Drug Delivery Systems: Study of Inclusion Complex Formation between Methylxanthines and Cyclodextrins and Their Thermodynamic and Transport Properties
Abstract
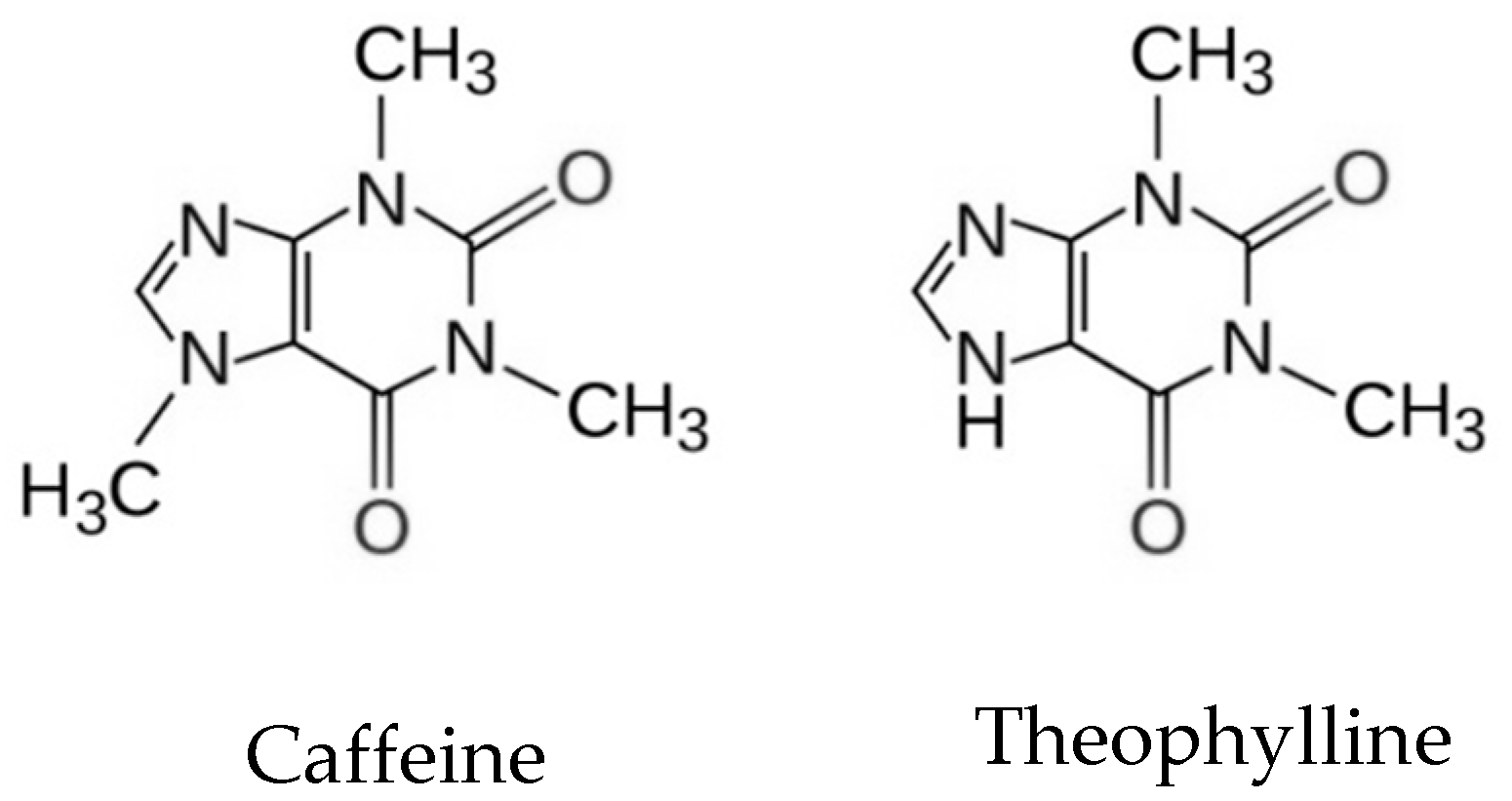
:1. Introduction
- (a)
- they have limited applicability due to solubility limits;
- (b)
- the administration of methylxanthines has high adverse effects at concentrations above the therapeutic range, which is especially important in the case of theophylline, due to its small therapeutic range, which requires the dose and the form of administration to be rigorously controlled until the effective concentrations are reached.
