Improvement of Mitochondrial Activity and Fibrosis by Resveratrol Treatment in Mice with Schistosoma japonicum Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Animals and Parasites
2.3. Establishment of Model Mice and RSV Treatment
2.4. Measurement of Mitochondrial Membrane Potential
2.5. Masson Staining
2.6. Western Blot Analysis
2.7. Electron Microscope Analysis
2.8. Statistical Analysis
3. Results
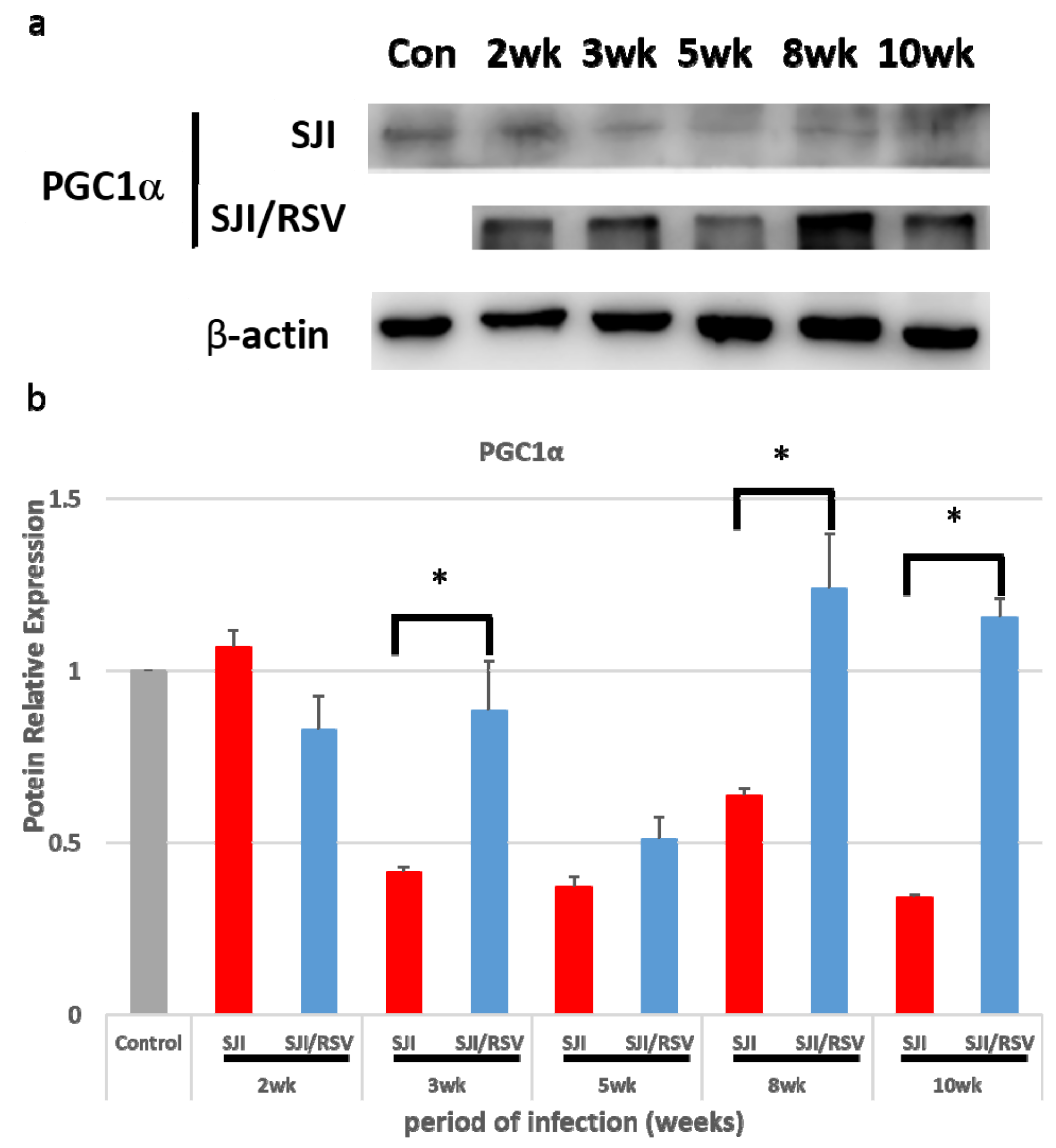
3.1. RSV Treatment Affects the Expression of Genes Involved in Mitochondrial Biogenesis
3.2. RSV Improves Mitochondrial Function in S. japonicum-Infected Mice
3.3. RSV Treatment Reduces SSLF
3.4. RSV Treatment Reduces Protein Expression of Fibrosis Markers
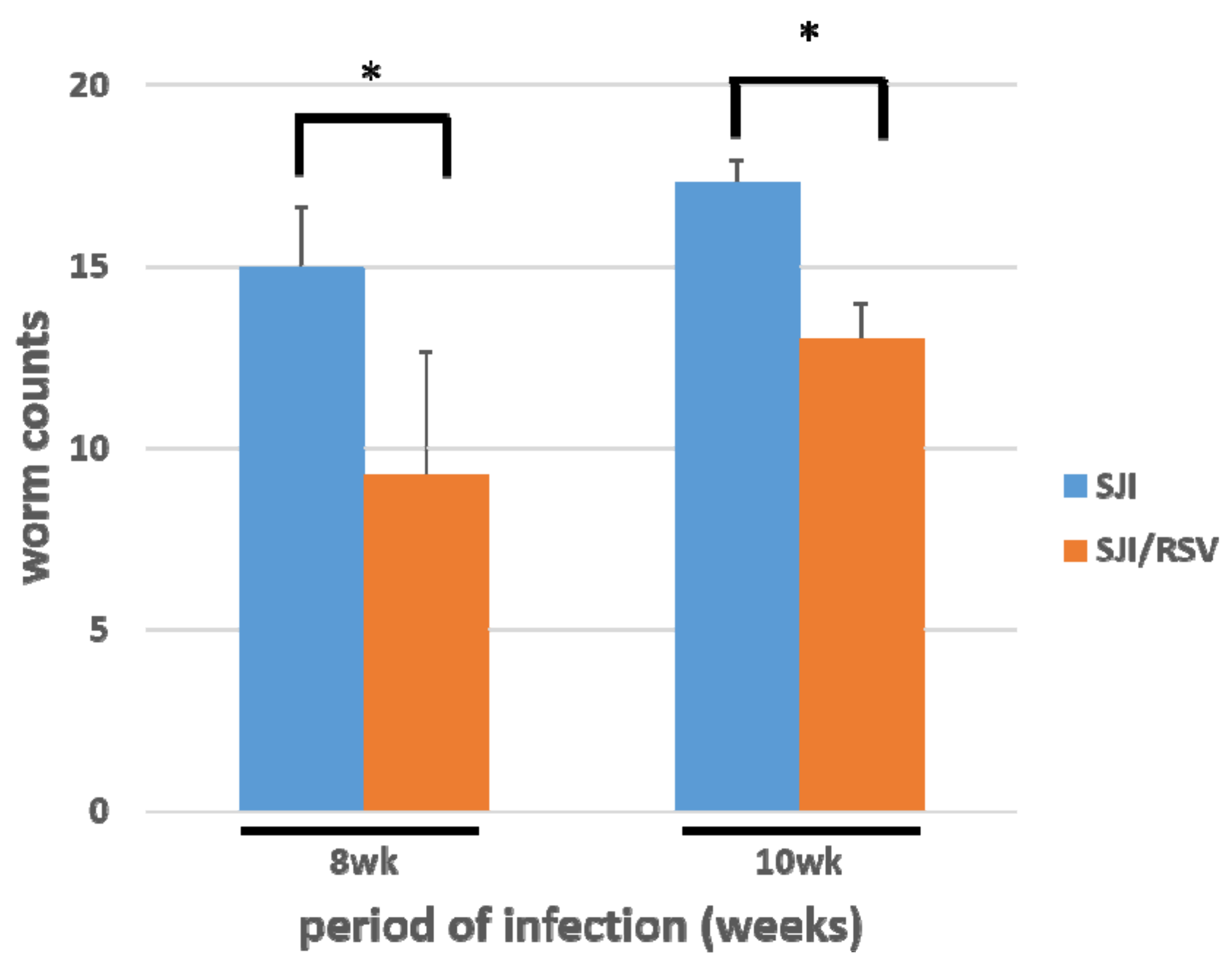
3.5. RSV Treatment Reduces the S. japonicum Worm Count in Infected Mice
3.6. RSV Treatment Causes Damage to S. japonicum Worms
3.7. RSV Causes Damage in S. japonicum Worms in a Dose-Dependent Manner
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gryseels, B.; Polman, K.; Clerinx, J.; Kestens, L. Human schistosomiasis. Lancet 2006, 368, 1106–1118. [Google Scholar] [CrossRef]
- Burke, M.L.; Jones, M.K.; Gobert, G.N.; Li, Y.S.; Ellis, M.K.; McManus, D.P. Immunopathogenesis of human schistosomiasis. Parasite Immunol. 2009, 31, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.L.; McManus, D.P.; Ramm, G.A.; Duke, M.; Li, Y.; Jones, M.K.; Gobert, G.N. Temporal expression of chemokines dictates the hepatic inflammatory infiltrate in a murine model of schistosomiasis. PLoS Negl. Trop. Dis. 2010, 4, e598. [Google Scholar] [CrossRef] [PubMed]
- Chuah, C.; Jones, M.K.; Burke, M.L.; McManus, D.P.; Gobert, G.N. Cellular and chemokine-mediated regulation in schistosome-induced hepatic pathology. Trends Parasitol. 2014, 30, 141–150. [Google Scholar] [CrossRef] [PubMed]
- McManus, D.P.; Gray, D.J.; Li, Y.; Feng, Z.; Williams, G.M.; Stewart, D.; Rey-Ladino, J.; Ross, A.G. Schistosomiasis in the People’s Republic of China: The era of the Three Gorges Dam. Clin. Microbiol. Rev. 2010, 23, 442–466. [Google Scholar] [CrossRef] [PubMed]
- La Flamme, A.C.; MacDonald, A.S.; Huxtable, C.R.; Carroll, M.; Pearce, E.J. Lack of C3 affects Th2 response development and the sequelae of chemotherapy in schistosomiasis. J. Immunol. 2003, 170, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.S.; Mentink-Kane, M.M.; Pesce, J.T.; Ramalingam, T.R.; Thompson, R.; Wynn, T.A. Immunopathology of schistosomiasis. Immunol. Cell Biol. 2007, 85, 148–154. [Google Scholar] [CrossRef]
