Essential Oils and Extracts of Juniperus macrocarpa Sm. and Juniperus oxycedrus L.: Comparative Phytochemical Composition and Anti-Proliferative and Antioxidant Activities
Abstract
:1. Introduction
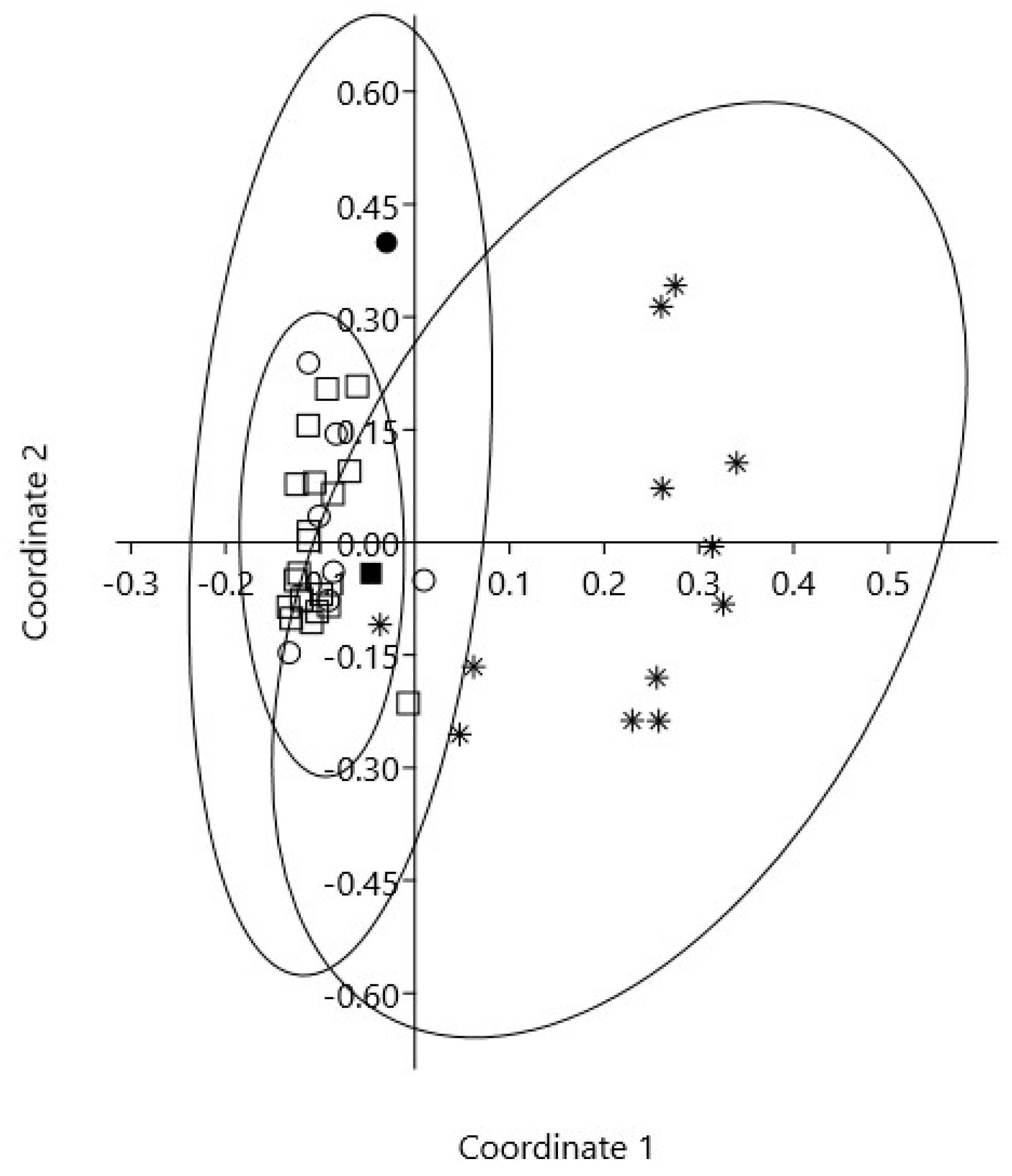
2. Results
2.1. Chemical Composition of Essential Oils
2.2. The Chemical Profiles of Juniperus Polar Extracts
2.3. Antioxidant and Anti-Proliferative Properties
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Materials and Extraction
4.3. Phytochemical Screening
4.4. In Vitro Antioxidant Activity
4.5. In Vitro Anti-Proliferative Activity
4.5.1. Cell Lines and Culture Conditions
4.5.2. Cell Viability Assay
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Adams, R.P.; Tashev, A.N.; Schwarzbach, A.E. Variation in Juniperus communis trees and shrubs from Bulgaria: Analyses of nrDNA and cpDNA regions plus leaf essential oil. Phytologia 2014, 96, 124–129. [Google Scholar]
- Mao, K.; Hao, G.; Liu, J.; Adams, R.P.; Milne, R.I. Diversification and biogeography of Juniperus (Cupressaceae): Variable diversification rates and multiple intercontinental dispersals. New Phytologist 2010, 188, 254–272. [Google Scholar] [CrossRef] [PubMed]
- Roma-Marzio, F.; Najar, B.; Alessandri, J.; Pistelli, L.; Peruzzi, L. Taxonomy of prickly juniper (Juniperus oxycedrus group): A phytochemical-morphometric combined approach at the contact zone of two cryptospecies. Phytochemistry 2017, 141, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Farhat, P.; Hidalgo, O.; Robert, T.; Siljak-Yakovlev, S.; Leitch, I.J.; Adams, R.P.; Bou Dagher-Kharrat, M. Polyploidy in the conifer genus Juniperus: An unexpectedly high rate. Front. Plant Sci. 2019, 10, 676–690. [Google Scholar] [CrossRef] [Green Version]
- Loizzo, M.R.; Tundis, R.; Conforti, F.; Saab, A.M.; Statti, G.A.; Menichini, F. Comparative chemical composition, antioxidant and hypoglycaemic activities of Juniperus oxycedrus ssp. oxycedrus L. berry and wood oils from Lebanon. Food Chem. 2007, 105, 572–578. [Google Scholar]
- Akkol, E.K.; Güvenç, A.; Yesilada, E. A comparative study on the antinociceptive and anti-inflammatory activities of five Juniperus taxa. J. Ethnopharmacol. 2009, 125, 330–336. [Google Scholar] [CrossRef]
- Sezik, E.; Kocakulaka, E.; Baserb, K.H.C.; Ozekb, T. Composition of the essential oils of Juniperus oxycedrus subsp. Macrocarpa from Turkey. Chem. Nat. Comp. 2005, 41, 352–354. [Google Scholar] [CrossRef]
- Oztürk, M.; Tümen, I.; Uğur, A.; Aydoğmuş-Öztürk, F.; Topçu, G. Evaluation of fruit extracts of six Turkish Juniperus species for their antioxidant, anticholinesterase and antimicrobial activities. J. Sci. Food Agric. 2010, 91, 867–876. [Google Scholar] [CrossRef]
- Orhan, N.; Aslan, M.; Peckan, M.; Orhan, D.D.; Bedir, E.; Ergun, F. Identification of hypoglycaemic compounds from berries of Juniperus oxycedrus subsp. Oxycedrus through bioactivity guided isolation technique. J. Ethnopharmacol. 2012, 139, 110–118. [Google Scholar]
