Sphingosine 1-Phosphate and Cancer: Lessons from Thyroid Cancer Cells
Abstract
:1. Introduction
2. Sphingosine 1-Phosphate and the Thyroid
3. Sphingosine 1-Phosphate and Thyroid Cancer
3.1. Receptor Profile in Thyroid Cancer Cells
3.2. Effects on Proliferation and Migration
3.3. Importance of Sphingosine Kinase
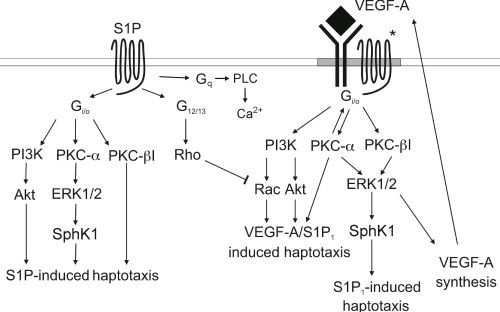
3.4. Signaling cross Talk with VEGF

4. Cross Talk with Ion Channels: HERG
5. Concluding Remarks
Acknowledgements
Conflict of Interest
References
- Breslow, D.K.; Weissman, J.S. Membranes in balance: Mechanisms of sphingolipid homeostasis. Mol. Cell 2010, 40, 267–279. [Google Scholar] [CrossRef]
- Szabo, I.; Adams, C.; Gulbins, E. Ion channels and membrane rafts in apoptosis. Pflüegers Arch. 2004, 448, 304–312. [Google Scholar] [CrossRef]
- Hait, N.C.; Oskeritzian, C.A.; Paugh, S.W.; Milstien, S.; Spiegel, S. Sphingosine kinase, sphingosine 1-phosphate, apoptosis and diseases. Biochem. Biophys. Acta 2006, 1758, 2016–2026. [Google Scholar] [CrossRef]
- Beech, D.J. Integration of transient receptor potential canonical channels with lipids. Acta Physiol. (Oxf) 2012, 204, 227–237. [Google Scholar] [CrossRef]
- Törnquist, K. Sphingosine 1-phosphate, sphingosine kinase and autocrine calcium signalling in thyroid cells. Acta Physiol. (Oxf) 2012, 204, 151–157. [Google Scholar] [CrossRef]
- Pitson, S.M. Regulation of sphingosine kinase and sphingolipid signaling. Trends Biochem. Sci. 2010, 36, 97–107. [Google Scholar] [CrossRef]
- Hait, N.C.; Allegood, J.; Maceyka, M.; Strub, G.M.; Harikumar, K.B.; Singh, S.K.; Luo, C.; Marmorstein, R.; Kordula, T.; Milstien, S.; Spiegel, S. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science 2009, 325, 1254–1257. [Google Scholar] [CrossRef]
- Strub, G.M.; Paillard, M.; Liang, J.; Gomez, L.; Allegood, J.C.; Hait, N.C.; Maceyka, M.; Price, M.M.; Chen, Q.; Simpson, D.C.; et al. Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. FASEB J. 2011, 25, 600–612. [Google Scholar] [CrossRef]
- Pappu, R.; Schwab, S.R.; Cornelissan, I.; Pereira, J.P.; Regard, J.B.; Xu, Y.; Camerer, E.; Zheng, Y.-W.; Huang, Y.; Cyster, J.G.; Coughlin, S.H. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science 2007, 316, 295–298. [Google Scholar] [CrossRef]
- Venkataraman, K.; Lee, Y.M.; Michaud, J.; Thangada, S.; Ai, Y.; Bonkovsky, H.L.; Parikh, N.S.; Habrukowich, C.; Hla, T. Vasular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ. Res. 2008, 102, 669–679. [Google Scholar] [CrossRef]
- Murata, N.; Sato, K.; Tomura, H.; Yanagita, M.; Kuwabara, A.; Ui, M.; Okajima, F. Interaction of sphingosine 1-phosphate with plasma components, including lipoproteins, regulates the lipid receptor-mediated actions. Biochem. J. 2000, 352, 809–815. [Google Scholar] [CrossRef]
- Takabe, K.; Paugh, S.W.; Milstien, S.; Spiegel, S. “Inside-out” signaling of sphingosine 1-phosphate: Therapeutic implications. Pharmacol. Rev. 2008, 60, 181–195. [Google Scholar] [CrossRef]
- Strub, G.M.; Maceyka, M.; Hait, N.C.; Milstein, S.; Spiegel, S. Extracellular and intracellular actions of sphingosine-1-phosphate. Adv. Exp. Med. Biol. 2010, 688, 141–155. [Google Scholar]
- Pyne, N.J.; Pyne, S. Sphingosine 1-phosphate and cancer. Nat. Rev. Cancer 2010, 10, 489–503. [Google Scholar] [CrossRef]