2. Mathematical Concepts
2.1. Molar Volume
2.2. Viscosity
2.3. Mutual Isothermal Diffusion
3. Experimental
3.1. Materials and Solutions
3.2. Equipment and Procedure
4. Results and Discussion
4.1. Characterization of Physicochemical Properties of a Drug in Aqueous Solution
4.1.1. Caffeine + Water System
4.1.2. Theophylline + Water System
4.1.3. β-Cyclodextrin (β-CD) + Water System
4.1.4. Hydroxypropyl-β-Cyclodextrin (HP-β-CD) + Water System
4.2. Influence of Cyclodextrins on the Physicochemical Properties of Methylxanthines in Aqueous Solution
4.2.1. Characterization of the Interactions between Cyclodextrins and Caffeine in Aqueous Solution
4.2.2. Characterization of the Interactions between Cyclodextrins and Theophylline in Aqueous Solution
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gallardo, V.C.; Ceja, J.P.M.; Arroyo, M.H.; Aureoles, G.A.; Galván, D.E.; Landa, J.F.R. Farmacognosia: Breve historia de sus orígenes y su relación con las ciencias médicas. Rev. Biomed. 2004, 15, 123–136. [Google Scholar] [CrossRef]
- Human Medicinal Agents from Plants; Kinghorn, A.D.; Balandrin, M.F. (Eds.) Proceedings of the ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1993; Volume 534. [Google Scholar]
- Lee, K.-H. Discovery and Development of Natural Product-Derived Chemotherapeutic Agents Based on a Medicinal Chemistry Approach. J. Nat. Prod. 2010, 73, 500–516. [Google Scholar] [CrossRef] [PubMed]
- Prentis, R.A.; Lis, Y.; Walker, S.R. Pharmaceutical innovation by the seven UK-owned pharmaceutical companies (1964–1985). Br. J. Clin. Pharmacol. 1988, 25, 387–396. [Google Scholar] [CrossRef]
- Schuster, D.; Laggner, C.; Langer, T. Why Drugs Fail—A Study on Side Effects in New Chemical Entities. Curr. Pharm. Des. 2005, 11, 3545–3559. [Google Scholar] [CrossRef] [PubMed]
- DiMasi, J.A. Risks in new drug development: Approval success rates for investigational drugs. Clin. Pharmacol. Ther. 2001, 69, 297–307. [Google Scholar] [CrossRef]
- DiMasi, J.A.; Hansenb, R.W.; Grabowskic, H.G. The price of innovation: New estimates of drug development costs. J. Health Econ. 2003, 22, 151–185. [Google Scholar] [CrossRef]
- Wu-Pong, S.; Rojanasakul, Y. Biopharmaceutical Drug Design and Development, 2nd ed.; Humana Press: New York, NY, USA, 2008. [Google Scholar]
- Han, C.; Wang, B. Factors that impact the developability of drug candidates-An overview. In Drug Delivery: Principles and Applications; Wang, B., Siahaan, T., Soltero, R., Eds.; Wiley Interscience: Hoboken, NJ, USA, 2005; Volume 1, pp. 1–15. [Google Scholar]
- Monografías de Formas Farmacéuticas. Formas Farmacéuticas. In Real Farmacopea Española, 3th ed.; Ministerio de Sanidad y Consumo: Madrid, Spain, 2005; Volume 645. [Google Scholar]
- Zhou, H. Water Insoluble Drug Formulation; CRC Press: Boca Raton, FL, USA, 2008; Volume 5, pp. 91–100. [Google Scholar]
- Uekama, K.; Hirayama, F.; Irie, T. Cyclodextrin Drug Carrier Systems. Chem. Rev. 1998, 98, 2045–2076. [Google Scholar] [CrossRef]
