The Role of Sonic Hedgehog in Craniofacial Patterning, Morphogenesis and Cranial Neural Crest Survival
Abstract
:1. Introduction—Formation of the Craniofacial Skeleton
2. Development of the Craniofacial Skeleton—The First Pharyngeal Arch
3. Defects of Craniofacial Development
4. A Key Pharyngeal-Arch Derived Regulator of Craniofacial Development—Sonic Hedgehog (Shh)
5. The Role of Shh in the Formation of the First Pharyngeal Arch and Maintaining Neural Crest Cell Fidelity
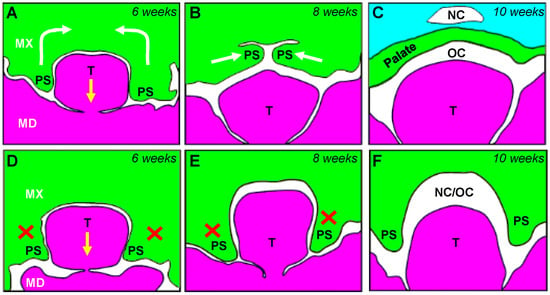
6. Role of Shh in Development and Fusion of the Palate (Maxilla/Upper Jaw) and Mandible (Lower Jaw)
7. Conclusions and Future Directions
Acknowledgments
Conflicts of Interest
References
- Cordero, D.R.; Brugmann, S.; Chu, Y.; Bajpai, R.; Jame, M.; Helms, J.A. Cranial neural crest cells on the move: Their roles in craniofacial development. Am. J. Med. Genet. 2011, 155A, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Lumsden, A.; Sprawson, N.; Graham, A. Segmental origin and migration of neural crest cells in the hindbrain region of the chick embryo. Development 1991, 113, 1281–1291. [Google Scholar] [PubMed]
- Murdoch, J.N.; Copp, A.J. The relationship between sonic hedgehog signaling, cilia, and neural tube defects. Birth Defects Res. Part A Clin. Mol. Teratol. 2010, 88, 633–652. [Google Scholar] [CrossRef] [PubMed]
- Gitton, Y.; Heude, E.; Vieux-Rochas, M.; Benouaiche, L.; Fontaine, A.; Sato, T.; Kurihara, Y.; Kurihara, H.; Couly, G.; Levi, G. Evolving maps in craniofacial development. Semin. Cell Dev. Biol. 2010, 21, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Cobourne, M.T.; Sharpe, P.T. Sonic hedgehog signaling and the developing tooth. Curr. Top. Dev. Biol. 2005, 65, 255–287. [Google Scholar] [PubMed]
- Lan, Y.; Jia, S.; Jiang, R. Molecular patterning of the mammalian dentition. Semin. Cell Dev. Biol. 2014, 25–26, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, M.; Tian, W. An inductive signalling network regulates mammalian tooth morphogenesis with implications for tooth regeneration. Cell Prolif. 2013, 46, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Xavier, G.M.; Seppala, M.; Barrell, W.; Birjandi, A.A.; Geoghegan, F.; Cobourne, M.T. Hedgehog receptor function during craniofacial development. Dev. Biol. 2016, 415, 198–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, J.M.; Moonis, G.; Green, G.E.; Carmody, R.; Burbank, H.N. Syndromes of the first and second branchial arches, part 1: Embryology and characteristic defects. Am. J. Neuroradiol. 2011, 32, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Grevellec, A.; Tucker, A.S. The pharyngeal pouches and clefts: Development, evolution, structure and derivatives. Semin. Cell Dev. Biol. 2010, 21, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Cobourne, M.T.; Sharpe, P.T. Tooth and jaw: Molecular mechanisms of patterning in the first branchial arch. Arch. Oral Biol. 2003, 48, 1–14. [Google Scholar] [CrossRef]
- Graham, A. The development and evolution of the pharyngeal arches. J. Anat. 2001, 199, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Graham, A.; Smith, A. Patterning the pharyngeal arches. BioEssays 2001, 23, 54–61. [Google Scholar] [CrossRef]