- Yester, J.W.; Tizazu, E.; Harikumar, K.B.; Kordula, T. Extracellular and intracellular sphingosine-1-phosphate in cancer. Cancer Metastasis Rev. 2011, 30, 577–597. [Google Scholar] [CrossRef]
- Törnquist, K.; Ekokoski, E. Effect of sphingosine derivatives on calcium fluxes in thyroid FRTL-5 cells. Biochem. J. 1994, 299, 213–218. [Google Scholar]
- Okajima, F.; Tomura, H.; Sho, K.; Kimura, T.; Sato, K.; Im, D.-S.; Akbar, M.; Kondo, Y. Sphingosine 1-phosphate stimulates hydrogen peroxide generation through activation of phospholipase C-Ca2+ system in FRTL-5 thyroid cells: Possible involvement of guanosine triphosphate-binding proteins in the lipid signaling. Endocrinology 1997, 138, 220–229. [Google Scholar] [CrossRef]
- Törnquist, K.; Saarinen, P.; Vainio, M.; Ahlström, M. Sphingosine 1-phosphate mobilizes sequestered calcium, activates calcium entry, and stimulates DNA synthesis in thyroid FRTL-5 cells. Endocrinology 1997, 138, 4049–4057. [Google Scholar] [CrossRef]
- Björklund, S.; Palmberg, S.; Rask, S.; Westerdahl, A.-C.; Törnquist, K. Effects of sphingosine 1-phosphate on calcium signaling, proliferation, and S1P2 receptor expression in PC Cl3 rat thyroid cells. Mol. Cell. Endocrinol. 2005, 231, 65–74. [Google Scholar] [CrossRef]
- Mattie, M.; Brooker, G.; Spiegel, S. Sphingosine-1-phosphate, a putative second messenger, mobilizes calcium from internal stores via an inositol trisphosphate-independent pathway. J. Biol. Chem. 1994, 269, 3181–3188. [Google Scholar]
- Högback, S.; Leppimäki, P.; Rudnäs, B.; Björklund, S.; Slotte, J.P.; Törnquist, K. Ceramide 1-phosphate increases intracellular free calcium concentrations in tyroid FRTL-5 cells. Evidence for an effect mediated by inositol 1,4,5-trisphosphate and intracellular sphingosine 1-phosphate. Biochem. J. 2003, 370, 111–119. [Google Scholar] [CrossRef]
- Meyer zu Heringdorf, D.; Lass, H.; Alemany, R.; Laser, K.T.; Neumann, E.; Zhang, C.; Schmidt, M.; Rauen, U.; Jakobs, K.H.; van Koppen, C.J. Sphingosine kinase mediated Ca2+ signalling by G-protein-coupled receptors. EMBO J. 1998, 17, 2830–2837. [Google Scholar] [CrossRef]
- Törnquist, K. Sphingosine 1-phosphate activates Na+-H+ exchange in thyroid FRTL-5 cells. Am. J. Physiol. 1997, 272, C1052–C1057. [Google Scholar]
- Kimura, T.; Okajima, F.; Kikuchi, T.; Kuwabara, A.; Tomura, H.; Sho, K.; Kobayashi, I.; Kondo, Y. Inhibition of TSH-induced hydrogen peroxide production by TNFa through a sphingomyelinase signaling pathway. Am. J. Physiol. 1997, 273, E638–E643. [Google Scholar]
- Kimura, T.O.F.; Sho, K.; Kobayashi, I.; Kondo, Y. Thyrotropin-induced hydrogen peroxide production in FRTL-5 thyroid cells is mediated not by adenosine 3',5'-monophosphate, but by Ca2+ signaling followed by phospholipase A2 activation and potentiated by an adenosine derivative. Endocrinology 1995, 136, 116–123. [Google Scholar] [CrossRef]
- Törnquist, K.; Malm, A.-M.; Kronqvist, R.; Pasternack, M.; Björklund, S.; Tuominen, R.; Slotte, J.P. Tumor necrosis factor a, sphingomyelinase and ceramide attenuate store-operated calcium entry in thyroid FRTL-5 cells. J. Biol. Chem. 1999, 274, 9370–9377. [Google Scholar] [CrossRef]
- Spiegel, S.; Milstien, S. Sphingosine-1-phosphate: An enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 2003, 4, 397–407. [Google Scholar] [CrossRef]
