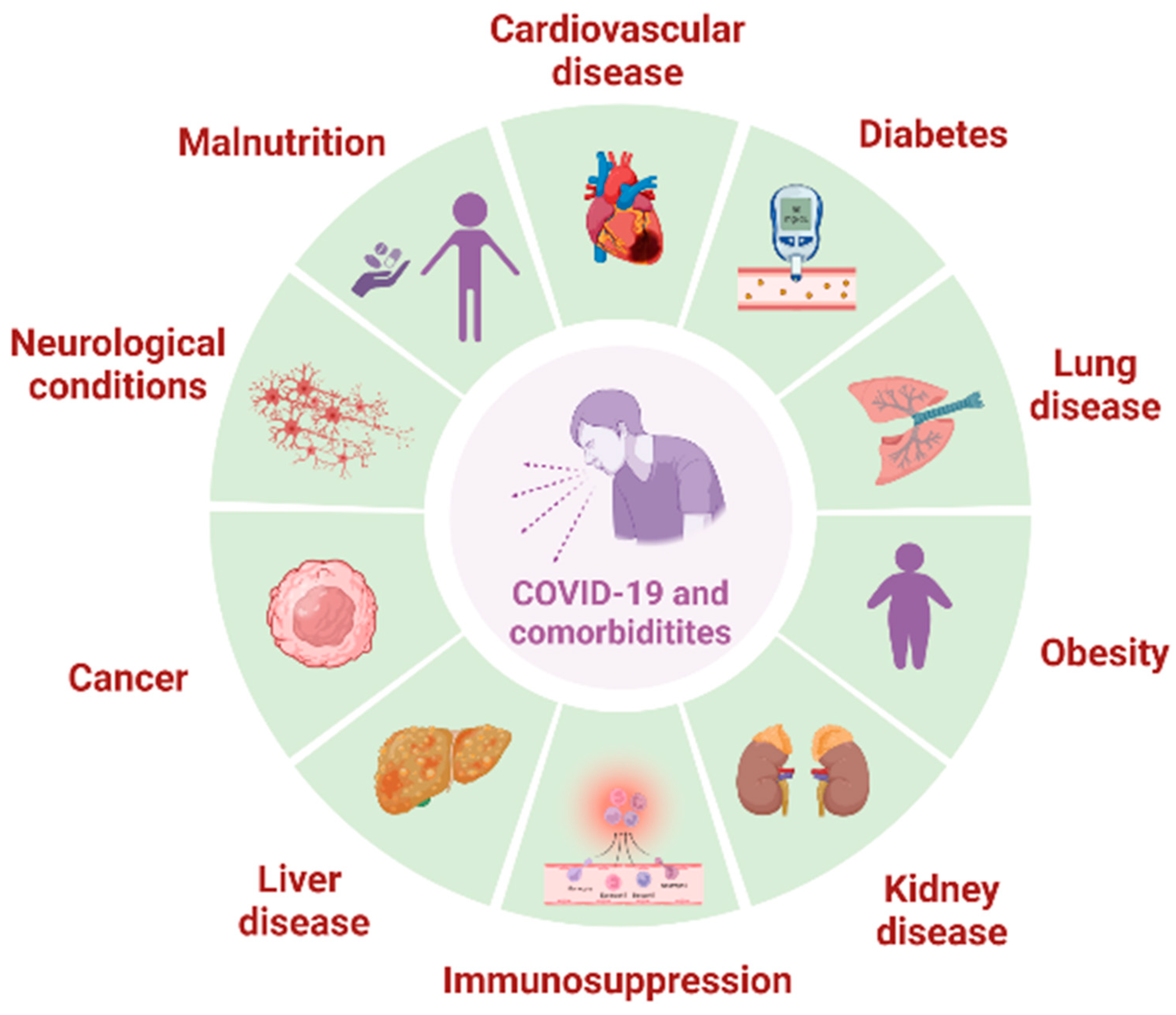
COVID-19 and Comorbidities: What Has Been Unveiled by Metabolomics?
Abstract
:1. Introduction
2. Methods
3. Results of the Selection Process
3.1. Diabetes
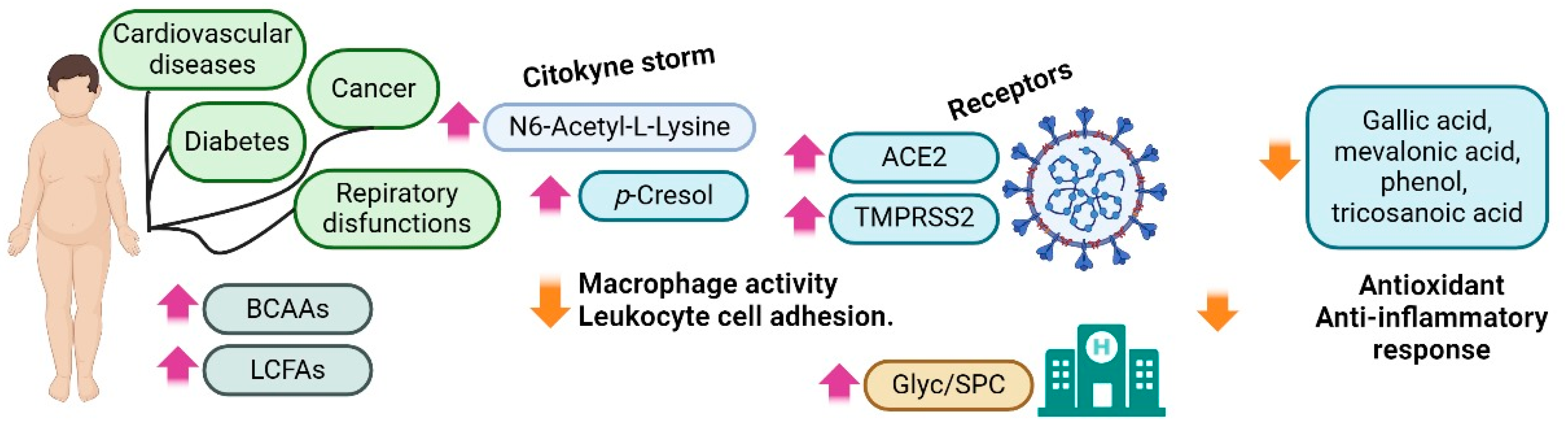
3.2. Obesity
3.3. Cancer
3.4. Kidney Disease
3.5. Cardiovascular Diseases and Blood Disorders
3.6. Alzheimer’s Disease
3.7. Thyroid Disorders
3.8. Respiratory Diseases
3.9. Inducers of Comorbidities
3.9.1. Malnutrition (Vitamins and Minerals)
3.9.2. Immunological System
3.9.3. Oxidative Stress
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Official COVID-19 Info. 2023. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed on 10 November 2023).