- Achak, N.; Romane, A.; Alifriqui, M.; Adams, P.R. Chemical studies of leaf essential oils of three species of Juniperus from Tensift Al Haouz-Marrakech Region (Morocco). J. Essent. Oil Res. 2009, 21, 337–341. [Google Scholar] [CrossRef]
- Adams, R.P. The leaf essential oils and chemotaxonomy of Juniperus sect. Juniperus. Biochem. Syst. Ecol. 1998, 26, 637–645. [Google Scholar] [CrossRef]
- Alan, S.; Kürkçüoğlu, M.; Şener, G. Composition of the essential oils of Juniperus oxycedrus L. subsp. oxycedrus growing in Turkey. Turk. J. Pharm. Sci. 2016, 13, 300–303. [Google Scholar] [CrossRef]
- Derwich, E.; Chabir, R. Identification of the volatile constituents of the essential oil of Juniperus oxycedrus L. (Cupressaceae) from the North Centre Region of Morocco. Asian J. Pharm. Clin. Res. 2011, 4, 50–54. [Google Scholar]
- Djebaili, H.; Zellagui, A.; Gherraf, N. Germacrene-D, a characteristic component of the essential oils from the leaves of Juniperus oxycedrus ssp. macrocarpa (S. et Sm.) BalI Growing in El Kala, Algeria. J. Nat. Prod. Plant Resour. 2013, 3, 40–44. [Google Scholar]
- Dob, T.; Dahmane, D.; Chelghoum, C. Essential oil composition of Juniperus oxycedrus growing in Algeria. Pharm. Biol. 2006, 44, 1–6. [Google Scholar] [CrossRef]
- Hayta, S.; Bagci, E. Essential oil constituents of the leaves, bark and cones of Juniperus oxycedrus subsp oxycedrus L. from Turkey. Acta Bot. Gall. Bot. Lett. 2014, 161, 201–207. [Google Scholar] [CrossRef]
- Lesjak, M.M.; Beara, I.N.; Orčić, D.Z.; Petar, K.N.; Simin, N.D.; Emilija, S.D.; Mimica-Dukić, N.M. Phytochemical composition and antioxidant, anti-inflammatory and antimicrobial activities of Juniperus macrocarpa Sibth. Et Sm. J. Funct. Foods 2014, 7, 257–268. [Google Scholar] [CrossRef]
- Medini, H.; Manongiu, B.; Aicha, N.; Chekir-Ghedira, L.; Harzalla-Skhiri, F.; Khouja, M.L. Chemical and antibacterial polymorphism of Juniperus oxycedrus ssp. oxycedrus and Juniperus oxycedrus ssp. macrocarpa (Cupressaceae) leaf essential oils from Tunisia. J. Chem. 2013, 2013, 389252. [Google Scholar] [CrossRef] [Green Version]
- Stassi, V.; Verykokidou, E.; Loukis, A.; Harvala, A.; Philianos, S. Essential oil of Juniperus oxycedrus L. subsp. macrocarpa (Sm.) Ball. J. Essent. Oil Res. 1995, 7, 675–676. [Google Scholar] [CrossRef]
- Valentini, G.; Bellomaria, B.; Maggi, F.; Manzi, A. The leaf and female cone oils of Juniperus oxycedrus L. subsp. Oxycedrus and J. oxycedrus subsp. Macrocarpa (Sibth. Et Sm.) Ball. from Abruzzo. J. Essent. Oil Res. 2003, 15, 418–421. [Google Scholar] [CrossRef]
- Vourlioti-Arapi, F.; Michaelakis, A.; Evergetis, E.; Koliopoulos, G.; Haroutounian, S.A. Essential oils of indigenous in Greece six Juniperus taxa. Parasitol. Res. 2012, 110, 1829–1839. [Google Scholar] [CrossRef]