- Chai, Y.; Maxson, R.E., Jr. Recent advances in craniofacial morphogenesis. Dev. Dyn. 2006, 235, 2353–2375. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Sasano, Y.; Bringas, P., Jr.; Mayo, M.; Kaartinen, V.; Heisterkamp, N.; Groffen, J.; Slavkin, H.; Shuler, C. Characterization of the fate of midline epithelial cells during the fusion of mandibular prominences in vivo. Dev. Dyn. 1997, 208, 526–535. [Google Scholar] [CrossRef]
- Greene, R.M.; Pisano, M.M. Palate morphogenesis: Current understanding and future directions. Birth Defects Res. C Embryo Today 2010, 90, 133–154. [Google Scholar] [CrossRef] [PubMed]
- M’Boneko, V.; Merker, H.J. Development and morphology of the periderm of mouse embryos (days 9–12 of gestation). Acta Anat. 1988, 133, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Fitchett, J.E.; Hay, E.D. Medial edge epithelium transforms to mesenchyme after embryonic palatal shelves fuse. Dev. Biol. 1989, 131, 455–474. [Google Scholar] [CrossRef]
- Martinez-Alvarez, C.; Blanco, M.J.; Perez, R.; Rabadan, M.A.; Aparicio, M.; Resel, E.; Martinez, T.; Nieto, M.A. Snail family members and cell survival in physiological and pathological cleft palates. Dev. Biol. 2004, 265, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Mori, C.; Nakamura, N.; Okamoto, Y.; Osawa, M.; Shiota, K. Cytochemical identification of programmed cell death in the fusing fetal mouse palate by specific labelling of DNA fragmentation. Anat. Embryol. 1994, 190, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Duke University School of Medicine. Craniofacial Embryology. Available online: https://web.duke.edu/anatomy/embryology/craniofacial/craniofacial.html (accessed on 27 July 2016).
- Jones, N.C.; Lynn, M.L.; Gaudenz, K.; Sakai, D.; Aoto, K.; Rey, J.P.; Glynn, E.F.; Ellington, L.; Du, C.; Dixon, J.; et al. Prevention of the neurocristopathy treacher collins syndrome through inhibition of p53 function. Nat. Med. 2008, 14, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski, T.; Ahn, D.G.; Schilling, T.F.; Nair, S.; Ruvinsky, I.; Geisler, R.; Rauch, G.J.; Haffter, P.; Zon, L.I.; Zhou, Y.; et al. The zebrafish van gogh mutation disrupts tbx1, which is involved in the digeorge deletion syndrome in humans. Development 2003, 130, 5043–5052. [Google Scholar] [CrossRef] [PubMed]
- Talbot, J.C.; Walker, M.B.; Carney, T.J.; Huycke, T.R.; Yan, Y.L.; BreMiller, R.A.; Gai, L.; Delaurier, A.; Postlethwait, J.H.; Hammerschmidt, M.; et al. Fras1 shapes endodermal pouch 1 and stabilizes zebrafish pharyngeal skeletal development. Development 2012, 139, 2804–2813. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Schutte, B.C.; Richardson, R.J.; Bjork, B.C.; Knight, A.S.; Watanabe, Y.; Howard, E.; de Lima, R.L.; Daack-Hirsch, S.; Sander, A.; et al. Mutations in irf6 cause van der woude and popliteal pterygium syndromes. Nat. Genet. 2002, 32, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Nusslein-Volhard, C.; Wieschaus, E. Mutations affecting segment number and polarity in drosophila. Nature 1980, 287, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Echelard, Y.; Epstein, D.J.; St-Jacques, B.; Shen, L.; Mohler, J.; McMahon, J.A.; McMahon, A.P. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of cns polarity. Cell 1993, 75, 1417–1430. [Google Scholar] [CrossRef]
- Riddle, R.D.; Johnson, R.L.; Laufer, E.; Tabin, C. Sonic hedgehog mediates the polarizing activity of the zpa. Cell 1993, 75, 1401–1416. [Google Scholar] [CrossRef]