- Luo, Y.M.; Wu, J.; Lu, J.Y.; Xu, X.; Long, W.; Yan, G.J.; Tang, M.Y.; Zou, L.; Xu, D.Z.; Zhuo, P.; et al. Investigation of COVID-19-related symptoms based on factor analysis. Ann. Palliat. Med. 2020, 9, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Xu, Y.; Sun, C.; Wang, X.; Guo, Y.; Qiu, S.; Ma, K. A systematic review of asymptomatic infections with COVID-19. J. Microbiol. Immunol. Infect. 2021, 54, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Yuan, J.-M. Predictive symptoms and comorbidities for severe COVID-19 and intensive care unit admission: A systematic review and meta-analysis. Int. J. Public Health 2020, 65, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Marzook, H.; Ahmad, F. Comorbidities and clinical complications associated with SARS-CoV-2 infection: An overview. Clin. Exp. Med. 2023, 23, 313–331. [Google Scholar] [CrossRef] [PubMed]
- Atabati, E.; Dehghani-Samani, A.; Mortazavimoghaddam, S.G. Association of COVID-19 and other viral infections with interstitial lung diseases, pulmonary fibrosis, and pulmonary hypertension: A narrative review. Can. J. Respir. Ther. 2020, 56, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Luo, R.; Wang, K.; Zhang, M.; Wang, Z.; Dong, L.; Li, J.; Yao, Y.; Ge, S.; Xu, G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020, 97, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Bhowmik, B.; do Vale Moreira, N.C. COVID-19 and diabetes: Knowledge in progress. Diabetes Res. Clin. Pract. 2020, 162, 108142. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, C.; Vlachogiannis, N.I.; Bakogiannis, C.; Spyridopoulos, I.; Stamatelopoulos, K.; Kanakakis, I.; Vassilikos, V.; Stellos, K. Involvement of cardiovascular system as the critical point in coronavirus disease 2019 (COVID-19) prognosis and recovery. Hell. J. Cardiol. 2020, 61, 381–395. [Google Scholar] [CrossRef]
- Popkin, B.M.; Du, S.; Green, W.D.; Beck, M.A.; Algaith, T.; Herbst, C.H.; Alsukait, R.F.; Alluhidan, M.; Alazemi, N.; Shekar, M. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes. Rev. 2020, 21, e13128. [Google Scholar] [CrossRef]
- Bedock, D.; Bel Lassen, P.; Mathian, A.; Moreau, P.; Couffignal, J.; Ciangura, C.; Poitou-Bernert, C.; Jeannin, A.-C.; Mosbah, H.; Fadlallah, J.; et al. Prevalence and severity of malnutrition in hospitalized COVID-19 patients. Clin. Nutr. ESPEN 2020, 40, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.G.; Chahine, L.M.; Goldman, S.M.; Korell, M.; Mann, E.; Kinel, D.R.; Arnedo, V.; Marek, K.L.; Tanner, C.M. The effect of the COVID-19 pandemic on people with Parkinson’s disease. J. Park. Dis. 2020, 10, 1365–1377. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, Y.; Liu, M.; Shi, S.; Tian, J. Impacts of immunosuppression and immunodeficiency on COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, e93–e95. [Google Scholar] [CrossRef]
- Linjawi, M.; Shakoor, H.; Hilary, S.; Ali, H.I.; Al-Dhaheri, A.S.; Ismail, L.C.; Apostolopoulos, V.; Stojanovska, L. Cancer Patients during COVID-19 Pandemic: A Mini-Review. Healthcare 2023, 11, 248. [Google Scholar] [CrossRef] [PubMed]
- Marjot, T.; Webb, G.J.; Barritt, A.S.; Moon, A.M.; Stamataki, Z.; Wong, V.W.; Barnes, E. COVID-19 and liver disease: Mechanistic and clinical perspectives. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 348–364. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O. Combining genomics, metabolome analysis, and biochemical modelling to understand metabolic networks. Comp. Funct. Genom. 2001, 2, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Gowda, D.; Kellarai, A.; Hegde, P.S.; Agni, M.B.; Lundstrom, K.; Barh, D. Chapter 5—Metabolites and metabolomics in COVID-19. In Omics Approaches and Technologies in COVID-19; Barh, D., Azevedo, V., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 87–99. [Google Scholar] [CrossRef]
- Marín-Corral, J.; Rodríguez-Morató, J.; Gomez-Gomez, A.; Pascual-Guardia, S.; Muñoz-Bermúdez, R.; Salazar-Degracia, A.; Pérez-Terán, P.; Restrepo, M.I.; Khymenets, O.; Haro, N. Metabolic signatures associated with severity in hospitalized COVID-19 patients. Int. J. Mol. Sci. 2021, 22, 4794. [Google Scholar] [CrossRef] [PubMed]
- Keen, B.; Cawley, A.; Reedy, B.; Fu, S.L. Metabolomics in clinical and forensic toxicology, sports anti-doping and veterinary residues. Drug Test. Anal. 2022, 14, 794–807. [Google Scholar] [CrossRef]
- Alboniga, O.E.; Jimenez, D.; Sanchez-Conde, M.; Vizcarra, P.; Ron, R.; Herrera, S.; Martinez-Sanz, J.; Moreno, E.; Moreno, S.; Barbas, C.; et al. Metabolic Snapshot of Plasma Samples Reveals New Pathways Implicated in SARS-CoV-2 Pathogenesis. J. Proteome Res. 2022, 21, 623–634. [Google Scholar] [CrossRef]
- Barberis, E.; Amede, E.; Khoso, S.; Castello, L.; Sainaghi, P.P.; Bellan, M.; Balbo, P.E.; Patti, G.; Brustia, D.; Giordano, M.; et al. Metabolomics Diagnosis of COVID-19 from Exhaled Breath Condensate. Metabolites 2021, 11, 847. [Google Scholar] [CrossRef]
- Schmelter, F.; Foeh, B.; Mallagaray, A.; Rahmoeller, J.; Ehlers, M.; Lehrian, S.; von Kopylow, V.; Kuensting, I.; Lixenfeld, A.S.; Martin, E.; et al. Metabolic and Lipidomic Markers Differentiate COVID-19 From Non-Hospitalized and Other Intensive Care Patients. Front. Mol. Biosci. 2021, 8, 737039. [Google Scholar] [CrossRef]
- Kale, N.S.; Haug, K.; Conesa, P.; Jayseelan, K.; Moreno, P.; Rocca-Serra, P.; Nainala, V.C.; Spicer, R.A.; Williams, M.; Li, X. MetaboLights: An open-access database repository for metabolomics data. Curr. Protoc. Bioinform. 2016, 53, 14.13.1–14.13.18. [Google Scholar] [CrossRef]
- Liebal, U.W.; Phan, A.N.; Sudhakar, M.; Raman, K.; Blank, L.M. Machine learning applications for mass spectrometry-based metabolomics. Metabolites 2020, 10, 243. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Yi, X.; Sun, Y.; Bi, X.; Du, J.; Zhang, C.; Quan, S.; Zhang, F.; Sun, R.; Qian, L.; et al. Proteomic and Metabolomic Characterization of COVID-19 Patient Sera. Cell 2020, 182, 59–72.e15. [Google Scholar] [CrossRef]
- Murillo, J.; Villegas, L.M.; Ulloa-Murillo, L.M.; Rodríguez, A.R. Recent trends on omics and bioinformatics approaches to study SARS-CoV-2: A bibliometric analysis and mini-review. Comput. Biol. Med. 2021, 128, 104162. [Google Scholar] [CrossRef]
- Su, Y.; Chen, D.; Yuan, D.; Lausted, C.; Choi, J.; Dai, C.L.; Voillet, V.; Duvvuri, V.R.; Scherler, K.; Troisch, P.; et al. Multi-Omics Resolves a Sharp Disease-State Shift between Mild and Moderate COVID-19. Cell 2020, 183, 1479–1495.e1420. [Google Scholar] [CrossRef]
- Suvarna, K.; Biswas, D.; Pai, M.G.J.; Acharjee, A.; Bankar, R.; Palanivel, V.; Salkar, A.; Verma, A.; Mukherjee, A.; Choudhury, M.; et al. Proteomics and Machine Learning Approaches Reveal a Set of Prognostic Markers for COVID-19 Severity with Drug Repurposing Potential. Front. Physiol. 2021, 12, 652799. [Google Scholar] [CrossRef] [PubMed]
- Heald, A.H.; Stedman, M.; Davies, M.; Livingston, M.; Alshames, R.; Lunt, M.; Rayman, G.; Gadsby, R. Estimating life years lost to diabetes: Outcomes from analysis of National Diabetes Audit and Office of National Statistics data. Cardiovasc. Endocrinol. Metab. 2020, 9, 183. [Google Scholar] [CrossRef] [PubMed]
- Toniolo, A.; Cassani, G.; Puggioni, A.; Rossi, A.; Colombo, A.; Onodera, T.; Ferrannini, E. The diabetes pandemic and associated infections: Suggestions for clinical microbiology. Rev. Med. Microbiol. 2019, 30, 1–17. [Google Scholar] [CrossRef]
- Berbudi, A.; Rahmadika, N.; Tjahjadi, A.I.; Ruslami, R. Type 2 diabetes and its impact on the immune system. Curr. Diabetes Rev. 2020, 16, 442. [Google Scholar] [CrossRef]
- World Health Organization. WHO Diabetes. 2023. Available online: https://www.who.int/health-topics/diabetes#tab=tab_1 (accessed on 10 November 2023).