- Balthasar, S.; Samulin, J.; Ahlgren, H.; Bergelin, N.; Lundqvist, M.; Toescu, E.C.; Eggo, M.C.; Törnquist, K. Sphingosine 1-phosphate receptor expression profile and regulation of migration in human thyroid cancer cells. Biochem. J. 2006, 398, 547–556. [Google Scholar] [CrossRef]
- Maceyka, M.; Harikumar, K.B.; Milstien, S.; Spiegel, S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012, 22, 50–60. [Google Scholar] [CrossRef]
- Guan, H.; Liu, L.; Cai, J.; Liu, J.; Ye, C.; Li, M.; Li, Y. Sphingosine kinase 1 is overexpressed and promotes proliferation in human thyroid cancer. Mol. Endocrinol. 2011, 25, 1858–1866. [Google Scholar] [CrossRef]
- Asghar, M.Y.; Viitanen, T.; Kemppainen, K.; Törnquist, K. Sphingosine 1-phosphate and human ether-a-go-go-related gene potassium channels modulate migration in human anaplastic thyroid cancer cells. Endoc-Relat. Cancer 2012, 19, 667–680. [Google Scholar] [CrossRef]
- Takuwa, N.; Du, W.; Kaneko, E.; Okamoto, H.; Yoshioka, K.; Takuwa, Y. Tumor-suppressive sphingosine-1-phosphate receptor-2 counteracting tumor-promoting sphingosine-1-phosphate receptor-1 and sphingosine kinase 1. Am. J. Cancer Res. 2011, 1, 460–481. [Google Scholar]
- Bergelin, N.; Blom, T.; Löf, C.; Alam, C.; Balthasar, S.; Heikkilä, J.; Slotte, J.P.; Hinkkanen, A.; Törnquist, K. Sphingosine kinase as an oncogene: Autocrine sphingosine 1-phosphate enhances ML-1 thyroid carcinoma cell migration by a mechanism dependent on PKC-α and Erk1/2. Endocrinology 2009, 150, 2055–2063. [Google Scholar]
- Xia, P.; Gamble, J.R.; Wang, L.; Pitson, S.M.; Moretti, P.A.; Wattenberg, B.W.; D'Andrea, R.J.; Vadas, M.A. An oncogenic role of sphingosine kinase. Curr. Biol. 2000, 10, 1527–1530. [Google Scholar] [CrossRef]
- Olivera, A.; Kohama, T.; Edsall, L.; Nava, V.; Cuvillier, O.; Poulton, S.; Spiegel, S. Sphingosine kinase expresion increases intracellular sphingosine-1-phosphate and promotes cell growth and survival. J. Cell Biol. 1999, 147, 545–558. [Google Scholar] [CrossRef]
- Ruckhäberle, E.; Rody, A.; Engels, K.; Gaetje, R.; von Minckwitz, G.; Schiffmann, S.; Grösch, S.; Geisslinger, G.; Holtrich, U.; Karn, T.; Kaufmann, M. Microarray analysis of altered sphingolipid metabolism reveals prognostic significance of sphingosine kinase 1 in breast cancer. Breast Cancer Res. Treat. 2008, 112, 41–52. [Google Scholar]
- Alvarez, J.; Milstien, S.; Spiegel, S. Autocrine and paracrine roles of sphingosine-1-phosphate. TRENDS Endocrinol. Metab. 2007, 18, 300–307. [Google Scholar] [CrossRef]
- Pyne, N.J.; Pyne, S. Receptor tyrosine kinase-G-protein-coupled receptor signalling platforms: Out of the shadow? Trends Pharmacol. Sci. 2011, 32, 443–450. [Google Scholar]
- Takabe, K.; Kim, R.H.; Allegood, J.C.; Mitra, P.; Ramachandran, S.; Nagahishi, M.; Harikumar, K.B.; Hait, N.C.; Milstien, S.; Spiegel, S. Estradiol induces export of sphingosine 1-phosphate from breast cancer cells via ABCC1 and ABCG2. J. Biol. Chem. 2010, 285, 10477–10486. [Google Scholar] [CrossRef]
- Sato, K.; Malchinkhuu, E.; Horiuchi, Y.; Mogi, C.; Tomura, H.; Tosaka, M.; Yoshimoto, Y.; Kuwabara, A.; Okajima, F. Citical role of ABCA1 transporter in sphingosine 1-phosphate release from astrocytes. J. Neurochem. 2007, 103, 2610–2619. [Google Scholar]
