205. [Google Scholar]
- Xu, J.; van Herwijnen, Z.O.; Drager, D.B.; Sui, C.; Haring, M.A.; Schuurink, R.C. SlMYC1 Regulates type VI glandular trichome formation and terpene biosynthesis in tomato glandular cells. Plant Cell 2018, 30, 2988–3005. [Google Scholar] [CrossRef]
- Penuelas, M.; Monte, I.; Schweizer, F.; Vallat, A.; Reymond, P.; Garcia-Casado, G.; Franco-Zorrilla, J.M.; Solano, R. Jasmonate-related MYC transcription factors are functionally conserved in Marchantia polymorpha. Plant Cell 2019, 31, 2491–2509. [Google Scholar] [CrossRef]
- Shen, Q.; Lu, X.; Yan, T.; Fu, X.; Lv, Z.; Zhang, F.; Pan, Q.; Wang, G.; Sun, X.; Tang, K. The jasmonate-responsive AaMYC2 transcription factor positively regulates artemisinin biosynthesis in Artemisia annua. New Phytol. 2016, 210, 1269–1281. [Google Scholar] [PubMed]
- Cardenas, P.D.; Sonawane, P.D.; Pollier, J.; Vanden Bossche, R.; Dewangan, V.; Weithorn, E.; Tal, L.; Meir, S.; Rogachev, I.; Malitsky, S.; et al. GAME9 regulates the biosynthesis of steroidal alkaloids and upstream isoprenoids in the plant mevalonate pathway. Nat. Commun. 2016, 7, 10654. [Google Scholar]
- Fürstenberg-Hägg, J.; Zagrobelny, M.; Bak, S. Plant defense against insect herbivores. Int. J. Mol. Sci. 2013, 14, 10242–10297. [Google Scholar]
- Gleadow, R.M.; Møller, B.L. Cyanogenic glycosides: Synthesis, physiology, and phenotypic plasticity. Annu. Rev. Plant Biol. 2014, 65, 155–185. [Google Scholar]
- Zust, T.; Agrawal, A.A. Mechanisms and evolution of plant resistance to aphids. Nat. Plants 2016, 2, 15206. [Google Scholar]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K.; et al. Plant secondary metabolites as defense tools against herbivores for sustainable crop protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef] [PubMed]
- De Boer, K.; Tilleman, S.; Pauwels, L.; Vanden Bossche, R.; De Sutter, V.; Vanderhaeghen, R.; Hilson, P.; Hamill, J.D.; Goossens, A. APETALA2/ETHYLENE RESPONSE FACTOR and basic helix-loop-helix tobacco transcription factors cooperatively mediate jasmonate-elicited nicotine biosynthesis. Plant J. 2011, 66, 1053–1065. [Google Scholar] [CrossRef]
- Todd, A.T.; Liu, E.; Polvi, S.L.; Pammett, R.T.; Page, J.E. A functional genomics screen identifies diverse transcription factors that regulate alkaloid biosynthesis in Nicotiana benthamiana. Plant J. 2010, 62, 589–600. [Google Scholar] [CrossRef]
- Schweizer, F.; Colinas, M.; Pollier, J.; Van Moerkercke, A.; Vanden Bossche, R.; de Clercq, R.; Goossens, A. An engineered combinatorial module of transcription factors boosts production of monoterpenoid indole alkaloids in Catharanthus roseus. Metab. Eng. 2018, 48, 150–162. [Google Scholar] [PubMed]
- Van Moerkercke, A.; Steensma, P.; Schweizer, F.; Pollier, J.; Gariboldi, I.; Payne, R.; Vanden Bossche, R.; Miettinen, K.; Espoz, J.; Purnama, P.C.; et al. The bHLH transcription factor BIS1 controls the iridoid branch of the monoterpenoid indole alkaloid pathway in Catharanthus roseus. Proc. Natl. Acad. Sci. USA 2015, 112, 8130–8135. [Google Scholar]