- Shah, A.D.; Langenberg, C.; Rapsomaniki, E.; Denaxas, S.; Pujades-Rodriguez, M.; Gale, C.P.; Deanfield, J.; Smeeth, L.; Timmis, A.; Hemingway, H. Type 2 diabetes and incidence of cardiovascular diseases: A cohort study in 1 9 million people. Lancet Diabetes Endocrinol. 2015, 3, 105–113. [Google Scholar] [CrossRef]
- Jitraknatee, J.; Ruengorn, C.; Nochaiwong, S. Prevalence and risk factors of chronic kidney disease among type 2 diabetes patients: A cross-sectional study in primary care practice. Sci. Rep. 2020, 10, 6205. [Google Scholar] [CrossRef]
- Ferlita, S.; Yegiazaryan, A.; Noori, N.; Lal, G.; Nguyen, T.; To, K.; Venketaraman, V. Type 2 diabetes mellitus and altered immune system leading to susceptibility to pathogens, especially Mycobacterium tuberculosis. J. Clin. Med. 2019, 8, 2219. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Khanal, V.K.; Jha, N.; Pyakurel, P.; Gurung, G.N. Study of the magnitude of diabetes and its associated risk factors among the tuberculosis patients of Morang, Eastern Nepal. BMC Public Health 2019, 19, 1545. [Google Scholar] [CrossRef]
- Daryabor, G.; Atashzar, M.R.; Kabelitz, D.; Meri, S.; Kalantar, K. The effects of type 2 diabetes mellitus on organ metabolism and the immune system. Front. Immunol. 2020, 11, 1582. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-Y.; Kuo, R.-L.; Huang, H.-I. Activation of type I interferon antiviral response in human neural stem cells. Stem Cell Res. Ther. 2019, 10, 387. [Google Scholar] [CrossRef]
- Schneider, W.M.; Chevillotte, M.D.; Rice, C.M. Interferon-stimulated genes: A complex web of host defenses. Annu. Rev. Immunol. 2014, 32, 513–545. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, P.; Gronke, K.; Diefenbach, A. A catch-22: Interleukin-22 and cancer. Eur. J. Immunol. 2018, 48, 15–31. [Google Scholar] [CrossRef]
- Hu, R.; Xia, C.-Q.; Butfiloski, E.; Clare-Salzler, M. Effect of high glucose on cytokine production by human peripheral blood immune cells and type I interferon signaling in monocytes: Implications for the role of hyperglycemia in the diabetes inflammatory process and host defense against infection. Clin. Immunol. 2018, 195, 139–148. [Google Scholar] [CrossRef]
- Wang, X.; Ota, N.; Manzanillo, P.; Kates, L.; Zavala-Solorio, J.; Eidenschenk, C.; Zhang, J.; Lesch, J.; Lee, W.P.; Ross, J. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature 2014, 514, 237–241. [Google Scholar] [CrossRef]
- Posso, M.; Comas, M.; Román, M.; Domingo, L.; Louro, J.; González, C.; Sala, M.; Anglès, A.; Cirera, I.; Cots, F. Comorbidities and mortality in patients with COVID-19 aged 60 years and older in a university hospital in Spain. Arch. De Bronconeumol. 2020, 56, 756. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Gillies, C.L.; Singh, R.; Singh, A.; Chudasama, Y.; Coles, B.; Seidu, S.; Zaccardi, F.; Davies, M.J.; Khunti, K. Prevalence of co-morbidities and their association with mortality in patients with COVID-19: A systematic review and meta-analysis. Diabetes Obes. Metab. 2020, 22, 1915–1924. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; Yu, T. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Elrayess, M.A.; Cyprian, F.S.; Abdallah, A.M.; Emara, M.M.; Diboun, I.; Anwardeen, N.; Schuchardt, S.; Yassine, H.M. Metabolic Signatures of Type 2 Diabetes Mellitus and Hypertension in COVID-19 Patients with Different Disease Severity. Front. Med. 2022, 8, 788687. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gómez, L.E.; Ibarra-González, I.; Fernández-Lainez, C.; Tusie, T.; Moreno-Macías, H.; Martinez-Armenta, C.; Jimenez-Gutierrez, G.E.; Vázquez-Cárdenas, P.; Vidal-Vázquez, P.; Ramírez-Hinojosa, J.P.; et al. Metabolic Reprogramming in SARS-CoV-2 Infection Impacts the Outcome of COVID-19 Patients. Front. Immunol. 2022, 13, 936106. [Google Scholar] [CrossRef] [PubMed]
- Maltais-Payette, I.; Lajeunesse-Trempe, F.; Pibarot, P.; Biertho, L.; Tchernof, A. Association between Circulating Amino Acids and COVID-19 Severity. Metabolites 2023, 13, 201. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, N.; Montazeri, H. On BCG Vaccine Protection from COVID-19: A Review. SN Compr. Clin. Med. 2021, 3, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.; Reandelar, M.; Fasciglione, K.; Roumenova, V.; Li, Y.; Otazu, G. Correlation between universal BCG vaccination policy and reduced morbidity and mortality for COVID-19: An epidemiological study. medRxiv 2020. [Google Scholar] [CrossRef]
- Anwardeen, N.R.; Cyprian, F.S.; Yassine, H.M.; Al-Thani, A.A.; Abdallah, A.M.; Emara, M.M.; Elrayess, M.A. The retrospective study of the metabolic patterns of BCG-vaccination in type-2 diabetic individuals in COVID-19 infection. Front. Immunol. 2023, 14, 1146443. [Google Scholar] [CrossRef]
- Tong, L.; Xiao, X.; Li, M.; Fang, S.; Ma, E.; Yu, X.; Zhu, Y.; Wu, C.; Tian, D.; Yang, F.; et al. A glucose-like metabolite deficient in diabetes inhibits cellular entry of SARS-CoV-2. Nat. Metab. 2022, 4, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, K.; Egea Rodrigues, C.; Floegel, A.; Ahrens, W. Omics Biomarkers in Obesity: Novel Etiological Insights and Targets for Precision Prevention. Curr. Obes. Rep. 2020, 9, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Payab, M.; Tayanloo-Beik, A.; Falahzadeh, K.; Mousavi, M.; Salehi, S.; Djalalinia, S.; Ebrahimpur, M.; Rezaei, N.; Rezaei-Tavirani, M.; Larijani, B.; et al. Metabolomics prospect of obesity and metabolic syndrome; a systematic review. J. Diabetes Metab. Disord. 2022, 21, 889–917. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Obesity. 2023. Available online: https://www.who.int/health-topics/obesity#tab=tab_1 (accessed on 10 November 2023).
- Rangel-Huerta, O.D.; Pastor-Villaescusa, B.; Gil, A. Are we close to defining a metabolomic signature of human obesity? A systematic review of metabolomics studies. Metabolomics 2019, 15, 93. [Google Scholar] [CrossRef] [PubMed]
- Sorrow, P.; Maguire, R.; Murphy, S.K.; Belcher, S.M.; Hoyo, C. Elevated metabolites of acetaminophen in cord blood of children with obesity. Pediatr. Obes. 2019, 14, e12465. [Google Scholar] [CrossRef] [PubMed]
- Szczerbinski, L.; Wojciechowska, G.; Olichwier, A.; Taylor, M.A.; Puchta, U.; Konopka, P.; Paszko, A.; Citko, A.; Goscik, J.; Fiehn, O.; et al. Untargeted Metabolomics Analysis of the Serum Metabolic Signature of Childhood Obesity. Nutrients 2022, 14, 214. [Google Scholar] [CrossRef] [PubMed]
- Marco-Ramell, A.; Tulipani, S.; Palau-Rodriguez, M.; Gonzalez-Dominguez, R.; Miñarro, A.; Jauregui, O.; Sanchez-Pla, A.; Macias-Gonzalez, M.; Cardona, F.; Tinahones, F.J.; et al. Untargeted Profiling of Concordant/Discordant Phenotypes of High Insulin Resistance and Obesity To Predict the Risk of Developing Diabetes. J. Proteome Res. 2018, 17, 2307–2317. [Google Scholar] [CrossRef] [PubMed]
- Tulipani, S.; Palau-Rodriguez, M.; Miñarro Alonso, A.; Cardona, F.; Marco-Ramell, A.; Zonja, B.; Lopez de Alda, M.; Muñoz-Garach, A.; Sanchez-Pla, A.; Tinahones, F.J.; et al. Biomarkers of Morbid Obesity and Prediabetes by Metabolomic Profiling of Human Discordant Phenotypes. Clin. Chim. Acta 2016, 463, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Zhang, J.; Wang, M.; Chen, L. Obesity is associated with severe COVID-19 but not death: A dose−response meta-analysis. Epidemiol. Infect. 2021, 149, e144. [Google Scholar] [CrossRef]
- Singh, R.; Rathore, S.S.; Khan, H.; Karale, S.; Chawla, Y.; Iqbal, K.; Bhurwal, A.; Tekin, A.; Jain, N.; Mehra, I.; et al. Association of Obesity with COVID-19 Severity and Mortality: An Updated Systemic Review, Meta-Analysis, and Meta-Regression. Front. Endocrinol. 2022, 13, 780872. [Google Scholar] [CrossRef]
- Stefan, N.; Birkenfeld, A.L.; Schulze, M.B.; Ludwig, D.S. Obesity and impaired metabolic health in patients with COVID-19. Nat. Rev. Endocrinol. 2020, 16, 341–342. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hu, J.; Zhu, C. Obesity aggravates COVID-19: A systematic review and meta-analysis. J. Med. Virol. 2021, 93, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Zorita, S.; Milton-Laskibar, I.; García-Arellano, L.; González, M.; Portillo, M.P. An Overview of Adipose Tissue ACE2 Modulation by Diet and Obesity. Potential Implications in COVID-19 Infection and Severity. Int. J. Mol. Sci. 2021, 22, 7975. [Google Scholar] [CrossRef] [PubMed]