- Karaman, I.; Sahin, F.; Güllüce, M.; Ogütçü, H.; Sengül, M.; Adigüzel, A. Antimicrobial activity of aqueous and methanol extracts of Juniperus oxycedrus L. J. Ethnopharmacol. 2003, 85, 231–235. [Google Scholar] [CrossRef]
- Spengler, G.; Gajdács, M.; Donadu, M.G.; Usai, M.; Marchetti, M.; Ferrari, M.; Mazzarello, V.; Zanetti, S.; Nagy, F.; Kovács, R. Evaluation of the antimicrobial and antivirulent potential of essential oils isolated from Juniperus oxycedrus L. ssp. macrocarpa aerial parts. Microorganisms 2022, 10, 758. [Google Scholar] [CrossRef]
- Moreno, L.; Bello, R.; Beltran, B.; Calatayud, S.; Promo-Yufera, E.; Esplugues, J. Pharmacological screening of different Juniperus oxycedrus L. extracts. Pharmacol. Toxicol. 1998, 82, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Amri, I.; Hamrouni, L.; Gargouri, S.; Banana, M.; Jamoussi, B. Chemical composition and antifungal activity of essential oils isolated from Juniperus oxycedrus L. Int. J. Appl. Biol. Pharm. Technol. 2013, 4, 227–233. [Google Scholar]
- George, S.; Abrahamse, H. Redox potential of antioxidants in cancer progression and prevention. Antioxidants 2020, 9, 1156. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Abouali, M.; Salehi, P.; Sonboli, A.; Kanani, M.; Menichini, F.; Tundis, R. In vitro antioxidant and anti-proliferative activities of nine Salvia species. Nat. Prod. Res. 2014, 28, 2278–2285. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Bonesi, M.; Menichini, F.; Dodaro, D.; Passalacqua, N.G.; Statti, G.A.; Menichini, F. In vitro cytotoxic effects of Senecio stabianus Lacaita (Asteraceae) on human cancer cell lines. Nat. Prod. Res. 2009, 23, 1707–1718. [Google Scholar] [CrossRef]
- Nagendra-Prasad, K.; Hao, J.; Shi, J.; Liu, T.; Li, J.; Wei, X.; Qiu, X.; Xue, S.; Jiang, Y. Antioxidant and anticancer activities of high pressure-assisted extract of longan (Dimocarpus longan Lour.) fruit pericarp. Innov. Food Sci. Emer. Tech. 2009, 10, 413–419. [Google Scholar] [CrossRef]
- Awney, H.A.; Sindi, H. The effect of rosemary on the mutagenic activity of heterocyclic amines extracted from common food consumed in Saudi Arabia. Int. J. Food Sci. Nutr. 2010, 61, 192–203. [Google Scholar] [CrossRef]
- Adams, R.P.; Morris, A.J.; Pandey, R.N.; Schwarzbachb, A.E. Cryptic speciation between Juniperus deltoides and Juniperus oxycedrus (Cupressaceae) in the Mediterranean. Biochem. Syst. Ecol. 2005, 33, 771–787. [Google Scholar] [CrossRef]
- Stassi, V.; Verykokidou, E.; Loukis, A.; Harvala, C. Polyphenolic compounds from the leaves of Juniperus oxycedrus L. subsp. Macrocarpa (Sm.) ball. Pharm. Acta Helv. 1998, 72, 311–312. [Google Scholar] [CrossRef]
- Taviano, M.F.; Marino, A.; Trovato, A.; Bellinghieri, V.; Melchini, A.; Dugo, P.; Cacciola, F.; Donato, P.; Mondello, L.; Güvenç, A.; et al. Juniperus oxycedrus L. subsp. Oxycedrus and Juniperus oxycedrus L. subsp. Macrocarpa (Sibth. & Sm.) Ball. “berries” from Turkey: Comparative evaluation of phenolic profile, antioxidant, cytotoxic and antimicrobial activities. Food Chem. Toxicol. 2013, 58, 22–29. [Google Scholar] [PubMed]