- Loftsson, T.; Duchêne, D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef]
- Vyas, A.; Saraf, S. Cyclodextrin based novel drug delivery systems. J. Incl. Phenom. Macrocycl. Chem. 2008, 62, 23–42. [Google Scholar] [CrossRef]
- Lazzara, G.; Milioto, S.; Schimmenti, R. Thermodynamics of cyclodextrin–star copolymer threading–dethreading process. J. Therm. Anal. Calorim. 2015, 121, 1345–1352. [Google Scholar] [CrossRef]
- Bertolino, V.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F. Crystallinity of block copolymer controlled by cyclodextrin. J. Therm. Anal. Calorim. 2018, 132, 191–196. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, H.; Ma, P.X. Host−guest interaction mediated polymeric assemblies: Multifunctional nanoparticles for drug and gene delivery. ACS Nano 2010, 4, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Gidwani, B.; Vyas, A. A Comprehensive Review on Cyclodextrin-Based Carriers for Delivery of Chemotherapeutic Cytotoxic Anticancer Drugs. BioMed Res. Int. J. 2015, 3, 1–15. [Google Scholar] [CrossRef]
- Szejtli, J. Comprehensive Supramolecular Chemistry; Pergamon: Oxford, UK, 1996; Volume 3. [Google Scholar]
- Bender, M.L.; Momiyama, M. Cyclodextrin Chemistry; Springer: Berlin, Germany, 1978. [Google Scholar]
- Jones, S.P.; Grant, D.J.W.; Hadgraft, J.; Parr, D. Cyclodextrins in the pharmaceutical sciences Part. 1. Preparation, structure, and properties of cyclodextrins and cyclodextrin inclusion compounds. Acta Pharm. Tech. 1984, 30, 213–223. [Google Scholar]
- Gilbert, R.M. Caffeine Consumption. Prog. Clin. Biol. Res. 1984, 158, 185–213. [Google Scholar]
- Ashihara, H.; Crozier, A. Biosynthesis and metabolism of caffeine and related purine alkaloids in plants. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 1999; Volume 30, pp. 117–205. [Google Scholar]
- Spiro, M.; Grandoso, D.M.; Price, W.E. Protonation constant of caffeine in aqueous solution. J. Chem. Soc. Faraday Trans. I 1989, 85, 4259–4267. [Google Scholar] [CrossRef]
- Nishijo, J.; Yonetani, I. The Interaction of Theophylline with Benzylamine in Aqueous Solution. Chem. Pharm. Bull. 1982, 30, 4507–4511. [Google Scholar] [CrossRef]
- Budavari, S.; O’Neil, M.J.; Smith, A.; Heckelman, P.E. The Merck Index, 14th ed.; Merck: Rahway, NJ, USA, 2006. [Google Scholar]
- Fredholm, B.B.; Battig, K.; Holmen, J.; Nehlig, A.; Zvartau, E.E. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol. Rev. 1999, 51, 83–133. [Google Scholar] [PubMed]
- Moratalla, R. Neurobiología de las metilxantinas. Trastor. Adict. 2008, 10, 201–207. [Google Scholar] [CrossRef]
- Peck, C.C.; Nichols, A.I.; Baker, J.; Lenert, L.L.; Ezra, D. Clinical pharmacodynamics of theophylline. J. Allergy Clin. Immunol. 1985, 76, 292–297. [Google Scholar] [CrossRef]
- Sharron, H.F.; Konjeti, R.S.; Hengming, K.; Jackie, D.C. Inhibition of Cyclic Nucleotide Phosphodiesterases by Methylxanthines. In Methylxanthines, Handbook of Experimental Pharmacology; Fredholm, B.B., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 93–134. [Google Scholar]