- Sarver, D.C.; Wong, G.W. Obesity alters Ace2 and Tmprss2 expression in lung, trachea, and esophagus in a sex-dependent manner: Implications for COVID-19. Biochem. Biophys. Res. Commun. 2021, 538, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Molani Gol, R.; Rafraf, M. Association between abdominal obesity and pulmonary function in apparently healthy adults: A systematic review. Obes. Res. Clin. Pract. 2021, 15, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Rössler, T.; Berezhnoy, G.; Singh, Y.; Cannet, C.; Reinsperger, T.; Schäfer, H.; Spraul, M.; Kneilling, M.; Merle, U.; Trautwein, C. Quantitative Serum NMR Spectroscopy Stratifies COVID-19 Patients and Sheds Light on Interfaces of Host Metabolism and the Immune Response with Cytokines and Clinical Parameters. Metabolites 2022, 12, 1277. [Google Scholar] [CrossRef]
- Jalaleddine, N.; Hachim, M.; Al-Hroub, H.; Saheb Sharif-Askari, N.; Senok, A.; Elmoselhi, A.; Mahboub, B.; Samuel Kurien, N.M.; Kandasamy, R.K.; Semreen, M.H.; et al. N6-Acetyl-L-Lysine and p-Cresol as Key Metabolites in the Pathogenesis of COVID-19 in Obese Patients. Front. Immunol. 2022, 13, 827603. [Google Scholar] [CrossRef]
- Lee, A.J.X.; Purshouse, K. COVID-19 and cancer registries: Learning from the first peak of the SARS-CoV-2 pandemic. Br. J. Cancer 2021, 124, 1777–1784. [Google Scholar] [CrossRef]
- Bourgin, M.; Derosa, L.; Silva, C.A.C.; Goubet, A.-G.; Dubuisson, A.; Danlos, F.-X.; Grajeda-Iglesias, C.; Cerbone, L.; Geraud, A.; Laparra, A. Circulating acetylated polyamines correlate with COVID-19 severity in cancer patients. Aging 2021, 13, 20860. [Google Scholar] [CrossRef]
- Yoo, H.C.; Yu, Y.C.; Sung, Y.; Han, J.M. Glutamine reliance in cell metabolism. Exp. Mol. Med. 2020, 52, 1496–1516. [Google Scholar] [CrossRef] [PubMed]
- Alberghina, L.; Gaglio, D. Redox control of glutamine utilization in cancer. Cell Death Dis. 2014, 5, e1561. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, T.; Yoshinaga, S.K.; Shibue, K.; Mak, T.W. Comorbidity-associated glutamine deficiency is a predisposition to severe COVID-19. Cell Death Differ. 2021, 28, 3199–3213. [Google Scholar] [CrossRef] [PubMed]
- Amere Subbarao, S. Cancer vs. SARS-CoV-2 induced inflammation, overlapping functions, and pharmacological targeting. Inflammopharmacology 2021, 29, 343–366. [Google Scholar] [CrossRef] [PubMed]
- Costantini, S.; Madonna, G.; Di Gennaro, E.; Capone, F.; Bagnara, P.; Capone, M.; Sale, S.; Nicastro, C.; Atripaldi, L.; Fiorentino, G.; et al. New Insights into the Identification of Metabolites and Cytokines Predictive of Outcome for Patients with Severe SARS-CoV-2 Infection Showed Similarity with Cancer. Int. J. Mol. Sci. 2023, 24, 4922. [Google Scholar] [CrossRef] [PubMed]
- Denkinger, C.M.; Janssen, M.; Schäkel, U.; Gall, J.; Leo, A.; Stelmach, P.; Weber, S.F.; Krisam, J.; Baumann, L.; Stermann, J.; et al. Anti-SARS-CoV-2 antibody-containing plasma improves outcome in patients with hematologic or solid cancer and severe COVID-19: A randomized clinical trial. Nat. Cancer 2023, 4, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Zhong, N.S. Clinical Characteristics of COVID-19 in China. Reply. N. Engl. J. Med. 2020, 382, 1861–1862. [Google Scholar] [CrossRef] [PubMed]
- Pei, G.; Zhang, Z.; Peng, J.; Liu, L.; Zhang, C.; Yu, C.; Ma, Z.; Huang, Y.; Liu, W.; Yao, Y.; et al. Renal Involvement and Early Prognosis in Patients with COVID-19 Pneumonia. J. Am. Soc. Nephrol. 2020, 31, 1157–1165. [Google Scholar] [CrossRef]
- Gupta, S.; Coca, S.G.; Chan, L.; Melamed, M.L.; Brenner, S.K.; Hayek, S.S.; Sutherland, A.; Puri, S.; Srivastava, A.; Leonberg-Yoo, A. AKI treated with renal replacement therapy in critically ill patients with COVID-19. J. Am. Soc. Nephrol. 2021, 32, 161–176. [Google Scholar] [CrossRef]
- Ronco, C.; Bellomo, R.; Kellum, J.A. Acute kidney injury. Lancet 2019, 394, 1949–1964. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Henry, B.M.; Lippi, G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int. Urol. Nephrol. 2020, 52, 1193–1194. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, J.S.; Ng, J.H.; Ross, D.W.; Sharma, P.; Shah, H.H.; Barnett, R.L.; Hazzan, A.D.; Fishbane, S.; Jhaveri, K.D.; Abate, M. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020, 98, 209–218. [Google Scholar] [CrossRef]
- Fisher, M.; Neugarten, J.; Bellin, E.; Yunes, M.; Stahl, L.; Johns, T.S.; Abramowitz, M.K.; Levy, R.; Kumar, N.; Mokrzycki, M.H. AKI in hospitalized patients with and without COVID-19: A comparison study. J. Am. Soc. Nephrol. 2020, 31, 2145–2157. [Google Scholar] [CrossRef]
- Kellum, J.A.; van Till, J.O.; Mulligan, G. Targeting acute kidney injury in COVID-19. Nephrol. Dial. Transplant. 2020, 35, 1652–1662. [Google Scholar] [CrossRef]
- Lin, L.; Wang, X.; Ren, J.; Sun, Y.; Yu, R.; Li, K.; Zheng, L.; Yang, J. Risk factors and prognosis for COVID-19-induced acute kidney injury: A meta-analysis. BMJ Open 2020, 10, e042573. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, J.; Fonseca, J.A.; Outerelo, C.; Lopes, J.A. Acute kidney injury: From diagnosis to prevention and treatment strategies. J. Clin. Med. 2020, 9, 1704. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef] [PubMed]
- Marx, D.; Metzger, J.; Pejchinovski, M.; Gil, R.B.; Frantzi, M.; Latosinska, A.; Belczacka, I.; Heinzmann, S.S.; Husi, H.; Zoidakis, J. Proteomics and metabolomics for AKI diagnosis. Semin. Nephrol. 2018, 38, 63–87. [Google Scholar] [CrossRef]
- Murali, R.; Wanjari, U.R.; Mukherjee, A.G.; Gopalakrishnan, A.V.; Kannampuzha, S.; Namachivayam, A.; Madhyastha, H.; Renu, K.; Ganesan, R. Crosstalk between COVID-19 Infection and Kidney Diseases: A Review on the Metabolomic Approaches. Vaccines 2023, 11, 489. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, H.; Qiu, S.; Wang, X. Metabolomics insights into pathophysiological mechanisms of nephrology. Int. Urol. Nephrol. 2014, 46, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Vergara, A.; Wang, K.; Colombo, D.; Gheblawi, M.; Rasmuson, J.; Mandal, R.; Del Nonno, F.; Chiu, B.; Scholey, J.W.; Soler, M.J. Urinary angiotensin-converting enzyme 2 and metabolomics in COVID-19-mediated kidney injury. Clin. Kidney J. 2023, 16, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, I.; Garrett, T.J. Mass spectrometry techniques in emerging pathogens studies: COVID-19 perspectives. J. Am. Soc. Mass Spectrom. 2020, 31, 2013–2024. [Google Scholar] [CrossRef] [PubMed]
- Porcheddu, R.; Serra, C.; Kelvin, D.; Kelvin, N.; Rubino, S. Similarity in Case Fatality Rates (CFR) of COVID-19/SARS-CoV-2 in Italy and China. J. Infect. Dev. Ctries 2020, 14, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of Cardiac Injury with Mortality in Hospitalized Patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802–810. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Marrie, T.J.; Shariatzadeh, M.R. Community-acquired pneumonia requiring admission to an intensive care unit: A descriptive study. Medicine 2007, 86, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.A.; Warren-Gash, C. Cardiovascular complications of acute respiratory infections: Current research and future directions. Expert Rev. Anti. Infect. Ther. 2019, 17, 939–942. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, K.; Salkar, A.; Palanivel, V.; Bankar, R.; Banerjee, N.; Gayathri, J.P.M.; Srivastava, A.; Singh, A.; Khatri, H.; Agrawal, S.; et al. A Multi-omics Longitudinal Study Reveals Alteration of the Leukocyte Activation Pathway in COVID-19 Patients. J. Proteome Res. 2021, 20, 4667–4680. [Google Scholar] [CrossRef]
- Li, T.; Ning, N.; Li, B.; Luo, D.; Qin, E.; Yu, W.; Wang, J.; Yang, G.; Nan, N.; He, Z.; et al. Longitudinal Metabolomics Reveals Ornithine Cycle Dysregulation Correlates with Inflammation and Coagulation in COVID-19 Severe Patients. Front. Microbiol. 2021, 12, 723818. [Google Scholar] [CrossRef]
- Rizvi, Z.A.; Dalal, R.; Sadhu, S.; Binayke, A.; Dandotiya, J.; Kumar, Y.; Shrivastava, T.; Gupta, S.K.; Aggarwal, S.; Tripathy, M.R.; et al. Golden Syrian hamster as a model to study cardiovascular complications associated with SARS-CoV-2 infection. Elife 2022, 11, e73522. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, T.T.; Li, Y.; Li, J.; Fan, Y.; Huang, F.Q.; Cai, Y.Y.; Ma, G.; Liu, J.F.; Chen, Q.Q.; et al. Functional Metabolomics Characterizes a Key Role for N-Acetylneuraminic Acid in Coronary Artery Diseases. Circulation 2018, 137, 1374–1390. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention. Blood Disorders. 2023. Available online: https://www.cdc.gov/ncbddd/blooddisorders/index.html (accessed on 10 November 2023).