- Yaglioglu, A.S.; Eser, F. Screening of some Juniperus extracts for the phenolic compounds and their antiproliferative activities. S. Afr. J. Bot. 2017, 113, 29–33. [Google Scholar] [CrossRef]
- Živić, N.; Milošević, S.; Dekić, V.; Dekić, B.; Ristić, N.; Ristić, M.; Sretić, L. Phytochemical and antioxidant screening of some extracts of Juniperus communis L. and Juniperus oxycedrus L. Czech J. Food Sci. 2019, 37, 351–358. [Google Scholar] [CrossRef]
- Boudiba, S.; Tamfu, A.; Berka, B.; Hanini, K.; Hioun, S.; Allaf, K.; Boudiba, L.; Ceylan, O. Anti-quorum sensing and antioxidant activity of essential oils extracted from Juniperus species, growing spontaneously in Tebessa region (East of Algeria). Nat. Prod. Commun. 2021, 16, 1–11. [Google Scholar] [CrossRef]
- Gök, H.N.; Orhan, N.; Özüpek, B.; Pekacar, S.; Selvi, Ş.N.; Orhan, D.D. Standardization of Juniperus macrocarpa Sibt. & Sm. and Juniperus excelsa M. Bieb. extracts with carbohydrate digestive enzyme inhibitory and antioxidant activities. Iran J. Pharm. Res. 2021, 20, 441–455. [Google Scholar]
- Calderón-Montaño, J.M.; Martínez-Sánchez, S.M.; Jiménez-González, V.; Burgos-Morón, E.; Guillén-Mancina, E.; Jiménez-Alonso, J.J.; Díaz-Ortega, P.; García, F.; Aparicio, A.; López-Lázaro, M. Screening for selective anticancer activity of 65 extracts of plants collected in western Andalusia, Spain. Plants 2021, 10, 2193. [Google Scholar] [CrossRef]
- Lai, W.L.; Lee, S.C.; Chang, K.F.; Huang, X.F.; Li, C.Y.; Lee, C.J.; Wu, C.Y.; Hsu, H.J.; Tsai, N.M. Juniperus communis extract induces cell cycle arrest and apoptosis of colorectal adenocarcinoma in vitro and in vivo. Braz. J. Med. Biol. Res. 2021, 54, e10891. [Google Scholar] [CrossRef]
- Lee, C.C.; Hsiao, C.Y.; Lee, S.C.; Huang, X.F.; Chang, K.F.; Lee, M.S.; Hsieh, M.C.; Tsai, N.M. Suppression of oral cancer by induction of cell cycle arrest and apoptosis using Juniperus communis extract. Biosci. Rep. 2020, 40, BSR20202083. [Google Scholar] [CrossRef]
- Maurya, A.K.; Devi, R.; Kumar, A.; Koundal, R.; Thakur, S.; Sharma, A.; Kumar, D.; Kumar, R.; Padwad, Y.S.; Chand, G.; et al. Chemical composition, cytotoxic and antibacterial activities of essential oils of cultivated clones of Juniperus communis and wild Juniperus species. Chem. Biodivers. 2018, 15, e1800183. [Google Scholar] [CrossRef] [PubMed]
- Yaglioglu, A.S.; Eser, F.; Yaglioglu, M.S.; Demirtas, I. The antiproliferative and antioxidant activities of the essential oils of Juniperus species from Turkey. Flavour Fragr. J. 2020, 35, 511–523. [Google Scholar] [CrossRef]
- Tundis, R.; Bonesi, M.; Loizzo, M.R. A Comparative study of phytochemical constituents and bioactivity of n-hexane and dichloromethane extracts of Juniperus macrocarpa and J. oxycedrus. Biol. Life Sci. Forum 2021, 4, 42–49. [Google Scholar]