- Pearce, E.J.; MacDonald, A.S. The immunobiology of schistosomiasis. Nat. Rev. Immunol. 2002, 2, 499–511. [Google Scholar] [CrossRef]
- Andrade, Z.A. Schistosomiasis and liver fibrosis. Parasite Immunol. 2009, 31, 656–663. [Google Scholar] [CrossRef]
- Lenzi, H.L.; Romanha Wde, S.; Santos, R.M.; Rosas, A.; Mota, E.M.; Manso, P.P.; Caputo, L.F.; Pelajo-Machado, M. Four whole-istic aspects of schistosome granuloma biology: Fractal arrangement, internal regulation, autopoietic component and closure. Mem. Inst. Oswaldo. Cruz. 2006, 101, 219–231. [Google Scholar] [CrossRef]
- Anthony, B.J.; Ramm, G.A.; McManus, D.P. Role of resident liver cells in the pathogenesis of schistosomiasis. Trends Parasitol. 2012, 28, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Chitsulo, L.; Loverde, P.; Engels, D. Schistosomiasis. Nat. Rev. Microbiol. 2004, 2, 12–13. [Google Scholar] [CrossRef] [PubMed]
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef]
- Chen, T.T.; Wu, L.S.; Hsu, P.W.; Pang, C.Y.; Lee, K.M.; Cheng, P.C.; Peng, S.Y. Mitochondrial dynamics in the mouse liver infected by Schistosoma mansoni. Acta Trop. 2015, 148, 13–23. [Google Scholar] [CrossRef]
- Yu, W.; Fu, Y.C.; Wang, W. Cellular and molecular effects of resveratrol in health and disease. J. Cell. Biochem. 2012, 113, 752–759. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef]
- Chan, M.M. Antimicrobial effect of resveratrol on dermatophytes and bacterial pathogens of the skin. Biochem. Pharmacol. 2002, 63, 99–104. [Google Scholar] [CrossRef]
- Pace-Asciak, C.R.; Hahn, S.; Diamandis, E.P.; Soleas, G.; Goldberg, D.M. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: Implications for protection against coronary heart disease. Clin. Chim. Acta 1995, 235, 207–219. [Google Scholar] [CrossRef]
- Docherty, J.J.; Fu, M.M.; Stiffler, B.S.; Limperos, R.J.; Pokabla, C.M.; DeLucia, A.L. Resveratrol inhibition of herpes simplex virus replication. Antivir. Res. 1999, 43, 145–155. [Google Scholar] [CrossRef]
- Kedzierski, L.; Curtis, J.M.; Kaminska, M.; Jodynis-Liebert, J.; Murias, M. In vitro antileishmanial activity of resveratrol and its hydroxylated analogues against Leishmania major promastigotes and amastigotes. Parasitol. Res. 2007, 102, 91–97. [Google Scholar] [CrossRef]