- Varjosalo, M.; Taipale, J. Hedgehog: Functions and mechanisms. Gene. Dev. 2008, 22, 2454–2472. [Google Scholar] [CrossRef] [PubMed]
- Krauss, S.; Concordet, J.P.; Ingham, P.W. A functionally conserved homolog of the drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell 1993, 75, 1431–1444. [Google Scholar] [CrossRef]
- Odent, S.; Atti-Bitach, T.; Blayau, M.; Mathieu, M.; Auge, J.; Delezo de, A.L.; Gall, J.Y.; le Marec, B.; Munnich, A.; David, V.; et al. Expression of the sonic hedgehog (shh) gene during early human development and phenotypic expression of new mutations causing holoprosencephaly. Hum. Mol. Genet. 1999, 8, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Litingtung, Y.; Lei, L.; Westphal, H.; Chiang, C. Sonic hedgehog is essential to foregut development. Nat. Genet. 1998, 20, 58–61. [Google Scholar] [PubMed]
- Chang, D.T.; Lopez, A.; von Kessler, D.P.; Chiang, C.; Simandl, B.K.; Zhao, R.; Seldin, M.F.; Fallon, J.F.; Beachy, P.A. Products, genetic linkage and limb patterning activity of a murine hedgehog gene. Development 1994, 120, 3339–3353. [Google Scholar] [PubMed]
- Currie, P.D.; Ingham, P.W. Induction of a specific muscle cell type by a hedgehog-like protein in zebrafish. Nature 1996, 382, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Marti, E.; Takada, R.; Bumcrot, D.A.; Sasaki, H.; McMahon, A.P. Distribution of sonic hedgehog peptides in the developing chick and mouse embryo. Development 1995, 121, 2537–2547. [Google Scholar] [PubMed]
- Belloni, E.; Muenke, M.; Roessler, E.; Traverso, G.; Siegel-Bartelt, J.; Frumkin, A.; Mitchell, H.F.; Donis-Keller, H.; Helms, C.; Hing, A.V.; et al. Identification of sonic hedgehog as a candidate gene responsible for holoprosencephaly. Nat. Genet. 1996, 14, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Roessler, E.; Belloni, E.; Gaudenz, K.; Jay, P.; Berta, P.; Scherer, S.W.; Tsui, L.C.; Muenke, M. Mutations in the human sonic hedgehog gene cause holoprosencephaly. Nat. Genet. 1996, 14, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Roessler, E.; Belloni, E.; Gaudenz, K.; Vargas, F.; Scherer, S.W.; Tsui, L.C.; Muenke, M. Mutations in the c-terminal domain of sonic hedgehog cause holoprosencephaly. Hum. Mol. Genet. 1997, 6, 1847–1853. [Google Scholar] [CrossRef] [PubMed]
- Roessler, E.; Ward, D.E.; Gaudenz, K.; Belloni, E.; Scherer, S.W.; Donnai, D.; Siegel-Bartelt, J.; Tsui, L.C.; Muenke, M. Cytogenetic rearrangements involving the loss of the sonic hedgehog gene at 7q36 cause holoprosencephaly. Hum. Genet. 1997, 100, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Muenke, M.; Gurrieri, F.; Bay, C.; Yi, D.H.; Collins, A.L.; Johnson, V.P.; Hennekam, R.C.; Schaefer, G.B.; Weik, L.; Lubinsky, M.S.; et al. Linkage of a human brain malformation, familial holoprosencephaly, to chromosome 7 and evidence for genetic heterogeneity. Proc. Natl. Acad. Sci. USA 1994, 91, 8102–8106. [Google Scholar] [CrossRef] [PubMed]
- Garavelli, L.; Zanacca, C.; Caselli, G.; Banchini, G.; Dubourg, C.; David, V.; Odent, S.; Gurrieri, F.; Neri, G. Solitary median maxillary central incisor syndrome: Clinical case with a novel mutation of sonic hedgehog. Am. J. Med. Genet. Part A 2004, 127A, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, H.; Kamegai, A.; Hata, T.; Kitamura, N.; Hosoda, M.; Tani, R.; Hayashido, Y.; Toratani, S.; Okamoto, T. Developmental signaling disorders in craniofacial anomalies and cancers. Oral Sci. Int. 2006, 3, 56–63. [Google Scholar] [CrossRef]