- Santos, C.I.A.V. Sistemas de Liberación Controlada de Fármacos, Propiedades Termodinámicas y de Transporte de Sistemas que Incluyen Ciclodextrinas y Fármacos a Dosis Terapêuticas. Ph.D. Thesis, Universidad de Alcalá, Alcalá de Henares, Spain, Universidade de Coimbra, Coimbra, Portugal, 2012. [Google Scholar]
- Masson, D.O. Solute molecular volumes in relation to solvation and ionization. Philos. Mag. 1929, 8, 218–235. [Google Scholar] [CrossRef]
- Redlich, O. Molar volumes of solute IV. J. Phys. Chem. 1940, 44, 619–629. [Google Scholar] [CrossRef]
- Terekhova, I.V.; De Lisi, R.; Lazzara, G.; Milioto, S.; Muratore, N. Volume and Heat Capacity Studies To Evidence Interactions Between Cyclodextrins And Nicotinic Acid In Water. J. Therm. Anal. Calorim. 2008, 92, 285–290. [Google Scholar] [CrossRef]
- Terekhova, I.V. Volumetric and calorimetric study on complex formation of cyclodextrins with aminobenzoic acids. Mendeleev Commun. 2009, 19, 110–112. [Google Scholar] [CrossRef]
- Jones, G.; Dole, M. The viscosity of aqueous solutions of strong electrolytes with special reference to barium chloride. J. Am. Chem. Soc. 1929, 51, 2950–2964. [Google Scholar] [CrossRef]
- Thomas, D.G. Transport characteristics of suspension: VIII. A note on the viscosity of Newtonian suspensions of uniform spherical particles. J. Colloid Sci. 1965, 20, 267–277. [Google Scholar] [CrossRef]
- Tibor, E.G. Transport. Phenomena in Aqueous Solutions; Adam Hilger: London, UK, 1974. [Google Scholar]
- Tyrrel, H.J.V.; Harris, K.R. Diffusion in Liquids; Butterworths: London, UK, 1984. [Google Scholar]
- Ribeiro, A.C.F.; Esteso, M.A. Controlled-release systems: A brief review of the importance of their study in biological systems. Biomolecules 2018, 8, 178. [Google Scholar] [CrossRef]
- Vitagliano, V.; Sartorio, R. Some aspects of diffusion in ternary systems. J. Phys. Chem. 1970, 74, 2949–2956. [Google Scholar] [CrossRef]
- Paduano, L.; Sartorio, R.; Vitagliano, V.; Castronuovo, G. Calorimetric and diffusional behavior of the system alpha-cyclodextrin l-phenylalanine in aqueous-solution. Thermochim. Acta 1990, 162, 155–161. [Google Scholar] [CrossRef]
- Paduano, L.; Sartorio, R.; Vitagliano, V.; Costantino, L. Diffusion-coefficients in systems with inclusion-compounds. 2. alpha-cyclodextrin-(DL)norleucine-water at 25 °C. Ber. Bunsenges. Phys. Chem. 1990, 94, 741–745. [Google Scholar] [CrossRef]
- Paduano, L.; Sartorio, R.; Vitagliano, V. Diffusion Coefficients of the Ternary System α-Cyclodextrin−Sodium Benzenesulfonate−Water at 25 °C: The Effect of Chemical Equilibrium and Complex. Formation on the Diffusion Coefficients of a Ternary System. J. Phys. Chem. B 1998, 102, 5023–5028. [Google Scholar] [CrossRef]
- Barthel, J.; Gores, H.J.; Lohr, C.M.; Seidl, J.J. Taylor dispersion measurements at low electrolyte concentrations. I. Tetraalkylammonium perchlorate aqueous solutions. J. Solution Chem. 1996, 25, 921–935. [Google Scholar] [CrossRef]