- Lucas, I.K.; Kolodziej, H. In vitro antileishmanial activity of resveratrol originates from its cytotoxic potential against host cells. Planta Med. 2013, 79, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Chávez, E.; Reyes-Gordillo, K.; Segovia, J.; Shibayama, M.; Tsutsumi, V.; Vergara, P.; Moreno, M.G.; Muriel, P. Resveratrol prevents fibrosis, NF-kappa B activation and TGF-beta increases induced by chronic CCl4 treatment in rats. J. Appl. Toxicol. 2008, 28, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, Q.; Xu, T.; Hong, L.; Fu, R.; Wu, J.; Ding, J. Resveratrol attenuates the progress of liver fibrosis via the Akt/nuclear factor-κB pathways. Mol. Med. Rep. 2016, 13, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Juan, M.E.; Vinardell, M.P.; Planas, J.M. The daily oral administration of high doses of trans-resveratrol to rats for 28 days is not harmful. J. Nutr. 2002, 132, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Scarpulla, R.C. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim. Biophys. Acta 2011, 1813, 1269–1278. [Google Scholar] [CrossRef] [Green Version]
- Peng, K.; Tao, Y.; Zhang, J.; Wang, J.; Ye, F.; Dan, G.; Zhao, Y.; Cai, Y.; Zhao, J.; Wu, Q.; et al. Resveratrol regulates mitochondrial biogenesis and fission/fusion to attenuate rotenone-induced neurotoxicity. Oxid. Med. Cell. Longev. 2016, 2016, 6705621. [Google Scholar] [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disorder by activating SIRT1 and PGC-1α. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improve health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef]
- Scarpulla, R.C. Nuclear control of respiratory chain expression by nuclear respiratory factors and PGC-1-related coactivator. Ann. NY Acad. Sci. 2008, 1147, 321–334. [Google Scholar] [CrossRef]
- Scarpulla, R.C. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol. Rev. 2008, 88, 611–638. [Google Scholar] [CrossRef]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef]
- Nilsson, M.I.; Greene, N.P.; Dobson, J.P.; Wiggs, M.P.; Gasier, H.G.; Macias, B.R.; Shimkus, K.L.; Fluckey, J.D. Insulin resistance syndrome blunts the mitochondrial anabolic response following resistance exercise. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E466–E474. [Google Scholar] [CrossRef] [PubMed]
- Greene, N.P.; Nilsson, M.I.; Washington, T.A.; Lee, D.E.; Brown, L.A.; Papineau, A.M.; Shimkus, K.L.; Greene, E.S.; Crouse, S.F.; Fluckey, J.D. Impaired exercise-induced mitochondrial biogenesis in the obese Zucker rat, despite PGC-1α induction, is due to compromised mitochondrial translation elongation. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E503–E511. [Google Scholar] [CrossRef] [PubMed]