- Van Moerkercke, A.; Steensma, P.; Gariboldi, I.; Espoz, J.; Purnama, P.C.; Schweizer, F.; Miettinen, K.; Vanden Bossche, R.; De Clercq, R.; Memelink, J.; et al. The basic helix-loop-helix transcription factor BIS2 is essential for monoterpenoid indole alkaloid production in the medicinal plant Catharanthus roseus. Plant J. 2016, 88, 3–12. [Google Scholar] [CrossRef]
- Jimenez Aleman, G.H.; Thirumalaikumar, V.P.; Jander, G.; Fernie, A.R.; Skirycz, A. OPDA, more than just a jasmonate precursor. Phytochemistry 2022, 204, 113432. [Google Scholar] [PubMed]
- Maynard, D.; Groger, H.; Dierks, T.; Dietz, K.J. The function of the oxylipin 12-oxophytodienoic acid in cell signaling, stress acclimation, and development. J. Exp. Bot. 2018, 69, 5341–5354. [Google Scholar] [CrossRef] [PubMed]
- Altiok, N.; Mezzadra, H.; Patel, P.; Koyuturk, M.; Altiok, S. A plant oxylipin, 12-oxo-phytodienoic acid, inhibits proliferation of human breast cancer cells by targeting cyclin D1. Breast Cancer Res. Treat. 2008, 109, 315–323. [Google Scholar]
- Taki-Nakano, N.; Kotera, J.; Ohta, H. 12-Oxo-phytodienoic acid, a plant-derived oxylipin, attenuates lipopolysaccharide-induced inflammation in microglia. Biochem. Biophys. Res. Commun. 2016, 473, 1288–1294. [Google Scholar] [CrossRef]
- Taki-Nakano, N.; Ohzeki, H.; Kotera, J.; Ohta, H. Cytoprotective effects of 12-oxo phytodienoic acid, a plant-derived oxylipin jasmonate, on oxidative stress-induced toxicity in human neuroblastoma SH-SY5Y cells. Biochim. Biophys. Acta 2014, 1840, 3413–3422. [Google Scholar] [CrossRef]
- Liu, W.; Park, S.W. 12-Oxo-phytodienoic acid: A fuse and/or switch of plant growth and defense responses? Front. Plant Sci. 2021, 12, 724079. [Google Scholar] [CrossRef] [PubMed]
- Kneeshaw, S.; Soriano, G.; Monte, I.; Hamberg, M.; Zamarreno, A.M.; Garcia-Mina, J.M.; Franco-Zorrilla, J.M.; Kato, N.; Ueda, M.; Rey-Stolle, M.F.; et al. Ligand diversity contributes to the full activation of the jasmonate pathway in Marchantia polymorpha. Proc. Natl. Acad. Sci. USA 2022, 119, e2202930119. [Google Scholar] [CrossRef]
- Monte, I.; Ishida, S.; Zamarreno, A.M.; Hamberg, M.; Franco-Zorrilla, J.M.; Garcia-Casado, G.; Gouhier-Darimont, C.; Reymond, P.; Takahashi, K.; Garcia-Mina, J.M.; et al. Ligand-receptor co-evolution shaped the jasmonate pathway in land plants. Nat. Chem. Biol. 2018, 14, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Soriano, G.; Kneeshaw, S.; Jimenez-Aleman, G.; Zamarreno, A.M.; Franco-Zorrilla, J.M.; Rey-Stolle, M.F.; Barbas, C.; Garcia-Mina, J.M.; Solano, R. An evolutionarily ancient fatty acid desaturase is required for the synthesis of hexadecatrienoic acid, which is the main source of the bioactive jasmonate in Marchantia polymorpha. New Phytol. 2022, 233, 1401–1413. [Google Scholar] [CrossRef] [PubMed]
- Bowman, J.L.; Kohchi, T.; Yamato, K.T.; Jenkins, J.; Shu, S.; Ishizaki, K.; Yamaoka, S.; Nishihama, R.; Nakamura, Y.; Berger, F.; et al. Insights into land plant evolution garnered from the Marchantia polymorpha Genome. Cell 2017, 171, 287–304. [Google Scholar] [CrossRef]
- Koch, T.; Krumm, T.; Jung, V.; Engelberth, J.; Boland, W. Differential induction of plant volatile biosynthesis in the lima bean by early and late intermediates of the octadecanoid-signaling pathway. Plant Physiol. 1999, 121, 153–162. [Google Scholar] [CrossRef]