- Morava, E.; Bartsch, O.; Czako, M.; Frensel, A.; Kalscheuer, V.; Karteszi, J.; Kosztolanyi, G. Small inherited terminal duplication of 7q with hydrocephalus, cleft palate, joint contractures, and severe hypotonia. Clin. Dysmorphol. 2003, 12, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Schimmenti, L.A.; de la Cruz, J.; Lewis, R.A.; Karkera, J.D.; Manligas, G.S.; Roessler, E.; Muenke, M. Novel mutation in sonic hedgehog in non-syndromic colobomatous microphthalmia. Am. J. Med. Genet. Part A 2003, 116A, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Ahlgren, S.C.; Bronner-Fraser, M. Inhibition of sonic hedgehog signaling in vivo results in craniofacial neural crest cell death. Curr. Biol. 1999, 9, 1304–1314. [Google Scholar] [CrossRef]
- Brito, J.M.; Teillet, M.A.; le Douarin, N.M. An early role for sonic hedgehog from foregut endoderm in jaw development: Ensuring neural crest cell survival. Proc. Natl. Acad. Sci. USA 2006, 103, 11607–11612. [Google Scholar] [CrossRef] [PubMed]
- Brito, J.M.; Teillet, M.A.; le Douarin, N.M. Induction of mirror-image supernumerary jaws in chicken mandibular mesenchyme by sonic hedgehog-producing cells. Development 2008, 135, 2311–2319. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.; Litingtung, Y.; Lee, E.; Young, K.E.; Corden, J.L.; Westphal, H.; Beachy, P.A. Cyclopia and defective axial patterning in mice lacking sonic hedgehog gene function. Nature 1996, 383, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Moore-Scott, B.A.; Manley, N.R. Differential expression of sonic hedgehog along the anterior-posterior axis regulates patterning of pharyngeal pouch endoderm and pharyngeal endoderm-derived organs. Dev. Biol. 2005, 278, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Testaz, S.; Jarov, A.; Williams, K.P.; Ling, L.E.; Koteliansky, V.E.; Fournier-Thibault, C.; Duband, J.L. Sonic hedgehog restricts adhesion and migration of neural crest cells independently of the patched-smoothened-gli signaling pathway. Proc. Natl. Acad. Sci. USA 2001, 98, 12521–12526. [Google Scholar] [CrossRef] [PubMed]
- Tobin, J.L.; di Franco, M.; Eichers, E.; May-Simera, H.; Garcia, M.; Yan, J.; Quinlan, R.; Justice, M.J.; Hennekam, R.C.; Briscoe, J.; et al. Inhibition of neural crest migration underlies craniofacial dysmorphology and hirschsprung’s disease in bardet-biedl syndrome. Proc. Natl. Acad. Sci. USA 2008, 105, 6714–6719. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, E.J.; Fernandez-Zapico, M.E.; Battiato, N.L.; Rovasio, R.A. Sonic hedgehog is a chemotactic neural crest cell guide that is perturbed by ethanol exposure. Eur. J. Cell Biol. 2016, 95, 136–152. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Helms, J.A. The role of sonic hedgehog in normal and abnormal craniofacial morphogenesis. Development 1999, 126, 4873–4884. [Google Scholar] [PubMed]