- Hartley, G.S.; Crank, J. Some fundamental definitions and concepts in diffusion processes. Trans. Faraday Soc. 1949, 45, 801–818. [Google Scholar] [CrossRef]
- Miller, D.G.; Albright, J.G. Measurements of the Transport Properties of Fluids: Experimental Thermodynamics; Wakeham, W.A., Nagashima, A., Sengers, J.V., Eds.; Blackwell: Oxford, UK, 1991. [Google Scholar]
- Cussler, E.L. Diffusion Mass Transfer in Fluid Systems; University of Minesota: Minneapolis, Minnesota, USA, 1984. [Google Scholar]
- Loh, W. A técnica de dispersão de Taylor para estudos de difusão em líquidos e suas aplicações. Quim. Nova 1997, 20, 541–545. [Google Scholar] [CrossRef]
- Leaist, D.G. Determination of Ternary Diffusion Coefficients by the Taylor Dispersion Method. J. Phys. Chem. 1990, 94, 5180–5183. [Google Scholar] [CrossRef]
- Barros, M.C.F.; Ribeiro, A.C.F.; Esteso, M.A. Cyclodextrins in Parkinson’s desease. Biomolecules 2019, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Guo, Q.X. The driving forces in the inclusion complexation of cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2002, 42, 1–14. [Google Scholar] [CrossRef]
- Stella, V.J.; Rajewski, R.A. Cyclodextrins: Their future in drug formulation and delivery. Pharm. Res. 1997, 14, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.I.A.V.; Teijeiro, C.; Ribeiro, A.C.F.; Rodrigues, D.F.S.L.; Romero, C.M.; Esteso, M.A. Drug delivery systems: Study of inclusion complex formation for ternary caffeine-beta-cyclodextrin-water mixtures from apparent molar volume values at 298.15 K and 310.15 K. J. Mol. Liq. 2016, 223, 209–216. [Google Scholar] [CrossRef]
- Cesaro, A.; Russo, E.; Crescenzl, V. Thermodynamics of caffeine aqueous solutions. J. Phys. Chem. 1976, 80, 335–339. [Google Scholar] [CrossRef]
- Falk, M.; Gil, M.; Iza, N. Self-association of caffeine in aqueous solution: An FT-IR study. Can. J. Chem. 1990, 68, 1293–1299. [Google Scholar] [CrossRef]
- Franks, F.; Smith, H.T. Volumetric properties of alcohols in dilute aqueous solutions. Trans. Faraday Soc. 1968, 64, 2962–2972. [Google Scholar] [CrossRef]
- Franks, F.; Smith, H.T. Precision densities of dilute aqueous solutions of the isomeric butanols. J. Chem. Eng. Data 1968, 13, 538–541. [Google Scholar] [CrossRef]
- Franks, F.; Desnoyers, J.E. Water Science Review; Franks, N.F., Desnoyers, J.E., Eds.; Cambridge University Press: Cambridge, UK, 1985; pp. 171–232. [Google Scholar]
- Frank, H.S.; Evans, M.W. Free volume and entropy in condensed systems. III. Entropy in binary liquid mixtures; partial molal entropy in dilute solutions; structure and thermodynamics in aqueous electrolytes. J. Chem. Phys. 1945, 13, 507–532. [Google Scholar] [CrossRef]
- Keronei, P.K. Intermolecular Interactions in Aqueous Binary Mixtures of Non-electrolytes. Ph.D. Thesis, University of Auckland, Auckland, New Zealand, 1995. Available online: http://researchspace.auckland.ac.nz (accessed on 15 April 2019).