- Vijenthira, A.; Gong, I.Y.; Fox, T.A.; Booth, S.; Cook, G.; Fattizzo, B.; Martín-Moro, F.; Razanamahery, J.; Riches, J.C.; Zwicker, J.; et al. Outcomes of patients with hematologic malignancies and COVID-19: A systematic review and meta-analysis of 3377 patients. Blood 2020, 136, 2881–2892. [Google Scholar] [CrossRef] [PubMed]
- Henry, B.M.; de Oliveira, M.H.S.; Benoit, S.; Plebani, M.; Lippi, G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): A meta-analysis. Clin. Chem. Lab. Med. 2020, 58, 1021–1028. [Google Scholar] [CrossRef]
- Bange, E.M.; Han, N.A.; Wileyto, P.; Kim, J.Y.; Gouma, S.; Robinson, J.; Greenplate, A.R.; Hwee, M.A.; Porterfield, F.; Owoyemi, O.; et al. CD8(+) T cells contribute to survival in patients with COVID-19 and hematologic cancer. Nat. Med. 2021, 27, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Bird, P.W.; Badhwar, V.; Kennedy, B.; Ladani, S.; Tang, J.W. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) seroconversion in hematology-oncology patients. J. Med. Virol. 2021, 93, 4585–4591. [Google Scholar] [CrossRef] [PubMed]
- Candoni, A.; Pizzano, U.; Fabris, M.; Curcio, F.; Fanin, R. Seroconversion and kinetic of anti SARS-CoV-2 antibodies in 25 patients with hematological malignancies who recovered from SARS-CoV-2 infection. Hematol. Oncol. 2021, 39, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, E.; Pellegrino, M.; Todisco, V.; Dolci, G.; Bettelli, F.; Meschiari, M.; Bedini, A.; Fregni-Serpini, G.; Grottola, A.; Guaraldi, G. Persistent SARS-CoV-2 infection with multiple clinical relapses in two patients with follicular lymphoma treated with bendamustine and obinutuzumab or rituximab. Infection 2023, 51, 1577–1581. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Chen, L.; Chen, L.; Yuan, G.; Fang, Y.; Chen, W.; Wu, D.; Liang, B.; Lu, X.; Ma, Y.; et al. COVID-19 in persons with haematological cancers. Leukemia 2020, 34, 1637–1645. [Google Scholar] [CrossRef]
- Mansi, L.; Spehner, L.; Daguindau, E.; Bouiller, K.; Almotlak, H.; Stein, U.; Bouard, A.; Kim, S.; Klajer, E.; Jary, M.; et al. Study of the SARS-CoV-2-specific immune T-cell responses in COVID-19-positive cancer patients. Eur. J. Cancer 2021, 150, 1–9. [Google Scholar] [CrossRef]
- Oliva, A.; Curtolo, A.; Volpicelli, L.; Cancelli, F.; Borrazzo, C.; Cogliati Dezza, F.; Marcelli, G.; Gavaruzzi, F.; Di Bari, S.; Ricci, P.; et al. Correction: Clinical course of Coronavirus Disease-19 in patients with haematological malignancies is characterized by a longer time to respiratory deterioration compared to non-haematological ones: Results from a case-control study. Infection 2022, 50, 1383. [Google Scholar] [CrossRef] [PubMed]
- Passamonti, F.; Romano, A.; Salvini, M.; Merli, F.; Porta, M.G.D.; Bruna, R.; Coviello, E.; Romano, I.; Cairoli, R.; Lemoli, R.; et al. COVID-19 elicits an impaired antibody response against SARS-CoV-2 in patients with haematological malignancies. Br. J. Haematol. 2021, 195, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Raja, M.; Natori, Y.; Chandorkar, A.; Camargo, J.F.; Simkins, J.; Andrews, D.; Bradley, T.; Watts, J.; Komanduri, K.; Morris, M.I.; et al. Discordance Between Radiologic Findings and Molecular Testing in Patients with Underlying Hematologic Malignancy and Coronavirus Disease 2019. Open Forum. Infect. Dis. 2020, 7, ofaa372. [Google Scholar] [CrossRef] [PubMed]
- 2020 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2020, 16, 391–460. [CrossRef] [PubMed]
- Jeremic, D.; Jiménez-Díaz, L.; Navarro-López, J.D. Past, present and future of therapeutic strategies against amyloid-β peptides in Alzheimer’s disease: A systematic review. Ageing Res. Rev. 2021, 72, 101496. [Google Scholar] [CrossRef] [PubMed]
- Quintero Escobar, M.; Pontes, J.G.d.M.; Tasic, L. Metabolomics in degenerative brain diseases. Brain Res. 2021, 1773, 147704. [Google Scholar] [CrossRef] [PubMed]
- Schumacher-Schuh, A.; Bieger, A.; Borelli, W.V.; Portley, M.K.; Awad, P.S.; Bandres-Ciga, S. Advances in proteomic and metabolomic profiling of neurodegenerative diseases. Front. Neurol. 2022, 12, 2545. [Google Scholar] [CrossRef] [PubMed]
- Bianchetti, A.; Rozzini, R.; Guerini, F.; Boffelli, S.; Ranieri, P.; Minelli, G.; Bianchetti, L.; Trabucchi, M. Clinical Presentation of COVID19 in Dementia Patients. J. Nutr. Health Aging 2020, 24, 560–562. [Google Scholar] [CrossRef] [PubMed]
- Lingor, P.; Demleitner, A.F.; Wolff, A.W.; Feneberg, E. SARS-CoV-2 and neurodegenerative diseases: What we know and what we don’t. J. Neural Transm. 2022, 129, 1155–1167. [Google Scholar] [CrossRef]
- Flores-Silva, F.D.; García-Grimshaw, M.; Valdés-Ferrer, S.I.; Vigueras-Hernández, A.P.; Domínguez-Moreno, R.; Tristán-Samaniego, D.P.; Michel-Chávez, A.; González-Duarte, A.; Vega-Boada, F.A.; Reyes-Melo, I.; et al. Neurologic manifestations in hospitalized patients with COVID-19 in Mexico City. PLoS ONE 2021, 16, e0247433. [Google Scholar] [CrossRef]
- García-Moncó, J.C.; Cabrera Muras, A.; Erburu Iriarte, M.; Rodrigo Armenteros, P.; Collía Fernández, A.; Arranz-Martínez, J.; Kapetanovic, S.; Lorenzo-García, A.; Bilbao González, A.; Gomez-Beldarrain, M. Neurologic Manifestations in a Prospective Unselected Series of Hospitalized Patients with COVID-19. Neurol. Clin. Pract. 2021, 11, e64–e72. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Patricio, F.; Patricio-Martínez, A.; Santos-López, G.; Cedillo, L.; Tizabi, Y.; Limón, I.D. Neuropathological Aspects of SARS-CoV-2 Infection: Significance for Both Alzheimer’s and Parkinson’s Disease. Front. Neurosci. 2022, 16, 867825. [Google Scholar] [CrossRef]
- Desforges, M.; Le Coupanec, A.; Dubeau, P.; Bourgouin, A.; Lajoie, L.; Dubé, M.; Talbot, P.J. Human Coronaviruses and Other Respiratory Viruses: Underestimated Opportunistic Pathogens of the Central Nervous System? Viruses 2020, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Chiricosta, L.; Gugliandolo, A.; Mazzon, E. SARS-CoV-2 Exacerbates Beta-Amyloid Neurotoxicity, Inflammation and Oxidative Stress in Alzheimer’s Disease Patients. Int. J. Mol. Sci. 2021, 22, 13603. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, R.C. The What and How of prefrontal cortical organization. Trends Neurosci. 2010, 33, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Ciaccio, M.; Lo Sasso, B.; Scazzone, C.; Gambino, C.M.; Ciaccio, A.M.; Bivona, G.; Piccoli, T.; Giglio, R.V.; Agnello, L. COVID-19 and Alzheimer’s Disease. Brain Sci. 2021, 11, 305. [Google Scholar] [CrossRef]
- Fernandes, A.M.; Vendramini, P.H.; Galaverna, R.; Schwab, N.V.; Alberici, L.C.; Augusti, R.; Castilho, R.F.; Eberlin, M.N. Direct Visualization of Neurotransmitters in Rat Brain Slices by Desorption Electrospray Ionization Mass Spectrometry Imaging (DESI—MS). J. Am. Soc. Mass. Spectrom. 2016, 27, 1944–1951. [Google Scholar] [CrossRef] [PubMed]
- Stathatos, N. Anatomy and Physiology of the Thyroid Gland. In The Thyroid and Its Diseases: A Comprehensive Guide for the Clinician; Luster, M., Duntas, L.H., Wartofsky, L., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 3–12. [Google Scholar] [CrossRef]