- Haworth, K.E.; Wilson, J.M.; Grevellec, A.; Cobourne, M.T.; Healy, C.; Helms, J.A.; Sharpe, P.T.; Tucker, A.S. Sonic hedgehog in the pharyngeal endoderm controls arch pattern via regulation of fgf8 in head ectoderm. Dev. Biol. 2007, 303, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Marcucio, R.S.; Cordero, D.R.; Hu, D.; Helms, J.A. Molecular interactions coordinating the development of the forebrain and face. Dev. Biol. 2005, 284, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Couly, G.; Creuzet, S.; Bennaceur, S.; Vincent, C.; le Douarin, N.M. Interactions between hox-negative cephalic neural crest cells and the foregut endoderm in patterning the facial skeleton in the vertebrate head. Development 2002, 129, 1061–1073. [Google Scholar] [PubMed]
- Kikuchi, Y.; Agathon, A.; Alexander, J.; Thisse, C.; Waldron, S.; Yelon, D.; Thisse, B.; Stainier, D.Y. Casanova encodes a novel sox-related protein necessary and sufficient for early endoderm formation in zebrafish. Genes Dev. 2001, 15, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.; Trinh, L.A.; Reiter, J.F.; Alexander, J.; Yelon, D.; Stainier, D.Y. The zebrafish bonnie and clyde gene encodes a mix family homeodomain protein that regulates the generation of endodermal precursors. Genes Dev. 2000, 14, 1279–1289. [Google Scholar] [PubMed]
- Wall, N.A.; Hogan, B.L. Expression of bone morphogenetic protein-4 (bmp-4), bone morphogenetic protein-7 (bmp-7), fibroblast growth factor-8 (fgf-8) and sonic hedgehog (shh) during branchial arch development in the chick. Mech. Dev. 1995, 53, 383–392. [Google Scholar] [CrossRef]
- Abu-Issa, R.; Smyth, G.; Smoak, I.; Yamamura, K.; Meyers, E.N. Fgf8 is required for pharyngeal arch and cardiovascular development in the mouse. Development 2002, 129, 4613–4625. [Google Scholar] [PubMed]
- Walshe, J.; Mason, I. Fgf signalling is required for formation of cartilage in the head. Dev. Biol. 2003, 264, 522–536. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.W.; Hernandez-Lagunas, L.; Feng, W.; Melvin, V.S.; Williams, T.; Artinger, K.B. Vgll2a is required for neural crest cell survival during zebrafish craniofacial development. Dev. Biol. 2011, 357, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Balczerski, B.; Matsutani, M.; Castillo, P.; Osborne, N.; Stainier, D.Y.; Crump, J.G. Analysis of sphingosine-1-phosphate signaling mutants reveals endodermal requirements for the growth but not dorsoventral patterning of jaw skeletal precursors. Dev. Biol. 2012, 362, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.S.; Werling, U.; Braunstein, E.M.; Liao, J.; Nowotschin, S.; Edelmann, W.; Hebert, J.M.; Morrow, B.E. Inactivation of tbx1 in the pharyngeal endoderm results in 22q11ds malformations. Development 2006, 133, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski, T.; Nusslein-Volhard, C. The endoderm plays an important role in patterning the segmented pharyngeal region in zebrafish (danio rerio). Dev. Biol. 2000, 225, 339–356. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, S.; Simkin, J.; Darido, C.; Partridge, D.D.; Georgy, S.R.; Caddy, J.; Wilanowski, T.; Lieschke, G.J.; Doggett, K.; Heath, J.K.; et al. Grainyhead-like 3 regulation of endothelin-1 in the pharyngeal endoderm is critical for growth and development of the craniofacial skeleton. Mech. Dev. 2014, 133, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z.; Zhao, X.; Yu, X.; Hu, Y.; Geronimo, B.; Fromm, S.H.; Chen, Y.P. A new function of bmp4: Dual role for bmp4 in regulation of sonic hedgehog expression in the mouse tooth germ. Development 2000, 127, 1431–1443. [Google Scholar] [PubMed]