- Franks, F.; Reid, D.S. Water: A Comprehensive Treatise; Franks, F., Ed.; Plenum Press: New York, NY, USA, 1973; Volume 2, pp. 330–374. [Google Scholar]
- Sinha, B.; Roy, P.K.; Sarkar, B.K.; Brahman, D.; Roy, M.N. Apparent molar volumes and viscosity B-coefficients of caffeine in aqueous thorium nitrate solutions at T = (298.15, 308.15 and 318.15) K. J. Chem. Thermodyn. 2010, 42, 380–386. [Google Scholar] [CrossRef]
- Price, W.E.; Trickett, K.A.; Harris, K.R. Association of caffeine in aqueous solution. Effects on caffeine intradiffusion. J. Chem. Soc. Faraday Trans. I 1989, 85, 3281–3288. [Google Scholar] [CrossRef]
- Zhao, H. Viscosity B-coefficients and standard partial molar volumes of amino acids, and their roles in interpreting the protein (enzyme) stabilization. Biophys. Chem. 2006, 122, 157–183. [Google Scholar] [CrossRef]
- Thompson, P.T.; Fisher, B.; Wood, R.H. Viscosities of Solutions of Electrolytes and Nonelectrolytes in Ethylene Carbonate at 40 °C. J. Solution Chem. 1982, 11, 1–15. [Google Scholar] [CrossRef]
- Nishijo, J.; Yonetani, I.; Tagahara, K.; Suzuta, Y.; Iwamoto, E. Influence of temperature and ionization on self association of theophylline in aqueous solution. Studies by 1-HRMN. Chem. Pharm. Bull. 1986, 34, 4451–4456. [Google Scholar] [CrossRef]
- Santos, C.I.A.V.; Lobo, V.M.M.; Esteso, M.A.; Ribeiro, A.C.F. Effect of Potassium Chloride on Diffusion of Theophylline at T = 298.15 K. J. Chem. Thermodyn. 2011, 43, 873–875. [Google Scholar] [CrossRef]
- Paduano, L.; Sartorio, R.; Vitagliano, V.; Costantino, L. Diffusion properties of cyclodextrins in aqueous solution at 25 °C. J. Solution Chem. 1990, 19, 31–39. [Google Scholar] [CrossRef]
- Nightingale, E.R. Chemical Physics of Electrolyte Solutions; Conway, B.E., Barradas, R.G., Eds.; Wiley: New York, NY, USA, 1966; Volume 87. [Google Scholar]
- Milioto, S.; Bakshi, M.S.; Crisantino, R.; De Lisi, R. Thermodynamic properties of water–β-cyclodextrin-dodecylsurfactant ternary systems. J. Solution Chem. 1995, 24, 103–120. [Google Scholar] [CrossRef]
- Stezowski, J.J.; Jogun, K.H.; Eckle, E.; Bartels, K. Dimeric β-cyclodextrin complexes may mimic membrane diffusion transport. Nature 1978, 274, 617–619. [Google Scholar] [CrossRef]
- Marcus, Y. Effect of ions on the structure of water: Structure making and breaking. Chem. Rev. 2009, 109, 1346–1370. [Google Scholar] [CrossRef]
- Castronuovo, G.; Niccoli, M. The influence of cosolvents on hydrophilic and hydrophobic interactions. Calorimetric studies of parent and alkylated cyclomaltooligosaccharides in concentrated aqueous solutions of ethanol or urea. Carbohydr. Res. 2008, 343, 2771–2775. [Google Scholar] [CrossRef]
- Ribeiro, A.C.F.; Leaist, D.G.; Lobo, V.M.M.; Esteso, M.A.; Valente, A.J.M.; Santos, C.I.A.V.; Cabral, A.M.T.D.P.V.; Veiga, F.J.B. Binary mutual diffusion coefficients of aqueous solutions of ß-cyclodextrin at temperatures from 298.15 K to 312.15 K. J. Chem. Eng. Data 2006, 51, 1368–1371. [Google Scholar] [CrossRef]
- Glasstone, S.; Laidler, K.J.; Eyring, H. Theory of Rate Processes; McGraw-Hill: New York, NY, USA, 1941; Chapter 9. [Google Scholar]
- Longsworth, L.G. Temperature dependence of diffusion in aqueous solutions. J. Phys. Chem. 1954, 58, 770–773. [Google Scholar] [CrossRef]