- Gessl, A.; Lemmens-Gruber, R.; Kautzky-Willer, A. Thyroid Disorders. In Sex and Gender Differences in Pharmacology; Regitz-Zagrosek, V., Ed.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2012; pp. 361–386. [Google Scholar] [CrossRef]
- Ruggeri, R.M.; Campennì, A.; Deandreis, D.; Siracusa, M.; Tozzoli, R.; Petranović Ovčariček, P.; Giovanella, L. SARS-CoV-2-related immune-inflammatory thyroid disorders: Facts and perspectives. Expert Rev. Clin. Immunol. 2021, 17, 737–759. [Google Scholar] [CrossRef]
- Wei, L.; Sun, S.; Xu, C.-h.; Zhang, J.; Xu, Y.; Zhu, H.; Peh, S.-c.; Korteweg, C.; McNutt, M.A.; Gu, J. Pathology of the thyroid in severe acute respiratory syndrome. Hum. Pathol. 2007, 38, 95–102. [Google Scholar] [CrossRef]
- Gavriatopoulou, M.; Korompoki, E.; Fotiou, D.; Ntanasis-Stathopoulos, I.; Psaltopoulou, T.; Kastritis, E.; Terpos, E.; Dimopoulos, M.A. Organ-specific manifestations of COVID-19 infection. Clin. Exp. Med. 2020, 20, 493–506. [Google Scholar] [CrossRef]
- Moore, J.B.; June, C.H. Cytokine release syndrome in severe COVID-19. Science 2020, 368, 473–474. [Google Scholar] [CrossRef] [PubMed]
- Amich, I.; Anguita, E.; Escribano-Serrat, S.; Alvarez, C.; Rodríguez-Muñoz, D.; García, V.; Bello, R.; Peña-Pedrosa, J.A.; Martínez-Micaelo, N.; Amigó, N.; et al. Free triiodothyronine levels and age influences the metabolic profile and COVID-19 severity parameters in euthyroid and levothyroxine-treated patients. Front. Endocrinol. 2022, 13, 1025032. [Google Scholar] [CrossRef] [PubMed]
- Khoo, B.; Tan, T.; Clarke, S.A.; Mills, E.G.; Patel, B.; Modi, M.; Phylactou, M.; Eng, P.C.; Thurston, L.; Alexander, E.C.; et al. Thyroid Function Before, During, and After COVID-19. J. Clin. Endocrinol. Metab. 2021, 106, e803–e811. [Google Scholar] [CrossRef] [PubMed]
- Baldelli, R.; Nicastri, E.; Petrosillo, N.; Marchioni, L.; Gubbiotti, A.; Sperduti, I.; Di Giacinto, P.; Rizza, L.; Rota, F.; Franco, M.; et al. Thyroid dysfunction in COVID-19 patients. J. Endocrinol. Investig. 2021, 44, 2735–2739. [Google Scholar] [CrossRef] [PubMed]
- Lui, D.T.W.; Lee, C.H.; Chow, W.S.; Lee, A.C.H.; Tam, A.R.; Fong, C.H.Y.; Law, C.Y.; Leung, E.K.H.; To, K.K.W.; Tan, K.C.B.; et al. Thyroid Dysfunction in Relation to Immune Profile, Disease Status, and Outcome in 191 Patients with COVID-19. J. Clin. Endocrinol. Metab. 2021, 106, e926–e935. [Google Scholar] [CrossRef] [PubMed]
- Moitra, S.; Bandyopadhyay, A.; Lacy, P. Metabolomics of Respiratory Diseases. Handb. Exp. Pharmacol. 2023, 277, 339–365. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wang, B.; Yuan, T.; Chen, X.; Ao, Y.; Fitzpatrick, T.; Li, P.; Zhou, Y.; Lin, Y.-f.; Duan, Q. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: A systematic review and meta-analysis. J. Infect. 2020, 80, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Aveyard, P.; Gao, M.; Lindson, N.; Hartmann-Boyce, J.; Watkinson, P.; Young, D.; Coupland, C.A.C.; Tan, P.S.; Clift, A.K.; Harrison, D.; et al. Association between pre-existing respiratory disease and its treatment, and severe COVID-19: A population cohort study. Lancet Respir. Med. 2021, 9, 909–923. [Google Scholar] [CrossRef] [PubMed]
- Beltramo, G.; Cottenet, J.; Mariet, A.-S.; Georges, M.; Piroth, L.; Tubert-Bitter, P.; Bonniaud, P.; Quantin, C. Chronic respiratory diseases are predictors of severe outcome in COVID-19 hospitalised patients: A nationwide study. Eur. Respir. J. 2021, 58, 2004474. [Google Scholar] [CrossRef]
- Diboun, I.; Cyprian, F.S.; Anwardeen, N.R.; Yassine, H.M.; Elrayess, M.A.; Rahmoon, S.M.; Sayed, S.K.; Schuchardt, S.; Khatib, M.; Bansal, D.; et al. Identification of Prognostic Metabolomic Biomarkers at the Interface of Mortality and Morbidity in Pre-Existing TB Cases Infected with SARS-CoV-2. Front. Cell. Infect. Microbiol. 2022, 12, 929689. [Google Scholar] [CrossRef]
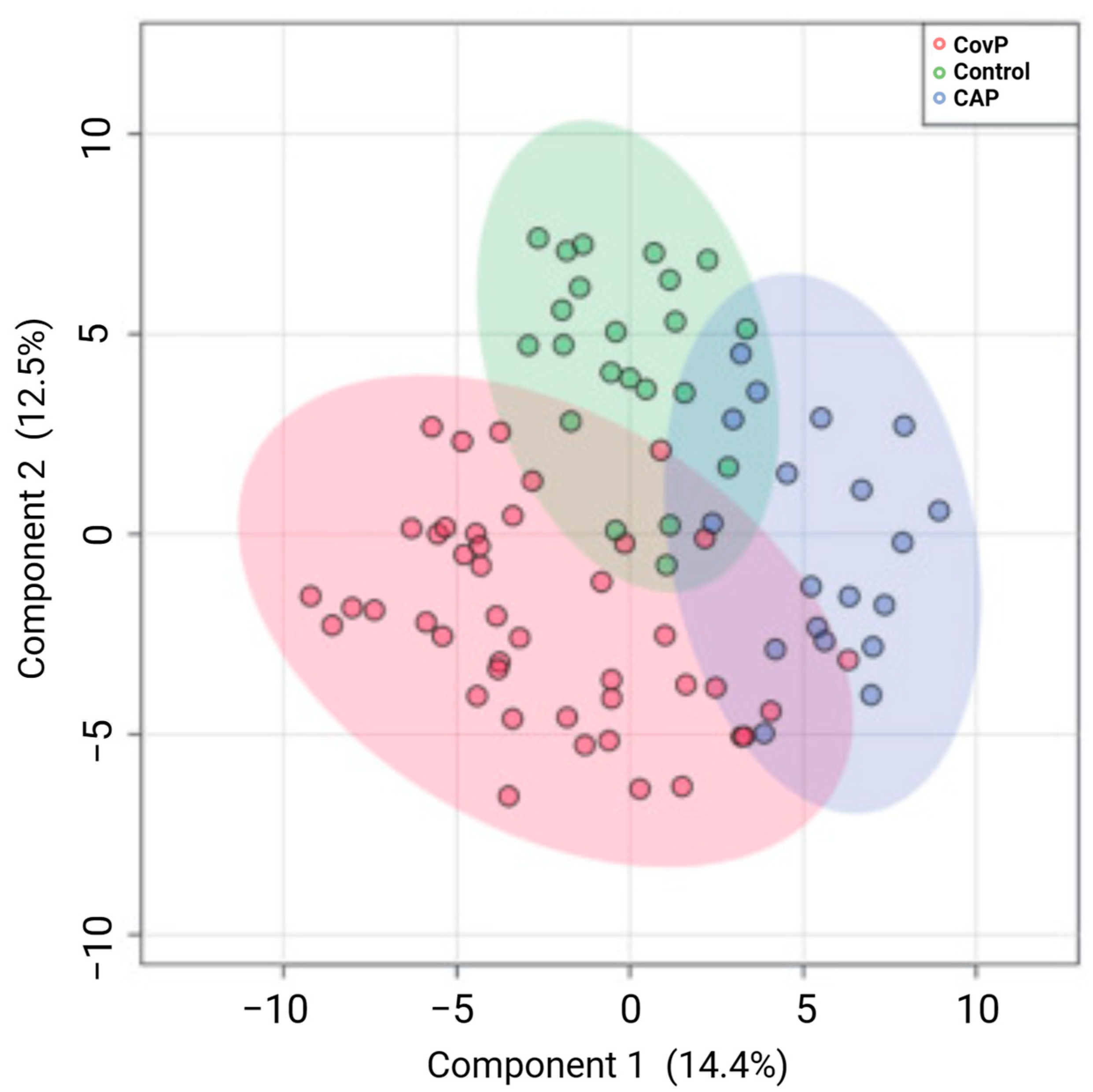
- More, T.H.; Mozafari, B.; Märtens, A.; Herr, C.; Lepper, P.M.; Danziger, G.; Volk, T.; Hoersch, S.; Krawczyk, M.; Guenther, K.; et al. Plasma Metabolome Alterations Discriminate between COVID-19 and Non-COVID-19 Pneumonia. Metabolites 2022, 12, 1058. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-Z.; Peng, C.-W.; Su, Z.-Q.; Huang, H.-T.; Liu, X.-H.; Zhan, S.-F.; Huang, X.-F. A practical strategy for exploring the pharmacological mechanism of luteolin against COVID-19/asthma comorbidity: Findings of system pharmacology and bioinformatics analysis. Front. Immunol. 2022, 12, 769011. [Google Scholar] [CrossRef] [PubMed]
- Kara, A.A.; Böncüoğlu, E.; Kıymet, E.; Arıkan, K.Ö.; Şahinkaya, Ş.; Düzgöl, M.; Cem, E.; Çelebi, M.; Ağın, H.; Bayram, S.N. Evaluation of predictors of severe-moderate COVID-19 infections at children: A review of 292 children. J. Med. Virol. 2021, 93, 6634–6640. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, S.A.; Lo, K.B.; Shah, S.; Zanoria, M.A.; Valiani, D.; Balogun, O.O.; Hiedra, R.; Azmaiparashvili, Z.; Patarroyo Aponte, G. Comparison of Patient Clinical characteristics and Outcomes Between Different COVID-19 Peak Periods: A Single Center Retrospective Propensity Matched Analysis. Cureus 2021, 13, e15777. [Google Scholar] [CrossRef] [PubMed]
- Adir, Y.; Saliba, W.; Beurnier, A.; Humbert, M. Asthma and COVID-19: An update. Eur. Respir. Rev. 2021, 30, 210152. [Google Scholar] [CrossRef] [PubMed]
- Skevaki, C.; Karsonova, A.; Karaulov, A.; Xie, M.; Renz, H. Asthma-associated risk for COVID-19 development. J. Allergy Clin. Immunol. 2020, 146, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Skevaki, C.; Karsonova, A.; Karaulov, A.; Fomina, D.; Xie, M.; Chinthrajah, S.; Nadeau, K.C.; Renz, H. SARS-CoV-2 infection and COVID-19 in asthmatics: A complex relationship. Nat. Rev. Immunol. 2021, 21, 202–203. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.B.; Theoharides, T.C. Methoxyluteolin inhibits neuropeptide-stimulated proinflammatory mediator release via mTOR activation from human mast cells. J. Pharmacol. Exp. Ther. 2017, 361, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Ma, L.; Wang, H.; Wu, S.; Huang, H.; Gu, Z.; Jiang, J.; Li, Y. Luteolin decreases the yield of influenza A virus in vitro by interfering with the coat protein I complex expression. J. Nat. Med. 2019, 73, 487–496. [Google Scholar] [CrossRef]
- Yi, L.; Li, Z.; Yuan, K.; Qu, X.; Chen, J.; Wang, G.; Zhang, H.; Luo, H.; Zhu, L.; Jiang, P. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. J. Virol. 2004, 78, 11334–11339. [Google Scholar] [CrossRef]
- Theoharides, T.C. COVID-19, pulmonary mast cells, cytokine storms, and beneficial actions of luteolin. Biofactors 2020, 46, 306. [Google Scholar] [CrossRef]
- Theoharides, T.C. Potential association of mast cells with coronavirus disease 2019. Ann. Allergy Asthma Immunol. 2021, 126, 217–218. [Google Scholar] [CrossRef]
- Das, M.; Ram, A.; Ghosh, B. Luteolin alleviates bronchoconstriction and airway hyperreactivity in ovalbumin sensitized mice. Inflamm. Res. 2003, 52, 101–106. [Google Scholar] [CrossRef]
- Rahlwes, K.C.; Dias, B.R.S.; Campos, P.C.; Alvarez-Arguedas, S.; Shiloh, M.U. Pathogenicity and virulence of Mycobacterium tuberculosis. Virulence 2023, 14, 2150449. [Google Scholar] [CrossRef]