- Pyne, N.J.; Waters, C.; Moughal, N.A.; Sambi, B.S.; Pyne, S. Receptor tyrosine kinase-GPCR signal complexes. Biochem. Soc. Trans. 2003, 31, 1220–1225. [Google Scholar] [CrossRef]
- Tanimoto, T.; Jin, Z.G.; Berk, B.C. Transactivation of vascular endothelial growth factor (VEGF) receptor Flk1/KFR is invovled in sphingosine 1-phosphate-stimulated phosphorylation of Akt and endothelial nitric-oxide synthase (eNOS). J Biol. Chem. 2002, 277, 42997–43001. [Google Scholar] [CrossRef]
- Waters, C.; Sambi, B.; Kong, K.C.; Thompson, D.; Pitson, S.M.; Pyne, S.; Pyne, N.J. Sphingosine 1-phosphate and platelet-derived growth factor (PDGF) act via PDGFβ receptor-sphingosine 1-phosphate receptor complex in airway smooth muscle cells. J. Biol. Chem 2003, 278, 6282–6290. [Google Scholar]
- El-Shewy, H.M.; Johnson, K.R.; Lee, M.H.; Jaffa, A.A.; Obeid, L.M.; Luttrell, L.M. Insulin-like growth factors mediate heterotrimeric G protein-dependent Erk1/2 activation by transactivating sphingosine 1-phosphate receptors. J. Biol. Chem. 2006, 281, 31399–31407. [Google Scholar]
- Miller, A.V.; Alvarez, S.E.; Spiege, S.; Lebman, D.A. Sphingosine kinase and sphingosine-1-phosphate are critical for transforming growth factor beta-induced extracellular signal-regulated kinase 1 and 2 activation and promotion of migration and invasion of esophageal cancer cells. Mol. Cell Biol. 2008, 28, 4142–4151. [Google Scholar] [CrossRef]
- Lebman, D.A.; Spiegel, S. Cross-talk at the crossroads of sphingosine-1-phosphate, growth factors, and cytokine signaling. J. Lipid Res. 2008, 49, 1388–1394. [Google Scholar] [CrossRef]
- Pyne, N.J.; Pyne, S. Sphingosine 1-phosphate, lysophosphatidic acid and growth factor signaling and termination. Biochi. Biophys. Acta 2008, 1781, 467–476. [Google Scholar] [CrossRef]
- Turner, H.E.; Harris, A.; Melmed, S.; Wass, J.A.H. Angiogenesis in endocrine tumors. Endocrine Rev. 2003, 24, 600–632. [Google Scholar] [CrossRef]
- Vieira, J.M.; Santos, S.C.R.; Espadinha, C.; Correia, I.; Vag, T.; Casalou, C.; Cavaco, B.M.; Catarino, A.S.; Dias, S.; Leite, V. Expression of vascular endothelial growth factor (VEGF) and its receptors in thyroid carcinomas of follicular origin: A potent autocrine loop. Eur. J. Endocrinol. 2005, 153, 701–709. [Google Scholar] [CrossRef]
- Kim, D.S.; Franklyn, J.A.; Boelaert, K.; Eggo, M.C.; Watkinson, J.C.; McCabe, C.J. Pituitary tumor transforming gene (PTTG) stimulates thyroid cell proliferation via vascular endothelial growth factor/kinesin insert domain receptor/inhibitor of DNA binding-3 autocrine pathway. J. Clin. Endocrinol. Metab. 2006, 91, 4603–4611. [Google Scholar] [CrossRef]
- Endo, A.; Nagashima, K.-I.; Kurose, H.; Mochizuki, S.; Matsuda, M.; Mochizuki, N. Sphingosine 1-phosphate induces membrane ruffling and increases motility of human umbilical vein endotjelial cells via vascular endothelial growth factor receptor and CRKII. J. Biol. Chem. 2002, 277, 23747–23754. [Google Scholar]
- Igarashi, J.; Erwin, P.A.; Dantas, A.P.V.; Chen, H.; Michel, T. VEGF induces S1P1 receptors in endothelial cells: Implications for cross-talk between sphingolipid and growth factor receptors. Proc. Natl. Acad. Sci. USA 2003, 100, 10664–10669. [Google Scholar]
- Hughes, S.K.; Wacker, B.K.; Kaneda, M.M.; Elbert, D.L. Fluid shear stress modulates cell migration induced by sphingosine 1-phosphate and vascular endothelial growth factor. Ann. Biomed. Eng. 2005, 33, 1004–1014. [Google Scholar]