- Seo, A.Y.; Joseph, A.M.; Dutta, D.; Hwang, J.C.; Aris, J.P.; Leeuwenburgh, C. New insights into the role of mitochondria in aging: Mitochondrial dynamics and more. J. Cell. Sci. 2010, 123, 2533–2542. [Google Scholar] [CrossRef]
- Spiegelman, B.M. Transcriptional control of energy homeostasis through the PGC1 coactivators. Novartis Found. Symp. 2007, 286, 3–6. [Google Scholar]
- Gouveia, M.J.; Brindley, P.J.; Rinaldi, G.; Gärtner, F.; Correia da Costa, J.M.; Vale, N. Combination Anthelmintic/Antioxidant Activity Against Schistosoma mansoni. Biomolecules 2019, 9, 54. [Google Scholar] [CrossRef]
- Njoroge, M.; Njuguna, N.M.; Mutai, P.; Ongarora, D.S.; Smith, P.W.; Chibale, K. Recent approaches to chemical discovery and development against malaria and the neglected tropical diseases human African trypanosomiasis and schistosomiasis. Chem. Rev. 2014, 114, 11138–11163. [Google Scholar] [CrossRef]
- Chatterjee, S.; Vrolix, G.; Depoortere, I.; Peeters, T.; van Marck, E. The therapeutic effect of the neuropeptide hormone somatostatin on Schistosoma mansoni caused liver fifibrosis. BMC Infect. Dis. 2005, 5, 45. [Google Scholar] [CrossRef]
- Cioli, D.; Pica-Mattoccia, L. Praziquantel. Parasitol. Res. 2003, 90, S3–S9. [Google Scholar]
- King, C.H.; Mahmoud, A.A. Drugs five years later: Praziquantel. Ann. Intern. Med. 1989, 110, 290–296. [Google Scholar] [CrossRef]
- Zhuo, X.H.; Sun, H.C.; Huang, B.; Yu, H.J.; Shan, Y.; Du, A.F. Evaluation of potential anti-toxoplasmosis efficiency of combined traditional herbs in a mouse model. J. Zhejiang Univ. Sci. B 2017, 18, 453–461. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.W.; Wang, B.; Zhang, Q.Z.; Xu, D.H.; Lin, D.J.; Yang, X.Y.; Zhu, S.Q.; Pan, J.Y.; Deng, Q.; Liu, Y.M.; et al. Combined effects of Chinese medicine feed and ginger extract bath on co-infection of Ichthyophthirius multifiliis and Dactylogyrus ctenopharyngodonid in grass carp. Parasitol. Res. 2017, 116, 2017–2025. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Wang, H.; Shan, D.; Li, B.; Liu, J.; Liu, Q. Activity of several kinds of drugs against Neospora caninum. Parasitol. Int. 2015, 64, 597–602. [Google Scholar] [CrossRef]
- Maizels, R.M.; Bundy, D.A.; Selkirk, M.E.; Smith, D.F.; Anderson, R.M. Immunological modulation and evasion by helminth parasites in human populations. Nature 1993, 365, 797–805. [Google Scholar] [CrossRef]