- Evans, D.F.; Tominaga, T.; Chan, C. Diffusion of symmetrical and spherical solutes in protic, aprotic and hydrocarbon solvents. J. Solution Chem. 1979, 8, 461–478. [Google Scholar] [CrossRef]
- Ribeiro, A.C.F.; Valente, A.J.M.; Santos, C.I.A.V.; Prazeres, P.M.R.A.; Lobo, V.M.M.; Burrows, H.D.; Esteso, M.A.; Cabral, A.M.T.D.P.V.; Veiga, F.J.B. Binary mutual diffusion coefficients of aqueous solutions of α-cyclodextrin, 2-hydroxypropyl-α-cyclodextrin and 2-hydroxypropyl-ß-cyclodextrin at temperatures from 298.15 to 312.15 K. J. Chem. Eng. Data 2007, 52, 586–590. [Google Scholar] [CrossRef]
- Loftsson, T.; Másson, M.; Brewester, M.E. Self-association of cyclodextrins and cyclodextrin complexes. J. Pharm. Sci. 2004, 93, 1091–1099. [Google Scholar] [CrossRef]
- Loftsson, T.; Másson, M.; Brewester, M.E. Self-association and cyclodextrin solubilization of drugs. J. Pharm. Sci. 2002, 91, 2307–2316. [Google Scholar] [CrossRef] [PubMed]
- Friedman, H.L.; Krishnan, C.V. Water: A Comprehensive Treatise; Franks, F., Ed.; Plenum Press: New York, NY, USA, 1973; Volume 3, Chapter 1. [Google Scholar]
- Bhat, R.; Kishore, N.; Ahluwalia, J.C. Thermodynamic studies of transfer of some amino acids and peptides from water to aqueous glucose and sucrose solutions at 298.15 K. J. Chem. Soc. Faraday Trans. I 1988, 84, 2651–2665. [Google Scholar] [CrossRef]
- Rekharsky, M.V.; Inoue, Y. Complexation Thermodynamics of Cyclodextrins. Chem. Rev. 1998, 98, 1875–1917. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.L.; Ding, L.H.; Dong, C.; Niu, W.P.; Shuang, S.M. Study on inclusion complex of cyclodextrin with methylxanthine derivatives by fluorimetry. Spectrochim. Acta Part A 2003, 59, 2697–2703. [Google Scholar] [CrossRef]
- Terekhova, I.V.; Kulikov, O.V. Calorimetric study of the molecular recognition of nucleic acid bases by β-cyclodextrin in aqueous solution. Mendeleev Commun. 2002, 12, 245–246. [Google Scholar] [CrossRef]
- Terekhova, I.V.; Kumeev, R.S.; Alper, G.A. The interaction of caffeine with substituted cyclodextrins in water. Russ. J. Phys. Chem. A 2007, 81, 1071–1075. [Google Scholar]
- Krishnamoorthy, R.; Mitra, A.K. Complexation of weak acids and bases with cyclodextrins: Effects of substrate ionization on the estimation and interpretation of association constants. Int. J. Pharm. Adv. 1996, 1, 329–343. [Google Scholar]
- Aicart, E.; Junquera, E. Complex formation between purine derivatives and cyclodextrins: A fluorescence spectroscopy study. J. Incl. Phenom. Macrocycl. Chem. 2003, 47, 161–165. [Google Scholar] [CrossRef]
- Pina, M.E.; Veiga, F. The influence of diluent on the release of theophylline from hydrophilic matrix tablets. Drug Dev. Ind. Pharm. 2000, 26, 1125–1128. [Google Scholar] [CrossRef]
- Terekhova, I.V.; Volkova, T.V.; Perlovich, G.L. Interactions of theophylline with cyclodextrins in water. Mendeleev Commun. 2007, 17, 244–246. [Google Scholar] [CrossRef]
- Wilson, L.D.; Verrall, R.E. A Volumetric Study of Cyclodextrin-α,ω-Alkyl Dicarboxylate Anion Complexes in Aqueous Solutions. J. Phys. Chem. B 2000, 104, 1880–1886. [Google Scholar] [CrossRef]
- Wilson, L.D.; Verrall, R.E. A Volumetric Study of β-cyclodextrin/Hydrocarbon and β-Cyclodextrin/Fluorocarbon Surfactant Inclusion Complexes in Aqueous Solutions. J. Phys. Chem. B 1997, 101, 9270–9279. [Google Scholar] [CrossRef]