- Shinya, T.; Miyamoto, K.; Uchida, K.; Hojo, Y.; Yumoto, E.; Okada, K.; Yamane, H.; Galis, I. Chitooligosaccharide elicitor and oxylipins synergistically elevate phytoalexin production in rice. Plant Mol. Biol. 2022, 109, 595–609. [Google Scholar] [CrossRef]
- Liu, S.; Li, T.; Zhang, P.; Zhao, L.; Yi, D.; Zhang, Z.; Cong, B. Insights into the jasmonate signaling in basal land plant revealed by the multi-omics analysis of an antarctic moss Pohlia nutans treated with OPDA. Int. J. Mol. Sci. 2022, 23, 13507. [Google Scholar] [CrossRef] [PubMed]
- Birkett, M.A.; Campbell, C.A.; Chamberlain, K.; Guerrieri, E.; Hick, A.J.; Martin, J.L.; Matthes, M.; Napier, J.A.; Pettersson, J.; Pickett, J.A.; et al. New roles for cis-jasmone as an insect semiochemical and in plant defense. Proc. Natl. Acad. Sci. USA 2000, 97, 9329–9334. [Google Scholar] [CrossRef]
- Loughrin, J.H.; Manukian, A.; Heath, R.R.; Tumlinson, J.H. Volatiles emitted by different cotton varieties damaged by feeding beet armyworm larvae. J. Chem. Ecol. 1995, 21, 1217–1227. [Google Scholar] [CrossRef]
- Rose, U.S.; Tumlinson, J.H. Volatiles released from cotton plants in response to Helicoverpa zea feeding damage on cotton flower buds. Planta 2004, 218, 824–832. [Google Scholar] [CrossRef]
- Lou, Y.; Baldwin, I.T. Manduca sexta recognition and resistance among allopolyploid Nicotiana host plants. Proc. Natl. Acad. Sci. USA 2003, 100 (Suppl. 2), 14581–14586. [Google Scholar] [CrossRef]
- Sobhy, I.S.; Erb, M.; Turlings, T.C. Plant strengtheners enhance parasitoid attraction to herbivore-damaged cotton via qualitative and quantitative changes in induced volatiles. Pest. Manag. Sci. 2015, 71, 686–693. [Google Scholar] [CrossRef]
- Rose, U.S.; Tumlinson, J.H. Systemic induction of volatile release in cotton: How specific is the signal to herbivory? Planta 2005, 222, 327–335. [Google Scholar] [CrossRef]
- Heil, M.; Kost, C. Priming of indirect defences. Ecol. Lett. 2006, 9, 813–817. [Google Scholar] [CrossRef]
- Ballhorn, D.J.; Kautz, S.; Schadler, M. Induced plant defense via volatile production is dependent on rhizobial symbiosis. Oecologia 2013, 172, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Dabrowska, P.; Boland, W. iso-OPDA: An early precursor of cis-jasmone in plants? Chembiochem 2007, 8, 2281–2285. [Google Scholar] [CrossRef]
- Bruce, T.J.; Matthes, M.C.; Chamberlain, K.; Woodcock, C.M.; Mohib, A.; Webster, B.; Smart, L.E.; Birkett, M.A.; Pickett, J.A.; Napier, J.A. cis-Jasmone induces Arabidopsis genes that affect the chemical ecology of multitrophic interactions with aphids and their parasitoids. Proc. Natl. Acad. Sci. USA 2008, 105, 4553–4558. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.; Covaci, A.D.; Roberts, J.M.; Sobhy, I.S.; Kirk, W.D.J.; Bruce, T.J.A. Effects of cis-Jasmone treatment of Brassicas on iInteractions with Myzus persicae aphids and their parasitoid Diaeretiella rapae. Front. Plant Sci. 2021, 12, 711896. [Google Scholar] [CrossRef] [PubMed]
- Moraes, M.C.B.; Laumann, R.A.; Pareja, M.; Sereno, F.T.P.S.; Michereff, M.F.F.; Birkett, M.A.; Pickett, J.A.; Borges, M. Attraction of the stink bug egg parasitoid Telenomus podisi to defence signals from soybean activated by treatment with cis-jasmone. Entomol. Exp. Appl. 2009, 131, 178–188. [Google Scholar] [CrossRef]