- Gharib, B.; Abdallahi, O.M.; Dessein, H.; De Reggi, M. Development of eosinophil peroxidase activity and concomitant alteration of the antioxidant defenses in the liver of mice infected with Schistosoma mansoni. J. Hepatol. 1999, 30, 594–602. [Google Scholar] [CrossRef]
- Chen, T.T.; Cheng, P.C.; Chang, K.C.; Feng, J.L.; Chen, C.C.; Lam, H.Y.; Cao, C.P.; Peng, S.Y. Activation of the NLRP3 and AIM2 inflammasomes in a mouse model of Schistosoma mansoni infection. J. Helminthol. 2019, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Valera Vera, E.A.; Sayé, M.; Reigada, C.; Damasceno, F.S.; Silber, A.M.; Miranda, M.R.; Pereira, C.A. Resveratrol inhibits Trypanosoma cruzi arginine kinase and exerts a trypanocidal activity. Int. J. Biol. Macromol. 2016, 87, 498–503. [Google Scholar] [CrossRef]
- Ferreira, C.; Soares, D.C.; Nascimento, M.T.; Pinto-da-Silva, L.H.; Sarzedas, C.G.; Tinoco, L.W.; Saraiva, E.M. Resveratrol is active against Leishmania amazonensis: In vitro effect of its association with Amphotericin B. Antimicrob. Agents Chemother. 2014, 58, 6197–6208. [Google Scholar] [CrossRef]
- Passos, C.L.; Ferreira, C.; Soares, D.C.; Saraiva, E. Leishmanicidal Effect of Synthetic trans-Resveratrol Analogs. PLoS ONE 2015, 10, e0141778. [Google Scholar] [CrossRef]
- Dal’Bó Pelegrini, M.; Pereira, J.B.; Dos Santos Costa, S.; Salazar Terreros, M.J.; Degrossoli, A. Evaluation of hypoxia inducible factor targeting pharmacological drugs as antileishmanial agents. Asian Pac. J. Trop. Med. 2016, 9, 652–657. [Google Scholar] [CrossRef] [Green Version]
- Roy, B.; Giri, B.R. α-Viniferin and resveratrol induced alteration in the activities of some energy metabolism related enzymes in the cestode parasite Raillietina echinobothrida. Acta Trop. 2016, 154, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Pais-Morales, J.; Betanzos, A.; García-Rivera, G.; Chávez-Munguía, B.; Shibayama, M.; Orozco, E. Resveratrol induces apoptosis-like death and prevents in vitro and in vivo virulence of Entamoeba histolytica. PLoS ONE 2016, 11, e0146287. [Google Scholar] [CrossRef]
- Duan, T.; Xu, Z.; Sun, F.; Wang, Y.; Zhang, J.; Luo, C.; Wang, M. HPA aptamer functionalized paclitaxel-loaded PLGA nanoparticles for enhanced anticancer therapy through targeted effects and microenvironment modulation. Biomed. Pharmacother. 2019, 117, 109121. [Google Scholar] [CrossRef]