- Spildo, K.; Hoiland, H. Complex Formation Between Alkane-α,ω-Diols and Cyclodextrins Studied by Partial Molar Volume and Compressibility Measurements. J. Solution Chem. 2002, 31, 149–164. [Google Scholar] [CrossRef]
- Gianni, P.; Lepori, L. Partial Molar Volumes of Ionic and Nonionic Organic Solutes in Water: A Simple Additivity Scheme Based on the Intrinsic Volume Approach. J. Solution Chem. 2000, 29, 405–447. [Google Scholar]
- Donald, H.; Jenkins, B.; Marcus, Y. Viscosity B-coefficients of ions in solution. Chem. Rev. 1995, 95, 2695–2724. [Google Scholar]
- Ribeiro, A.C.F.; Santos, C.I.A.V.; Lobo, V.M.M.; Cabral, A.M.T.D.P.V.; Veiga, F.J.B.; Esteso, M.A. Diffusion coefficients of the ternary system β-Cyclodextrin + caffeine + water at 298.15 K. J. Chem. Eng. Data 2009, 54, 115–117. [Google Scholar] [CrossRef]
- Ribeiro, A.C.F.; Santos, C.I.A.V.; Lobo, V.M.M.; Cabral, A.M.T.D.P.V.; Veiga, F.J.B.; Esteso, M.A. Diffusion Coefficients of the Ternary System (2-hydroxypropyl-β-cyclodextrin + Caffeine + Water) at T = 298.15 K. J. Chem. Thermodyn. 2009, 41, 1324–1328. [Google Scholar] [CrossRef]
- Ribeiro, A.C.F.; Santos, C.I.A.V.; Lobo, V.M.M.; Esteso, M.A. Diffusion of caffeine in different aqueous media at physiological temperature. In Caffeine: Chemistry, Analysis, Function and Effects; Food and Nutritional Components in Focus; Preedy, V.R., Ed.; The Royal Society of Chemistry Publishing: London, UK, 2012; Volume 2, pp. 89–100. [Google Scholar]
- Horiuchi, Y.; Abe, K.; Hirayama, F.; Uekama, K. Release control of theophylline by β-cyclodextrin derivatives: Hybridizing effect of hydrophilic, hydrophobic and ionizable β-cyclodextrin complexes. J. Control. Release 1991, 15, 177–183. [Google Scholar] [CrossRef]
- Ammar, H.O.; Ghorab, M.; El-nahhas, S.A.; Omar, S.M.; Ghorab, M.M. Improvement of some pharmaceutical properties of drugs by cyclodextrin complexation. 5. Theophylline. Pharmazie 1996, 51, 42–46. [Google Scholar]
- Santos, C.I.A.V.; Esteso, M.A.; Sartorio, R.; Ortona, O.; Sobral, A.J.N.; Arranja, C.T.; Lobo, V.M.M.; Ribeiro, A.C.F. A Comparison between the Diffusion Properties of Theophylline/β-Cyclodextrin and Theophylline/2-Hydroxypropyl-β-Cyclodextrin in Aqueous Systems. J. Chem. Eng. Data 2012, 57, 1881–1886. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, C.I.A.V.; Ribeiro, A.C.F.; Esteso, M.A. Drug Delivery Systems: Study of Inclusion Complex Formation between Methylxanthines and Cyclodextrins and Their Thermodynamic and Transport Properties. Biomolecules 2019, 9, 196. https://doi.org/10.3390/biom9050196
Santos CIAV, Ribeiro ACF, Esteso MA. Drug Delivery Systems: Study of Inclusion Complex Formation between Methylxanthines and Cyclodextrins and Their Thermodynamic and Transport Properties. Biomolecules. 2019; 9(5):196. https://doi.org/10.3390/biom9050196
Chicago/Turabian StyleSantos, Cecília I. A. V., Ana C. F. Ribeiro, and Miguel A. Esteso. 2019. "Drug Delivery Systems: Study of Inclusion Complex Formation between Methylxanthines and Cyclodextrins and Their Thermodynamic and Transport Properties" Biomolecules 9, no. 5: 196. https://doi.org/10.3390/biom9050196
APA StyleSantos, C. I. A. V., Ribeiro, A. C. F., & Esteso, M. A. (2019). Drug Delivery Systems: Study of Inclusion Complex Formation between Methylxanthines and Cyclodextrins and Their Thermodynamic and Transport Properties. Biomolecules, 9(5), 196. https://doi.org/10.3390/biom9050196