- Tundis, R.; Loizzo, M.R.; Bonesi, M.; Menichini, F.; Mastellone, V.; Colica, C.; Menichini, F. Comparative study on the antioxidant capacity and cholinesterase inhibitory activity of Citrus aurantifolia Swingle, C. aurantium L., and C. bergamia Risso and Poit. peel essential oils. J. Food Sci. 2012, 77, H40–H46. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 2007. [Google Scholar]
- Tranchant, J. Manuel Pratique de Chromatographie en Phase Gazeuse; Masson: Paris, France, 1995. [Google Scholar]
- Romeo, R.; De Bruno, A.; Imeneo, V.; Piscopo, A.; Poiana, M. Evaluation of enrichment with antioxidants from olive oil mill wastes in hydrophilic model system. J. Food Process. Preserv. 2019, 43, e14211. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Pugliese, A.; Bonesi, M.; De Luca, D.; O’Brien, N.; Menichini, F.; Tundis, R. Influence of drying and cooking process on the phytochemical content, antioxidant and hypoglycaemic properties of two bell Capsicum annum L. cultivars. Food Chem. Toxicol. 2013, 53, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Leporini, M.; Bonesi, M.; Loizzo, M.R.; Passalacqua, N.G.; Tundis, R. The essential oil of Salvia rosmarinus Spenn. from Italy as a source of health-promoting compounds: Chemical profile and antioxidant and cholinesterase inhibitory activity. Plants 2020, 9, 798. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Statti, G.A.; Menichini, F. Jacaranone: A cytotoxic constituent from Senecio ambiguus subsp. ambiguus (Biv.) DC. against renal adenocarcinoma ACHN and prostate carcinoma LNCaP cells. Arch. Pharm. Res. 2007, 30, 701–707. [Google Scholar]
- De Groot, P.M.; Wu, C.C.; Carter, B.W.; Munden, R.F. The epidemiology of lung cancer. Transl. Lung Cancer Res. 2019, 7, 220–233. [Google Scholar] [CrossRef]


| Compound | Class | RI a | % | I.M b | Sign | |
|---|---|---|---|---|---|---|
| J. macrocarpa | J. oxycedrus | |||||
| Tricyclene | mh | 928 | 0.2 ± 0.02 | n.d. | 1,2 | ** |
| α-Pinene | mh | 938 | 25.3 ± 2.5 | 36.9 ± 2.5 | 1,2,3 | ** |
| Camphene | mh | 953 | 0.6 ± 0.04 | 0.6 ± 0.03 | 1,2,3 | ns |
| β-Pinene | mh | 980 | 2.6 ± 0.9 | 5.5 ± 0.9 | 1,2,3 | ** |
| Myrcene | mh | 993 | 3.1 ± 0.7 | 2.7 ± 0.1 | 1,2,3 | ** |
| Sabinene | mh | 973 | 8.2 ± 1.1 | 0.7 ± 0.03 | 1,2,3 | ** |
| α-Phellandrene | mh | 1005 | 3.3 ± 0.8 | 1.1 ± 0.04 | 1,2 | ** |
| δ-3-Carene | mh | 1009 | 2.4 ± 0.02 | 0.6 ± 0.03 | 1,2 | ** |
| α-Terpinene | mh | 1012 | 0.7 ± 0.05 | 1.9 ± 0.5 | 1,2,3 | ** |
| p-Cymene | mh | 1025 | 3.2 ± 1.1 | 0.5 ± 0.04 | 1,2 | ** |
| Limonene | mh | 1030 | 3.4 ± 0.03 | 6.3 ± 0.9 | 1,2,3 | ** |
| (E)-β-Ocimene | mh | 1048 | 2.8 ± 0.7 | 0.2 ± 0.01 | 1,2 | ** |
| γ-Terpinene | mh | 1057 | 0.5 ± 0.01 | 0.2 ± 0.01 | 1,2,3 | ns |
| Terpinolene | mh | 1086 | 1.5 ± 0.7 | 0.3 ± 0.02 | 1,2,3 | ** |