- Bagcchi, S. WHO’s global tuberculosis report 2022. Lancet Microbe 2023, 4, e20. [Google Scholar] [CrossRef]
- Long, R.; Maycher, B.; Dhar, A.; Manfreda, J.; Hershfield, E.; Anthonisen, N. Pulmonary tuberculosis treated with directly observed therapy: Serial changes in lung structure and function. Chest 1998, 113, 933–943. [Google Scholar] [CrossRef]
- Halezeroglu, S.; Keles, M.; Uysal, A.; Celik, M.; Senol, C.; Haciibrahimoglu, G.; Arman, B. Factors affecting postoperative morbidity and mortality in destroyed lung. Ann. Thorac. Surg. 1997, 64, 1635–1638. [Google Scholar] [CrossRef]
- Antonio, G.E.; Wong, K.; Hui, D.S.; Wu, A.; Lee, N.; Yuen, E.H.; Leung, C.; Rainer, T.H.; Cameron, P.; Chung, S.S. Thin-section CT in patients with severe acute respiratory syndrome following hospital discharge: Preliminary experience. Radiology 2003, 228, 810–815. [Google Scholar] [CrossRef]
- Yu, M.; Liu, Y.; Xu, D.; Zhang, R.; Lan, L.; Xu, H. Prediction of the development of pulmonary fibrosis using serial thin-section CT and clinical features in patients discharged after treatment for COVID-19 pneumonia. Korean J. Radiol. 2020, 21, 746. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, M.; Luo, P.; Yin, Z.; Wang, S.; Liao, T.; Yang, F.; Wang, Z.; Yang, D.; Peng, Y.; et al. Plasma Metabolomic Profiling of Patients Recovered From Coronavirus Disease 2019 (COVID-19) with Pulmonary Sequelae 3 Months After Discharge. Clin. Infect. Dis. 2021, 73, 2228–2239. [Google Scholar] [CrossRef]
- Borczuk, A.C.; Salvatore, S.P.; Seshan, S.V.; Patel, S.S.; Bussel, J.B.; Mostyka, M.; Elsoukkary, S.; He, B.; Del Vecchio, C.; Fortarezza, F. COVID-19 pulmonary pathology: A multi-institutional autopsy cohort from Italy and New York City. Mod. Pathol. 2020, 33, 2156–2168. [Google Scholar] [CrossRef]
- Montes-Andujar, L.; Tinoco, E.; Baez-Pravia, O.; Martin-Saborido, C.; Blanco-Schweizer, P.; Segura, C.; Silva, E.P.; Reyes, V.; Cobo, A.R.; Zurdo, C. Empiric antibiotics for community-acquired pneumonia in adult patients: A systematic review and a network meta-analysis. Thorax 2021, 76, 1020–1031. [Google Scholar] [CrossRef]
- Guo, Y.; Xia, W.; Peng, X.; Shao, J. Features discriminating COVID-19 from community-acquired pneumonia in pediatric patients. Front. Pediatr. 2020, 8, 602083. [Google Scholar] [CrossRef]
- Tian, J.; Xu, Q.; Liu, S.; Mao, L.; Wang, M.; Hou, X. Comparison of clinical characteristics between coronavirus disease 2019 pneumonia and community-acquired pneumonia. Curr. Med. Res. Opin. 2020, 36, 1747–1752. [Google Scholar] [CrossRef]
- Dorr, F.; Chaves, H.; Serra, M.M.; Ramirez, A.; Costa, M.E.; Seia, J.; Cejas, C.; Castro, M.; Eyheremendy, E.; Slezak, D.F. COVID-19 pneumonia accurately detected on chest radiographs with artificial intelligence. Intell.-Based Med. 2020, 3, 100014. [Google Scholar] [CrossRef]
- Harmon, S.A.; Sanford, T.H.; Xu, S.; Turkbey, E.B.; Roth, H.; Xu, Z.; Yang, D.; Myronenko, A.; Anderson, V.; Amalou, A.; et al. Artificial intelligence for the detection of COVID-19 pneumonia on chest CT using multinational datasets. Nat. Commun. 2020, 11, 4080. [Google Scholar] [CrossRef]
- Li, L.; Qin, L.; Xu, Z.; Yin, Y.; Wang, X.; Kong, B.; Bai, J.; Lu, Y.; Fang, Z.; Song, Q.; et al. Using Artificial Intelligence to Detect COVID-19 and Community-acquired Pneumonia Based on Pulmonary CT: Evaluation of the Diagnostic Accuracy. Radiology 2020, 296, E65–E71. [Google Scholar] [CrossRef]
- Grassin-Delyle, S.; Roquencourt, C.; Moine, P.; Saffroy, G.; Carn, S.; Heming, N.; Fleuriet, J.; Salvator, H.; Naline, E.; Couderc, L.-J. Metabolomics of exhaled breath in critically ill COVID-19 patients: A pilot study. EBioMedicine 2021, 63, 103154. [Google Scholar] [CrossRef]
- Jayes, L.; Haslam, P.L.; Gratziou, C.G.; Powell, P.; Britton, J.; Vardavas, C.; Jimenez-Ruiz, C.; Leonardi-Bee, J.; Dautzenberg, B.; Lundbäck, B. SmokeHaz: Systematic reviews and meta-analyses of the effects of smoking on respiratory health. Chest 2016, 150, 164–179. [Google Scholar] [CrossRef]
- Gülsen, A.; Yigitbas, B.A.; Uslu, B.; Drömann, D.; Kilinc, O. The Effect of Smoking on COVID-19 Symptom Severity: Systematic Review and Meta-Analysis. Pulm. Med. 2020, 2020, 7590207. [Google Scholar] [CrossRef]
- Russo, P.; Bonassi, S.; Giacconi, R.; Malavolta, M.; Tomino, C.; Maggi, F. COVID-19 and smoking: Is nicotine the hidden link? Eur. Respir. J. 2020, 55, 2001116. [Google Scholar] [CrossRef]
- Cui, T.; Miao, G.; Jin, X.; Yu, H.; Zhang, Z.; Xu, L.; Wu, Y.; Qu, G.; Liu, G.; Zheng, Y. The adverse inflammatory response of tobacco smoking in COVID-19 patients: Biomarkers from proteomics and metabolomics. J. Breath Res. 2022, 16, 046002. [Google Scholar] [CrossRef]
- Wessels, I.; Rink, L. Micronutrients in autoimmune diseases: Possible therapeutic benefits of zinc and vitamin D. J. Nutr. Biochem. 2020, 77, 108240. [Google Scholar] [CrossRef]
- Mandal, S.K.; Tare, M.; Deepa, P.R. COVID-19 infection and metabolic comorbidities: Mitigating role of nutritional sufficiency and drug—Nutraceutical combinations of vitamin D. Hum. Nutr. Metab. 2023, 31, 200179. [Google Scholar] [CrossRef]
- Finzi, E. Treatment of SARS-CoV-2 with high dose oral zinc salts: A report on four patients. Int. J. Infect. Dis. 2020, 99, 307–309. [Google Scholar] [CrossRef]
- McCartney, D.M.; Byrne, D.G. Optimisation of vitamin D status for enhanced Immuno-protection against COVID-19. Ir. Med. J. 2020, 113, 58. [Google Scholar]
- Louca, P.; Murray, B.; Klaser, K.; Graham, M.S.; Mazidi, M.; Leeming, E.R.; Thompson, E.; Bowyer, R.; Drew, D.A.; Nguyen, L.H.; et al. Modest effects of dietary supplements during the COVID-19 pandemic: Insights from 445,850 users of the COVID-19 Symptom Study app. BMJ Nutr. Prev. Health 2021, 4, 149–157. [Google Scholar] [CrossRef]
- Dror, A.A.; Morozov, N.; Daoud, A.; Namir, Y.; Yakir, O.; Shachar, Y.; Lifshitz, M.; Segal, E.; Fisher, L.; Mizrachi, M. Pre-infection 25-hydroxyvitamin D3 levels and association with severity of COVID-19 illness. PLoS ONE 2022, 17, e0263069. [Google Scholar] [CrossRef]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef]
- Nair, R.; Maseeh, A. Vitamin D: The “sunshine” vitamin. J. Pharmacol. Pharmacother. 2012, 3, 118–126. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Hollis, B.W. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: Implications for establishing a new effective dietary intake recommendation for vitamin D. J. Nutr. 2005, 135, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Sidiropoulou, P.; Docea, A.O.; Nikolaou, V.; Katsarou, M.-S.; Spandidos, D.A.; Tsatsakis, A.; Calina, D.; Drakoulis, N. Unraveling the roles of vitamin D status and melanin during COVID-19. Int. J. Mol. Med. 2021, 47, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.W.; Paris, P.W.; Clemens, T.L.; Nolan, J.; Holick, M.F. Vitamin D absorption in healthy subjects and in patients with intestinal malabsorption syndromes. Am. J. Clin. Nutr. 1985, 42, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, L.M.; Edwards, Y.S.; Murray, A.W. Alveolar type II cell apoptosis. Comp. Biochem. Physiol. A-Mol. Integr. Physiol. 2001, 129, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.G.; Qin, L.; Puah, S.H. COVID-19 acute respiratory distress syndrome (ARDS): Clinical features and differences from typical pre-COVID-19 ARDS. Med. J. Aust. 2020, 213, 54–56.e51. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.E.; McDonagh, S.T.; McManus, R.J.; Martin, U. COVID-19 and hypertension: Risks and management. A scientific statement on behalf of the British and Irish Hypertension Society. J. Hum. Hypertens. 2021, 35, 304–307. [Google Scholar] [CrossRef]