- Kessoku, T.; Imajo, K.; Honda, Y.; Kato, T.; Ogawa, Y.; Tomeno, W.; Kato, S.; Mawatari, H.; Fujita, K.; Yoneda, M.; et al. Resveratrol ameliorates fibrosis and inflammation in a mouse model of nonalcoholic steatohepatitis. Sci. Rep. 2016, 6, 22251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, B.; Xiao, Z.; Zhang, W.; Chen, H.; Liu, H.; Pan, J.; Cai, X.; Liang, G.; Zhou, B.; Shan, X.; et al. A novel resveratrol-curcumin hybrid, a19, attenuates high fat diet-induced nonalcoholic fatty liver disease. Biomed. Pharmacother. 2019, 110, 951–960. [Google Scholar] [CrossRef]
- Chang, D.; Ramalho, L.N.; Ramalho, F.S.; Martinelli, A.L.; Zucoloto, S. Hepatic stellate cells in human schistosomiasis mansoni: A comparative immunohistochemical study with liver cirrhosis. Acta Trop. 2006, 97, 318–323. [Google Scholar] [CrossRef]
- Shen, K.; Feng, X.; Su, R.; Xie, H.; Zhou, L.; Zheng, S. Epigallocatechin 3-gallate ameliorates bile duct ligation induced liver injury in mice by modulation of mitochondrial oxidative stress and inflammation. PLoS ONE 2015, 10, e0126278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, W.; Zhang, T.; Zhou, Q.; Liu, J.; Liu, Y.; Kong, D.; Yu, W.; Liu, R.; Hai, C. TGF-β1 induces epithelial-to-mesenchymal transition via inhibiting mitochondrial functions in A549 cells. Free Radic. Res. 2018, 52, 1432–1444. [Google Scholar] [CrossRef]
- Fang, W.J.; Wang, C.J.; He, Y.; Zhou, Y.L.; Peng, X.D.; Liu, S.K. Resveratrol alleviates diabetic cardiomyopathy in rats by improving mitochondrial function through PGC-1α deacetylation. Acta Pharmacol. Sin. 2018, 39, 59–73. [Google Scholar] [CrossRef]
- Wang, H.; Guan, Y.; Karamercan, M.A.; Ye, L.; Bhatti, T.; Becker, L.B.; Baur, J.A.; Sims, C.A. Resveratrol rescues kidney mitochondrial function following hemorrhagic shock. Shock 2015, 44, 173–180. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Y.Y.; Liu, X.Y.; Jia, S.W.; Zhao, J.; Cui, D.; Wang, L. Resveratrol protects neurons and the myocardium by reducing oxidative stress and ameliorating mitochondria damage in a cerebral ischemia rat model. Cell. Physiol. Biochem. 2014, 34, 854–864. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, R.; Liu, Y.; Zhang, X.; Zhang, M.; Wu, L.; Gao, X.; Lan, T.; Wang, Y. Polydatin protects against carbon tetrachloride-induced liver fibrosis in mice. Arch. Biochem. Biophys. 2017, 629, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elgawad, H.; Abu-Elsaad, N.; El-Karef, A.; Ibrahim, T. Piceatannol increases the expression of hepatocyte growth factor and IL-10 thereby protecting hepatocytes in thioacetamide-induced liver fibrosis. Can. J. Physiol. Pharmacol. 