| Nonanal | oc | 1102 | 0.1 ± 0.01 | 0.3 ± 0.01 | 1,2 | * |
| α-Campholene aldehyde | om | 1132 | 1.2 ± 0.6 | 0.5 ± 0.02 | 1,2 | ** |
| Camphor | om | 1145 | 0.3 ± 0.03 | tr | 1,2 | ** |
| p-Mentha-1,5-dien-8-ol | om | 1167 | 0.4 ± 0.04 | n.d. | 1,2 | ** |
| Terpinen-4-ol | om | 1176 | 0.3 ± 0.02 | tr | 1,2 | ** |
| α-Terpineol | om | 1189 | 2.9 ± 0.9 | 0.3 ± 0.02 | 1,2,3 | ** |
| Myrtenal | om | 1196 | n.d. | 0.2 ± 0.01 | 1,2 | ** |
| Decanal | oc | 1205 | 0.2 ± 0.02 | 0.3 ± 0.03 | 1,2 | ns |
| Verbenone | om | 1206 | 0.5 ± 0.05 | n.d. | 1,2 | ** |
| (−)-Carvone | om | 1242 | 0.2 ± 0.03 | n.d. | 1,2 | * |
| Piperitone | om | 1254 | 0.2 ± 0.02 | n.d. | 1,2 | * |
| Phellandral | om | 1281 | 0.3 ± 0.02 | 0.2 ± 0.01 | 1,2 | ns |
| (−)-Bornyl acetate | om | 1286 | 0.2 ± 0.01 | n.d. | 1,2 | * |
| α-Cubebene | sh | 1352 | 0.2 ± 0.01 | 0.5 ± 0.01 | 1,2 | ns |
| α-Copaene | sh | 1377 | 0.2 ± 0.02 | 0.3 ± 0.02 | 1,2 | ns |
| β-Bourbonene | sh | 1385 | 0.3 ± 0.02 | 0.9 ± 0.02 | 1,2 | ** |
| β-Cubebene | sh | 1387 | 1.8 ± 0.6 | n.d. | 1,2 | ** |
| α-Gurjunene | sh | 1407 | n.d. | 1.1 ± 0.04 | 1,2 | ** |
| trans-Caryophyllene | sh | 1415 | 0.2 ± 0.01 | 2.0 ± 0.2 | 1,2,3 | ** |
| trans-α-Bergamotene | sh | 1438 | n.d. | 0.4 ± 0.01 | 1,2 | ** |
| α-Humulene | sh | 1455 | 0.2 ± 0.01 | 1.5 ± 0.08 | 1,2 | ** |
| Germacrene D | sh | 1477 | 4.5 ± 0.03 | 2.0 ± 0.07 | 1,2 | ** |
| γ-Cadinene | sh | 1515 | 0.2 ± 0.01 | 5.4 ± 0.6 | 1,2 | ** |
| δ-Cadinene | sh | 1526 | 0.4 ± 0.01 | 2.7 ± 0.6 | 1,2 | ** |
| (E)-β-Farnesene | sh | 1452 | 0.4 ± 0.02 | 0.7 ± 0.01 | 1,2 | ** |
| α-Muurolene | sh | 1500 | 0.3 ± 0.01 | 0.4 ± 0.01 | 1,2 | ns |
| Caryophyllene oxide | os | 1580 | 0.9 ± 0.04 | 3.8 ± 0.5 | 1,2 | ** |
| (Z,E)-Farnesol | os | 1722 | 2.8 ± 0.2 | 6.5 ± 0.8 | 1,2,3 | ** |
| Manoyl oxide | di | 1989 | 6.6 ± 0.6 | 2.4 ± 0.4 | 1,2 | ** |
| 13-epi-Manoyl oxide | di | 1994 | 0.6 ± 0.2 | 0.6 ± 0.02 | 1,2 | ns |
| (Z)-Phytol | di | 1950 | n.d. | 0.5 ± 0.01 | 1,2 | ** |
| Abietatriene | di | 2054 | 2.9 ± 0.7 | 2.6 ± 0.3 | 1,2 | * |
| Abietadiene | di | 2080 | 1.8 ± 0.6 | 1.7 ± 0.3 | 1,2 | ns |
| Heneicosane | oc | 2100 | n.d. | 0.2 ± 0.02 | 1,2,3 | * |
| Tricosane | oc | 2300 | 0.3 ± 0.02 | 0.2 ± 0.01 | 1,2,3 | ns |
| Pentacosane | oc | 2500 | 0.2 ± 0.01 | 0.5 ± 0.01 | 1,2,3 | * |
| Heptacosane | oc | 2700 | 0.4 ± 0.01 | 0.5 ± 0.02 | 1,2,3 | ns |
| Nonacosane | oc | 2900 | 0.4 ± 0.03 | 0.3 ± 0.01 | 1,2,3 | ns |
| Monoterpene hydrocarbons | mh | 57.8 | 57.5 | |||
| Oxygenated monoterpens | om | 6.5 | 1.2 | |||
| Sesquiterpene hydrocarbons | sh | 8.7 | 17.9 | |||
| Oxygenated sesquiterpenes | os | 3.7 | 10.3 | |||
| Diterpenes | di | 11.9 | 7.8 | |||
| Other constituents | oc | 1.6 | 2.3 | |||
| Total | 90.2 | 97.0 | ||||
| J. macrocarpa | J. oxycedrus | ||||
|---|---|---|---|---|---|
| Compound | Ethyl Acetate Extract | Methanol Extract | Ethyl Acetate Extract | Methanol Extract | Sign |