- Vieira, C.R.; Moraes, M.C.B.; Borges, M.; Sujii, E.R.; Laumann, R.A. cis-Jasmone indirect action on egg parasitoids (Hymenoptera: Scelionidae) and its application in biological control of soybean stink bugs (Hemiptera: Pentatomidae). Biol. Control 2013, 64, 75–82. [Google Scholar] [CrossRef]
- Sobhy, I.S.; Woodcock, C.M.; Powers, S.J.; Caulfield, J.C.; Pickett, J.A.; Birkett, M.A. cis-Jasmone Elicits Aphid-Induced Stress Signalling in Potatoes. J. Chem. Ecol. 2017, 43, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Disi, J.O.; Zebelo, S.; Ngumbi, E.; Fadamiro, H.Y. cis-Jasmone primes defense pathways in tomato via emission of volatile organic compounds and regulation of genes with consequences for Spodoptera exigua oviposition. Arthropod-Plant Interact. 2017, 11, 591–602. [Google Scholar] [CrossRef]
- Hegde, M.; Oliveira, J.N.; da Costa, J.G.; Loza-Reyes, E.; Bleicher, E.; Santana, A.E.; Caulfield, J.C.; Mayon, P.; Dewhirst, S.Y.; Bruce, T.J.; et al. Aphid antixenosis in cotton is activated by the natural plant defence elicitor cis-jasmone. Phytochemistry 2012, 78, 81–88. [Google Scholar] [CrossRef]
- Dewhirst, S.Y.; Birkett, M.A.; Loza-Reyes, E.; Martin, J.L.; Pye, B.J.; Smart, L.E.; Hardie, J.; Pickett, J.A. Activation of defence in sweet pepper, Capsicum annum, by cis-jasmone, and its impact on aphid and aphid parasitoid behaviour. Pest. Manag. Sci. 2012, 68, 1419–1429. [Google Scholar] [CrossRef]
- Bruce, T.J.; Martin, J.L.; Pickett, J.A.; Pye, B.J.; Smart, L.E.; Wadhams, L.J. cis-Jasmone treatment induces resistance in wheat plants against the grain aphid, Sitobion avenae (Fabricius) (Homoptera: Aphididae). Pest. Manag. Sci. 2003, 59, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Oluwafemi, S.; Dewhirst, S.Y.; Veyrat, N.; Powers, S.; Bruce, T.J.; Caulfield, J.C.; Pickett, J.A.; Birkett, M.A. Priming of production in maize of volatile organic defence compounds by the natural plant activator cis-Jasmone. PLoS ONE 2013, 8, e62299. [Google Scholar] [CrossRef] [PubMed]
- Delaney, K.J.; Wawrzyniak, M.; Lemanczyk, G.; Wrzesinska, D.; Piesik, D. Synthetic cis-jasmone exposure induces wheat and barley volatiles that repel the pest cereal leaf beetle, Oulema melanopus L. J. Chem. Ecol. 2013, 39, 620–629. [Google Scholar] [CrossRef]
- Moraes, M.C.; Birkett, M.A.; Gordon-Weeks, R.; Smart, L.E.; Martin, J.L.; Pye, B.J.; Bromilow, R.; Pickett, J.A. cis-Jasmone induces accumulation of defence compounds in wheat, Triticum aestivum. Phytochemistry 2008, 69, 9–17. [Google Scholar] [CrossRef]
- Egger, B.; Koschier, E.H. Behavioural responses of Frankliniella occidentalis Pergande larvae to methyl jasmonate and cis-jasmone. J. Pest. Sci. 2014, 87, 53–59. [Google Scholar] [CrossRef]
- Egger, B.; Spangl, B.; Koschier, E.H. Continuous exposure to the deterrents cis-jasmone and methyl jasmonate does not alter the behavioural responses of Frankliniella occidentalis. Entomol. Exp. Appl. 2016, 158, 78–86. [Google Scholar] [CrossRef]
- Pope, T.W.; Campbell, C.A.; Hardie, J.; Wadhams, L.J. Treating hop plants with (Z)-jasmone increases colonization by Phorodon humuli (Hemiptera: Aphididae) spring migrants. Bull. Entomol. Res. 2007, 97, 317–319. [Google Scholar] [CrossRef]