2016, 94, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Wang, H.; Wang, M.; Bai, L.; Yu, P.; Wu, W. Resveratrol alleviates chronic “real-world” ambient particulate matter-induced lung inflammation and fibrosis by inhibiting NLRP3 inflammasome activation in mice. Ecotoxicol. Environ. Saf. 2019, 182, 109425. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, M.; Zhou, Y.; Wang, C.; Yuan, Y.; Li, L.; Bresette, W.; Chen, Y.; Cheng, J.; Lu, Y.; et al. Resveratrol exerts dose-dependent anti-fibrotic or pro-fibrotic effects in kidneys: A potential risk to individuals with impaired kidney function. Phytomedicine 2019, 57, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xiao, Z. Therapeutic effect of resveratrol as well as resveratrol combined with praziquantel on the liver fibrosis due to Schistosoma japonicum infection in mice. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 2013, 31, 337–341. [Google Scholar]
- Cai, W.; Chen, Z.; Chen, F.; Zhou, C.; Liu, R.; Wang, J. Changes of ultrasoanography and two serum biochemical indices for hepatic fibrosis in schistosomiasis japonica patients one year after praziquantel treatment. Chin. Med. J. 1997, 110, 797–800. [Google Scholar]
- Yu, T.; Wang, L.; Lee, H.; O’Brien, D.K.; Bronk, S.F.; Gores, G.J.; Yoon, Y. Decreasing mitochondrial fission prevents cholestatic liver injury. J. Biol. Chem. 2014, 289, 34074–34088. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.T.; Peng, S.; Wang, Y.; Hu, Y.; Shen, Y.; Xu, Y.; Yin, J.; Liu, C.; Cao, J. Improvement of Mitochondrial Activity and Fibrosis by Resveratrol Treatment in Mice with Schistosoma japonicum Infection. Biomolecules 2019, 9, 658. https://doi.org/10.3390/biom9110658
Chen TT, Peng S, Wang Y, Hu Y, Shen Y, Xu Y, Yin J, Liu C, Cao J. Improvement of Mitochondrial Activity and Fibrosis by Resveratrol Treatment in Mice with Schistosoma japonicum Infection. Biomolecules. 2019; 9(11):658. https://doi.org/10.3390/biom9110658
Chicago/Turabian StyleChen, Tina Tuwen, Shihyi Peng, Yanjuan Wang, Yuan Hu, Yujuan Shen, Yuxin Xu, Jianhai Yin, Congshan Liu, and Jianping Cao. 2019. "Improvement of Mitochondrial Activity and Fibrosis by Resveratrol Treatment in Mice with Schistosoma japonicum Infection" Biomolecules 9, no. 11: 658. https://doi.org/10.3390/biom9110658
APA StyleChen, T. T., Peng, S., Wang, Y., Hu, Y., Shen, Y., Xu, Y., Yin, J., Liu, C., & Cao, J. (2019). Improvement of Mitochondrial Activity and Fibrosis by Resveratrol Treatment in Mice with Schistosoma japonicum Infection. Biomolecules, 9(11), 658. https://doi.org/10.3390/biom9110658