| Apigenin | 41.6 ± 1.7 dN | 82.7 ± 3.6 cM | 243.6 ± 5.3 bC | 324.8 ± 8.2 aF | ** |
| Caffeic acid | 43.5 ± 1.2 aM | 31.4 ± 2.1 bP | 19.3 ± 4.3 Cm | 10.7 ± 0.2 dN | ** |
| (+)-Catechin | 645.4 ± 5.6 bD | 915.5 ± 2.1 aC | 108.4 ± 7.7 dF | 537.0 ± 5.4 cD | ** |
| Chlorogenic acid | 313.6 ± 2.5 aE | 141.3 ± 6.8 cH | 45.8 ± 6.2 dI | 246.2 ± 9.2 bG | ** |
| (−)-Epicatechin | 161.0 ± 1.0 dF | 211.4 ± 4.6 cE | 4237.6 ± 5.7 aA | 3874.5 ± 4.2 bB | ** |
| Gallic acid | 713.7 ± 6.6 aC | 684.3 ± 8.8 bD | 0 cQ | 0 cQ | ** |
| Kaempferol | 35.4 ± 0.9 bO | 10.8 ± 4.5 dR | 15.6 ± 1.3 cN | 48.6 ± 3.5 aI | ** |
| Kaempferol-3-O-glucoside | 66.2 ± 2.2 bL | 189.3 ± 7.3 aF | 2.7 ± 0.1 dP | 8.6 ± 0.8 cO | ** |
| Luteolin | 10.1 ± 0.1 dQ | 78.5 ± 3.3 cN | 155.7 ± 8.4 bE | 329.6 ± 8.7 aE | ** |
| Neochlorogenic acid | 34.0 ± 1.3 cO | 130.9 ± 4.2 aL | 28.5 ± 0.9 dL | 40.5 ± 1.5 bL | ** |
| Protocatechuic acid | 1091.0 ± 7.2 bB | 1142.0 ± 9.2 aB | 0 cQ | 0 cP | ** |
| Quercetin | 137.2 ± 5.3 cH | 133.6 ± 5.3 dI | 192.4 ± 10.1 bD | 201.5 ± 5.5 aH | ** |
| Quercetin-3-O-glucoside | 1533.4 ± 9.12 cA | 1769.5 ± 4.3 bA | 2937.3 ± 5.6 aB | 1404.5 ± 7.2 dC | ** |
| Rutin | 149.3 ± 5.5 cG | 168.4 ± 3.8 bG | 65.6 ± 4.2 dH | 4016.4 ± 3.8 aA | ** |
| Syringic acid | 24.0 ± 1.2 bP | 21.1 ± 2.0 cQ | 13.5 ± 0.8 dO | 26.71 ± 0.4 aM | ** |
| Vanillic acid | 85.4 ± 15.3 aI | 57.4 ± 2.8 dO | 71.4 ± 5.7 bG | 65.3 ± 0.8 cH | ** |
| Sign | ** | ** | ** | ** | |
| Sample | ABTS IC50 (μg/mL) | DPPH IC50 (μg/mL) | FRAP Test μM Fe(II)/g c | β-Carotene Bleaching Test IC50 (μg/mL) | |
|---|---|---|---|---|---|
| 30 min | 60 min | ||||
| J. macrocarpa | |||||
| Essential oil | 20.4% a | 34.1% b | 2.4 ± 0.2 | 54.8 ± 3.4 | 49.4 ± 2.8 |
| Ethyl acetate extract | 147.6 ± 4.8 | 40.9 ± 2.4 | 26.4 ± 1.8 | 84.9 ± 3.5 | 95.7 ± 3.9 |
| Methanol extract | 39.1 ± 1.7 | 29.3 ± 1.5 | 23.6 ± 1.5 | 65.1 ± 2.2 | 62.5 ± 2.8 |
| J. oxycedrus | |||||
| Essential oil | 5.2% a | 31.6% b | 3.8 ± 0.3 | 47.5 ± 2.6 | 5.9 ± 3.4 |
| Ethyl acetate extract | 9.3 ± 1.3 | 20.6 ± 2.3 | 99.5 ± 3.7 | 15.1 ± 1.1 | 13.2 ± 0.8 |
| Methanol extract | 6.2 ± 1.1 | 19.7 ± 2.5 | 101.9 ± 3.9 | 23.1 ± 1.2 | 17.1 ± 0.9 |
| Positive control | |||||
| Ascorbic acid | 1.7 ± 0.4 | 5.1 ± 0.8 | |||
| BHT | 63.2 ± 4.4 | ||||
| Propyl gallate | 1.1 ± 0.05 | 1.0 ± 0.06 | |||
| Sample | MCF-7 | MDA-MB-231 | A549 | COR-L23 |
|---|---|---|---|---|
| J. macrocarpa | ||||
| Essential oil | 85.4 ± 3.2 ** | 96.4 ± 3.8 ** | >200 | 101.0 ± 3.9 ** |
| Ethyl acetate extract | 163.4 ± 4.9 ** | 186.2 ± 5.1 ** | >200 | >200 |
| Methanol extract | >200 | >200 | >200 | >200 |
| J. oxycedrus | ||||
| Essential oil | >200 | >200 | >200 | >200 |
| Ethyl acetate extract | 147.9 ± 4.6 ** | 158.1 ± 5.1 ** | >200 | 39.1 ± 1.4 ** |
| Methanol extract | >200 | >200 | 87.9 ± 4.7 ** | 26.0 ± 1.3 ** |
| Positive control | ||||
| Taxol | 0.08 ± 0.004 | 1.6 ± 0.03 | ||