- Kessler, A.; Baldwin, I.T. Defensive function of herbivore-induced plant volatile emissions in nature. Science 2001, 291, 2141–2144. [Google Scholar] [CrossRef]
- Loreto, F.; D’Auria, S. How do plants sense volatiles sent by other plants? Trends Plant Sci. 2022, 27, 29–38. [Google Scholar] [CrossRef]
- Matthes, M.C.; Bruce, T.J.; Ton, J.; Verrier, P.J.; Pickett, J.A.; Napier, J.A. The transcriptome of cis-jasmone-induced resistance in Arabidopsis thaliana and its role in indirect defence. Planta 2010, 232, 1163–1180. [Google Scholar] [CrossRef] [PubMed]
- Stotz, H.U.; Mueller, S.; Zoeller, M.; Mueller, M.J.; Berger, S. TGA transcription factors and jasmonate-independent COI1 signalling regulate specific plant responses to reactive oxylipins. J. Exp. Bot. 2013, 64, 963–975. [Google Scholar] [CrossRef]
- Koster, J.; Thurow, C.; Kruse, K.; Meier, A.; Iven, T.; Feussner, I.; Gatz, C. Xenobiotic- and jasmonic acid-inducible signal transduction pathways have become interdependent at the Arabidopsis CYP81D11 promoter. Plant Physiol. 2012, 159, 391–402. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Webster, S.; He, S.Y. Growth-defense trade-offs in plants. Curr. Biol. 2022, 32, 634–639. [Google Scholar] [CrossRef]
- Yang, D.L.; Yao, J.; Mei, C.S.; Tong, X.H.; Zeng, L.J.; Li, Q.; Xiao, L.T.; Sun, T.P.; Li, J.; Deng, X.W.; et al. Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proc. Natl. Acad. Sci. USA 2012, 109, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zheng, J.; Li, S.; Huang, G.; Skilling, S.J.; Wang, L.; Li, L.; Li, M.; Yuan, L.; Liu, P. Transporter-mediated nuclear entry of jasmonoyl-isoleucine is essential for jasmonate signaling. Mol. Plant 2017, 10, 695–708. [Google Scholar] [CrossRef]
- Guo, Q.; Yoshida, Y.; Major, I.T.; Wang, K.; Sugimoto, K.; Kapali, G.; Havko, N.E.; Benning, C.; Howe, G.A. JAZ repressors of metabolic defense promote growth and reproductive fitness in Arabidopsis. Proc. Natl. Acad. Sci. USA 2018, 115, 10768–10777. [Google Scholar] [CrossRef]
- Pratelli, R.; Pilot, G. Regulation of amino acid metabolic enzymes and transporters in plants. J. Exp. Bot. 2014, 65, 5535–5556. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, I.; Kissen, R.; Bones, A.M. Phytoalexins in defense against pathogens. Trends Plant Sci. 2012, 17, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Dudareva, N. The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef]
- Aniszewski, T. Alkaloids-Secrets of Life: Alkaloid Chemistry, Biological Significance, Applications and Ecological Role; Elsevier Science: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Savchenko, T.V.; Rolletschek, H.; Dehesh, K. Jasmonates-mediated rewiring of central metabolism regulates adaptive responses. Plant Cell Physiol. 2019, 60, 2613–2620. [Google Scholar] [CrossRef] [PubMed]
- Balcke, G.U.; Bennewitz, S.; Bergau, N.; Athmer, B.; Henning, A.; Majovsky, P.; Jimenez-Gomez, J.M.; Hoehenwarter, W.; Tissier, A. Multi-omics of tomato glandular trichomes reveals distinct features of central carbon metabolism supporting high productivity of specialized metabolites. Plant Cell 2017, 29, 960–983. [Google Scholar] [CrossRef]