- Zhang, Z.; Song, Y.; Zhao, X.; Zhang, X.; Fermin, C.; Chen, Y. Rescue of cleft palate in msx1-deficient mice by transgenic bmp4 reveals a network of bmp and shh signaling in the regulation of mammalian palatogenesis. Development 2002, 129, 4135–4146. [Google Scholar] [PubMed]
- Hu, D.; Young, N.M.; Li, X.; Xu, Y.; Hallgrimsson, B.; Marcucio, R.S. A dynamic shh expression pattern, regulated by shh and bmp signaling, coordinates fusion of primordia in the amniote face. Development 2015, 142, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Jiang, R. Sonic hedgehog signaling regulates reciprocal epithelial-mesenchymal interactions controlling palatal outgrowth. Development 2009, 136, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Garg, V.; Yamagishi, C.; Hu, T.; Kathiriya, I.S.; Yamagishi, H.; Srivastava, D. Tbx1, a digeorge syndrome candidate gene, is regulated by sonic hedgehog during pharyngeal arch development. Dev. Biol. 2001, 235, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, V.S.; Carpenter, C.; Freyer, L.; Liao, J.; Petti, M.; Morrow, B.E. Mesodermal tbx1 is required for patterning the proximal mandible in mice. Dev. Biol. 2010, 344, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Kang, J.S.; Cole, F.; Yi, M.J.; Krauss, R.S. Cdo functions at multiple points in the sonic hedgehog pathway, and cdo-deficient mice accurately model human holoprosencephaly. Dev. Cell 2006, 10, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Delloye-Bourgeois, C.; Rama, N.; Brito, J.; le Douarin, N.; Mehlen, P. Sonic hedgehog promotes the survival of neural crest cells by limiting apoptosis induced by the dependence receptor cdon during branchial arch development. Biochem. Biophys. Res. Commum. 2014, 452, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Helms, J.A.; Kim, C.H.; Hu, D.; Minkoff, R.; Thaller, C.; Eichele, G. Sonic hedgehog participates in craniofacial morphogenesis and is down-regulated by teratogenic doses of retinoic acid. Dev. Biol. 1997, 187, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Long, A.B.; Kaiser, W.J.; Mocarski, E.S.; Caspary, T. Apaf1 apoptotic function critically limits sonic hedgehog signaling during craniofacial development. Cell Death Differ. 2013, 20, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Rice, R.; Connor, E.; Rice, D.P. Expression patterns of hedgehog signalling pathway members during mouse palate development. Gene. Expr. Patterns 2006, 6, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Cobourne, M.T.; Green, J.B. Hedgehog signalling in development of the secondary palate. Front. Oral Biol. 2012, 16, 52–59. [Google Scholar] [PubMed]
- Hu, D.; Marcucio, R.S.; Helms, J.A. A zone of frontonasal ectoderm regulates patterning and growth in the face. Development 2003, 130, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Melnick, M.; Witcher, D.; Bringas, P., Jr.; Carlsson, P.; Jaskoll, T. Meckel’s cartilage differentiation is dependent on hedgehog signaling. Cells Tissues Organ 2005, 179, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Billmyre, K.K.; Klingensmith, J. Sonic hedgehog from pharyngeal arch 1 epithelium is necessary for early mandibular arch cell survival and later cartilage condensation differentiation. Dev. Dyn. 2015, 244, 564–576. [Google Scholar] [CrossRef] [PubMed]
- Haworth, K.E.; Healy, C.; Morgan, P.; Sharpe, P.T. Regionalisation of early head ectoderm is regulated by endoderm and prepatterns the orofacial epithelium. Development 2004, 131, 4797–4806. [Google Scholar] [CrossRef] [PubMed]