- Hambidge, M. Human Zinc Deficiency. J. Nutr. 2000, 130, 1344S–1349S. [Google Scholar] [CrossRef]
- Yao, J.S.; Paguio, J.A.; Dee, E.C.; Tan, H.C.; Moulick, A.; Milazzo, C.; Jurado, J.; Della Penna, N.; Celi, L.A. The minimal effect of zinc on the survival of hospitalized patients with COVID-19: An observational study. Chest 2021, 159, 108–111. [Google Scholar] [CrossRef]
- Pvsn, K.K.; Tomo, S.; Purohit, P.; Sankanagoudar, S.; Charan, J.; Purohit, A.; Nag, V.; Bhatia, P.; Singh, K.; Dutt, N.; et al. Comparative Analysis of Serum Zinc, Copper and Magnesium Level and Their Relations in Association with Severity and Mortality in SARS-CoV-2 Patients. Biol. Trace Elem. Res. 2023, 201, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Gleason, J.E.; Zhang, S.X.; Bruno, V.M.; Cormack, B.P.; Culotta, V.C. Candida albicans adapts to host copper during infection by swapping metal cofactors for superoxide dismutase. Proc. Natl. Acad. Sci. USA 2015, 112, E5336–E5342. [Google Scholar] [CrossRef] [PubMed]
- Larvie, D.Y.; Perrin, M.T.; Donati, G.L.; Armah, S.M. COVID-19 Severity Is Associated with Selenium Intake among Young Adults with Low Selenium and Zinc Intake in North Carolina. Curr. Dev. Nutr. 2023, 7, 100044. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.M.; Hardigan, P.C. A Case-Control Study for the Effectiveness of Oral Zinc in the Prevention and Mitigation of COVID-19. Front. Med. 2021, 8, 756707. [Google Scholar] [CrossRef] [PubMed]
- Sobczyk, M.K.; Gaunt, T.R. The Effect of Circulating Zinc, Selenium, Copper and Vitamin K1 on COVID-19 Outcomes: A Mendelian Randomization Study. Nutrients 2022, 14, 233. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.; Kim, H. The role of vitamin C, vitamin D, and selenium in immune system against COVID-19. Molecules 2020, 25, 5346. [Google Scholar] [CrossRef]
- Fath, M.K.; Naderi, M.; Hamzavi, H.; Ganji, M.; Shabani, S.; Ghahroodi, F.N.; Khalesi, B.; Pourzardosht, N.; Hashemi, Z.S.; Khalili, S. Molecular mechanisms and therapeutic effects of different vitamins and minerals in COVID-19 patients. J. Trace Elem. Med. Biol. 2022, 73, 127044. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Squitti, R.; Picozza, M.; Pawar, A.; Rongioletti, M.; Dutta, A.K.; Sahoo, S.; Goswami, K.; Sharma, P.; Prasad, R. Zinc and COVID-19: Basis of Current Clinical Trials. Biol. Trace Elem. Res. 2021, 199, 2882–2892. [Google Scholar] [CrossRef]
- Zabetakis, I.; Lordan, R.; Norton, C.; Tsoupras, A. COVID-19: The Inflammation Link and the Role of Nutrition in Potential Mitigation. Nutrients 2020, 12, 1466. [Google Scholar] [CrossRef]
- Rawat, D.; Roy, A.; Maitra, S.; Gulati, A.; Khanna, P.; Baidya, D.K. Vitamin C and COVID-19 treatment: A systematic review and meta-analysis of randomized controlled trials. Diabetes Metab. Syndr. 2021, 15, 102324. [Google Scholar] [CrossRef]
- Medicine, J.H. The Immune System. 2023. Available online: https://www.hopkinsmedicine.org/health/conditions-and-diseases/the-immune-system (accessed on 10 November 2023).
- Henderson, B. How Does the Immune System Work? 2023. Available online: https://www.livi.co.uk/your-health/how-the-immune-system-works/ (accessed on 10 November 2023).
- Shutterstock, I. Innate Images, Stock Photos & Vectors. 2023. Available online: https://www.shutterstock.com/pt/search/innate (accessed on 10 November 2023).
- Xiao, N.; Nie, M.; Pang, H.; Wang, B.; Hu, J.; Meng, X.; Li, K.; Ran, X.; Long, Q.; Deng, H.; et al. Integrated cytokine and metabolite analysis reveals immunometabolic reprogramming in COVID-19 patients with therapeutic implications. Nat. Commun. 2021, 12, 1618. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.; van Reenen, M.; Rossouw, T.; Lindeque, Z.; Louw, R. Phenylalanine metabolism and tetrahydrobiopterin bio-availability in COVID-19 and HIV. Heliyon 2023, 9, e15010. [Google Scholar] [CrossRef] [PubMed]
- Ansone, L.; Briviba, M.; Silamikelis, I.; Terentjeva, A.; Perkons, I.; Birzniece, L.; Rovite, V.; Rozentale, B.; Viksna, L.; Kolesova, O. Amino acid metabolism is significantly altered at the time of admission in hospital for severe COVID-19 patients: Findings from longitudinal targeted metabolomics analysis. Microbiol. Spectr. 2021, 9, e0033821. [Google Scholar] [CrossRef] [PubMed]
- Bakadia, B.M.; Boni, B.O.O.; Ahmed, A.A.Q.; Yang, G. The impact of oxidative stress damage induced by the environmental stressors on COVID-19. Life Sci. 2021, 264, 118653. [Google Scholar] [CrossRef] [PubMed]
- Betteridge, D.J. What is oxidative stress? Metabolism 2000, 49, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhou, M.; Li, L.; Luo, P.; Fan, W.; Xu, J.; Chen, Q.; Pan, F.; Lei, P.; Zheng, C. Characteristics of mental health implications and plasma metabolomics in patients recently recovered from COVID-19. Transl. Psychiatry 2021, 11, 307. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E. Hamilton rating scale for anxiety (HAM-A). Occup. Med. 2015, 65, 601. [Google Scholar] [CrossRef]
- Karu, N.; Kindt, A.; van Gammeren, A.J.; Ermens, A.A.; Harms, A.C.; Portengen, L.; Vermeulen, R.C.; Dik, W.A.; Langerak, A.W.; van der Velden, V.H. Severe COVID-19 is characterised by perturbations in plasma amines correlated with immune response markers, and linked to inflammation and oxidative stress. Metabolites 2022, 12, 618. [Google Scholar] [CrossRef]
- Beltrán-García, J.; Osca-Verdegal, R.; Pallardó, F.V.; Ferreres, J.; Rodríguez, M.; Mulet, S.; Sanchis-Gomar, F.; Carbonell, N.; García-Giménez, J.L. Oxidative stress and inflammation in COVID-19-associated sepsis: The potential role of anti-oxidant therapy in avoiding disease progression. Antioxidants 2020, 9, 936. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camelo, A.L.M.; Zamora Obando, H.R.; Rocha, I.; Dias, A.C.; Mesquita, A.d.S.; Simionato, A.V.C. COVID-19 and Comorbidities: What Has Been Unveiled by Metabolomics? Metabolites 2024, 14, 195. https://doi.org/10.3390/metabo14040195
Camelo ALM, Zamora Obando HR, Rocha I, Dias AC, Mesquita AdS, Simionato AVC. COVID-19 and Comorbidities: What Has Been Unveiled by Metabolomics? Metabolites. 2024; 14(4):195. https://doi.org/10.3390/metabo14040195
Chicago/Turabian StyleCamelo, André Luiz Melo, Hans Rolando Zamora Obando, Isabela Rocha, Aline Cristina Dias, Alessandra de Sousa Mesquita, and Ana Valéria Colnaghi Simionato. 2024. "COVID-19 and Comorbidities: What Has Been Unveiled by Metabolomics?" Metabolites 14, no. 4: 195. https://doi.org/10.3390/metabo14040195
APA StyleCamelo, A. L. M., Zamora Obando, H. R., Rocha, I., Dias, A. C., Mesquita, A. d. S., & Simionato, A. V. C. (2024). COVID-19 and Comorbidities: What Has Been Unveiled by Metabolomics? Metabolites, 14(4), 195. https://doi.org/10.3390/metabo14040195