- Obata, T.; Fernie, A.R. The use of metabolomics to dissect plant responses to abiotic stresses. Cell Mol. Life Sci. 2012, 69, 3225–3243. [Google Scholar] [CrossRef]
- Savchenko, T.; Rolletschek, H.; Heinzel, N.; Tikhonov, K.; Dehesh, K. Waterlogging tolerance rendered by oxylipin-mediated metabolic reprogramming in Arabidopsis. J. Exp. Bot. 2019, 70, 2919–2932. [Google Scholar] [CrossRef]
- Hanik, N.; Gomez, S.; Best, M.; Schueller, M.; Orians, C.M.; Ferrieri, R.A. Partitioning of new carbon as 11C in Nicotiana tabacum reveals insight into methyl jasmonate induced changes in metabolism. J. Chem. Ecol. 2010, 36, 1058–1067. [Google Scholar] [CrossRef]

| Plant Species | CJ-Induced VOCs | Refs. |
|---|---|---|
| Arabidopsis thaliana | Fatty acid derivatives: (Z)-3-hexen-1-ol, 1-octen-3-ol Terpenoids: 6-methyl-5-hepten-2-one Phenylpropanoids/benzenoids: ethylbenzene, 4-ethyltoluene Specific compounds: heptyl isothiocyanate | [89] |
| Glycine max | Terpenoids: camphene, myrcene, (E)-ocimene, TMTT Phenylpropanoids/benzenoids: MeSA | [91] |
| Capsicum annum | Fatty acid derivatives: (Z)-3-hexenyl acetate, (Z)-3-hexenyl butyrate Terpenoids: sabinene, myrcene, geranylacetone, 6-methyl-5-hepten-2-one | [96] |
| Gossypium hirsutum | Fatty acid derivatives: (Z)-3-hexenyl acetate Terpenoids: DMNT, TMTT Phenylpropanoids/benzenoids: MeSA | [95] |
| Zea mays | Terpenoids: (E)-(1R,9S)-caryophyllene, (E)-a-bergamotene, (E)-β-farnesene, DMNT | [98] |
| Solanum lycopersicon | Fatty acid derivatives: (E)-2-hexenal, (Z)-3-hexen-1-yl butyrate, nonanal, decanal Terpenoids: α-pinene, α-copaene, (E)-β-farnesene, DMNT, TMTT Phenylpropanoids/benzenoids: MeBA, MeSA Aromatic compounds: indole | [94] |
| Brassica napus, B. rapa and B. oleracea | Fatty acid derivatives: 2-ethyl-1-hexanol, 1-octanol, 2-butyl-1-octanol, nonanal, decanal, cis-3-hexenyl acetate, dihydrojasmone, cis-jasmone Terpenoids: citronellol, p-cymen-7-ol, α-elemene, α-curcumene, (E, E)-β-farnesene, DMNT Phenylpropanoids/benzenoids: MeSA, benzothiazole Specific compounds: methyl isothiocyanate, benzyl nitrile Aliphatic hydrocarbons: dodecane, (E)-3-tetradecene | [90] |
| Triticum aestivum L. and Hordeum vulgare L. | Fatty acid derivatives: (Z)-3-hexanal, (Z)-3-hexanol, (Z)-3-hexanyl acetate Terpenoids: (Z)-β-ocimene, linalool, β-caryophyllene, (E)-β–farnesene Aromatic compounds: indole | [99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, C.; Qiu, J.; Zhang, Y.; Li, M.; Liu, P. Jasmonates Coordinate Secondary with Primary Metabolism. Metabolites 2023, 13, 1008. https://doi.org/10.3390/metabo13091008
Luo C, Qiu J, Zhang Y, Li M, Liu P. Jasmonates Coordinate Secondary with Primary Metabolism. Metabolites. 2023; 13(9):1008. https://doi.org/10.3390/metabo13091008
Chicago/Turabian StyleLuo, Chen, Jianfang Qiu, Yu Zhang, Mengya Li, and Pei Liu. 2023. "Jasmonates Coordinate Secondary with Primary Metabolism" Metabolites 13, no. 9: 1008. https://doi.org/10.3390/metabo13091008
APA StyleLuo, C., Qiu, J., Zhang, Y., Li, M., & Liu, P. (2023). Jasmonates Coordinate Secondary with Primary Metabolism. Metabolites, 13(9), 1008. https://doi.org/10.3390/metabo13091008