- Trumpp, A.; Depew, M.J.; Rubenstein, J.L.; Bishop, J.M.; Martin, G.R. Cre-mediated gene inactivation demonstrates that fgf8 is required for cell survival and patterning of the first branchial arch. Genes Dev. 1999, 13, 3136–3148. [Google Scholar] [CrossRef] [PubMed]
- Parada, C.; Chai, Y. Mandible and tongue development. Curr. Top. Dev. Biol. 2015, 115, 31–58. [Google Scholar] [PubMed]
- Amano, O.; Doi, T.; Yamada, T.; Sasaki, A.; Sakiyama, K.; Kanegae, H.; Kindaichi, K. Meckel’s cartilage: Discovery, embryology and evolution—Overview of the specificity of meckel’s cartilage. J. Oral Biosci. 2010, 52, 125–135. [Google Scholar]
- Lee, S.K.; Kim, Y.S.; Oh, H.S.; Yang, K.H.; Kim, E.C.; Chi, J.G. Prenatal development of the human mandible. Anat. Rec. 2001, 263, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Muhlhauser, J. Resorption of the unmineralized proximal part of meckel’s cartilage in the rat. A light and electron microscopic study. J. Submicrosc. Cytol. 1986, 18, 717–724. [Google Scholar] [PubMed]
- Garg, A.; Townsend, G. Anatomical variation of the sphenomandibular ligament. Aust. Endod. J. 2001, 27, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, Y.; Chen, D.; Chen, Y. Enhanced bmp signaling prevents degeneration and leads to endochondral ossification of meckel’s cartilage in mice. Dev. Biol. 2013, 381, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Mao, J.; Tenzen, T.; Kottmann, A.H.; McMahon, A.P. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev. 2004, 18, 937–951. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, P.; Sarmah, S.; Zhou, F.C.; Marrs, J.A. Fetal alcohol spectrum disorder (fasd) associated neural defects: Complex mechanisms and potential therapeutic targets. Brain Sci. 2013, 3, 964–991. [Google Scholar] [CrossRef] [PubMed]
- Ahlgren, S.C.; Thakur, V.; Bronner-Fraser, M. Sonic hedgehog rescues cranial neural crest from cell death induced by ethanol exposure. Proc. Natl. Acad. Sci. USA 2002, 99, 10476–10481. [Google Scholar] [CrossRef] [PubMed]
- Dixon, M.J.; Marazita, M.L.; Beaty, T.H.; Murray, J.C. Cleft lip and palate: Understanding genetic and environmental influences. Nat. Rev. Genet. 2011, 12, 167–178. [Google Scholar] [CrossRef] [PubMed]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dworkin, S.; Boglev, Y.; Owens, H.; Goldie, S.J. The Role of Sonic Hedgehog in Craniofacial Patterning, Morphogenesis and Cranial Neural Crest Survival. J. Dev. Biol. 2016, 4, 24. https://doi.org/10.3390/jdb4030024
Dworkin S, Boglev Y, Owens H, Goldie SJ. The Role of Sonic Hedgehog in Craniofacial Patterning, Morphogenesis and Cranial Neural Crest Survival. Journal of Developmental Biology. 2016; 4(3):24. https://doi.org/10.3390/jdb4030024
Chicago/Turabian StyleDworkin, Sebastian, Yeliz Boglev, Harley Owens, and Stephen J. Goldie. 2016. "The Role of Sonic Hedgehog in Craniofacial Patterning, Morphogenesis and Cranial Neural Crest Survival" Journal of Developmental Biology 4, no. 3: 24. https://doi.org/10.3390/jdb4030024
APA StyleDworkin, S., Boglev, Y., Owens, H., & Goldie, S. J. (2016). The Role of Sonic Hedgehog in Craniofacial Patterning, Morphogenesis and Cranial Neural Crest Survival. Journal of Developmental Biology, 4(3), 24. https://doi.org/10.3390/jdb4030024