- Fieber, C.B.; Eldridge, J.; Taha, T.A.; Obeid, L.M.; Muise-Helmericks, R.C. Modulation of total Akt kinase by increased expression of a single isoform: Requirement of the sphingosine-1-phosphate receptor EDG3/S1P3, for the VEGF-dependent expression of Akt3 in primary endothelial cells. Exp. Cell Res. 2006, 312, 1164–1173. [Google Scholar] [CrossRef]
- Shu, X.; Wu, W.; Mosteller, R.D.; Broek, D. Sphingosine kinase mediates vascular endothelial growth factor-induced activation of ras and mitogen-activated protein kinases. Mol. Cell. Biol. 2002, 22, 7758–7768. [Google Scholar] [CrossRef]
- Balthasar, S.; Bergelin, N.; Löf, C.; Vainio, M.; Andersson, S.; Törnquist, K. Interaction between sphingosine 1-phosphate and vascular endothelial growth factor signalling in ML-1 follicular thyroid cancer cells. Endoc-Relat. Cancer 2008, 15, 521–534. [Google Scholar] [CrossRef]
- Bergelin, N.; Löf, C.; Balthasar, S.; Kalhori, V.; Törnquist, K. S1P1 and VEGFR-2 form a signaling complex with extracellular regulated kinase 1/2 and protein kinase C-α regulating ML-1 thyroid carcinoma cell migration. Endocrinology 2010, 151, 2994–3005. [Google Scholar] [CrossRef]
- Waters, C.M.; Connell, M.C.; Pyne, S.; Pyne, N.J. c-Src is involved in regulating signal transmission from PDGFβ receptor-GPCR(s) complexes in mammalian cells. Cell. Signal. 2005, 17, 263–277. [Google Scholar] [CrossRef]
- Sukocheva, O.; Wadham, C.; Holmes, A.; Albanese, N.; Verrier, E.; Feng, F.; Bernal, A.; Derian, C.K.; Ullrich, A.; Vadas, M.A.; Xia, P. Estrogen transactivates EGFR via the sphingosine 1-phosphate receptor EDG-3: The role of sphingosine kinase-1. J. Cell Biol. 2006, 173, 301–310. [Google Scholar] [CrossRef]
- Arcangeli, A. Ion channels and transporters in cancer. 3. Ion channels in tumor cell-microenvironment cross talk. Am. J. Physiol. Cell Physiol. 2011, 301, C762–C771. [Google Scholar] [CrossRef]
- Jehle, J.; Schweizer, P.A.; Katus, H.A.; Thomas, D. Novel roles for HERG K+ channels in cell proliferation and apoptosis. Cell Death Dis. 2011, 2, e193. [Google Scholar] [CrossRef]
- Masi, A.; Bechetti, A.; Restano-Cassulini, R.; Polvani, S.; Hofmann, G.; Buccoliero, A.M.; Paglierani, M.; Pollo, B.; Taddei, G.L.; Gallini, P.; et al. HERG1 channels are overexpressed in glioblastoma multiforme and modulate VEGF secretion in glioblastoma cell lines. Br. J. Cancer 2005, 93, 781–792. [Google Scholar] [CrossRef]
- Ramström, C.; Chapman, H.; Viitanen, T.; Afrasiabi, E.; Fox, H.; Kivelä, J.; Soini, S.; Korhonen, L.; Lindholm, D.; Pasternack, M.; Törnquist, K. Regulation of HERG (KCNH2) potassium channel surface expression by diacylglycerol. Cell. Mol. Life Sci. 2010, 67, 157–169. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Törnquist, K. Sphingosine 1-Phosphate and Cancer: Lessons from Thyroid Cancer Cells. Biomolecules 2013, 3, 303-315. https://doi.org/10.3390/biom3020303
Törnquist K. Sphingosine 1-Phosphate and Cancer: Lessons from Thyroid Cancer Cells. Biomolecules. 2013; 3(2):303-315. https://doi.org/10.3390/biom3020303
Chicago/Turabian StyleTörnquist, Kid. 2013. "Sphingosine 1-Phosphate and Cancer: Lessons from Thyroid Cancer Cells" Biomolecules 3, no. 2: 303-315. https://doi.org/10.3390/biom3020303