| Vinblastine sulfate | 67.3 ± 2.0 | 45.5 ± 0.7 | ||
| Compounds | Origin | Ref. |
|---|---|---|
| J. macrocarpa | ||
| α-Pinene (25.3%), p-cimene (13.2%), sabinene (8.2%) | Italy | Our data |
| Manoyl oxide (7.7–21.9%), α-pinene (7.2–11.1%), α-cedrol (2.3–9.7%) | Turkey | [7] |
| Sabinene (26.5%), α-pinene (22.6%), terpinen-4-ol (7.3%) | Spain | [9] |
| Gemacrene D (21.3%), (Z,E)-farnesol (10.9%), 8,13-epoxy-14,15-dinorlabdane (8.8%) | Algeria | [12] |
| α-Pinene (49.4%), gemacrene D (18.1%), β-phellandrene (3.8%) | Croatia | [15] |
| α-Pinene (15.9%), sabinene (12.1%), δ-3-carene (5.9%) | Tunisia | [16] |
| α-Pinene (22.8%), sabinene (9.1%), p-cimene (7.3%) | Tunisia | [16] |
| α-Pinene (26.9%), cedrolo (13.9%), dihydro-p-cimen-8-ol (8.5%) | Greece | [17] |
| α-Pinene (22.8%), α-terpineol (18.7%), 1,8-cineole (9.1%) | Italy | [18] |
| α-Pinene (81.3%), γ-muurolene (2.6%), β-pinene (2.1%) | Italy | [18] |
| α-Pinene (73.5%), α-terpineol (3.3%), β-pinene (2.1%) | Italy | [18] |
| α-Pinene (58.0%), cedrol (7.3%), α-muurolene (2.4%) | Greece | [19] |
| J. oxycedrus | ||
| α-Pinene (36.9%), limonene (6.3%), (Z,E)-farnesol (6.5%) | Italy | Our data |
| α-Pinene (17.1%), 13-epi-manoyl oxide (12.5%), (Z)-6-pentadecen-2-one (11.5%) | Morocco | [8] |
| α-Pinene (41.3%), α-phellandrene (8.2%), p-cymene (6.2%) | Spain | [9] |
| Limonene (27.7%), α-pinene (25.3%), myrcene (3.8%) | Greece | [9] |
| α-Pinene (42.7%), limonene (17.1%), δ-3-carene (13.7%) | Greece | [9] |
| Manoyl oxide (32.8%), caryophyllene oxide (11.9%), germacrene D (5.7%) | Turkey | [10] |
| α-Pinene (31.2%), sabinene (5.2%), limonene (5.0%) | Morocco | [11] |
| trans-Pinocarveol (7.0%), cis-verbenol (6.3%), manoyl oxide (6.0%) | Algeria | [13] |
| α-Pinene (42.9%), limonene (17.8%), caryophyllene oxide (5.1%) | Turkey | [14] |
| α-Pinene (49.5%), germacrene D (8.9%), 13-epi-manoil ossido (3.6%) | Tunisia | [16] |
| Limonene (30.0%), α-pinene (26.3%), (Z,E)-farnesol (5.1%) | Italy | [18] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meringolo, L.; Bonesi, M.; Sicari, V.; Rovito, S.; Passalacqua, N.G.; Loizzo, M.R.; Tundis, R. Essential Oils and Extracts of Juniperus macrocarpa Sm. and Juniperus oxycedrus L.: Comparative Phytochemical Composition and Anti-Proliferative and Antioxidant Activities. Plants 2022, 11, 1025. https://doi.org/10.3390/plants11081025
Meringolo L, Bonesi M, Sicari V, Rovito S, Passalacqua NG, Loizzo MR, Tundis R. Essential Oils and Extracts of Juniperus macrocarpa Sm. and Juniperus oxycedrus L.: Comparative Phytochemical Composition and Anti-Proliferative and Antioxidant Activities. Plants. 2022; 11(8):1025. https://doi.org/10.3390/plants11081025
Chicago/Turabian StyleMeringolo, Luciano, Marco Bonesi, Vincenzo Sicari, Simone Rovito, Nicodemo Giuseppe Passalacqua, Monica Rosa Loizzo, and Rosa Tundis. 2022. "Essential Oils and Extracts of Juniperus macrocarpa Sm. and Juniperus oxycedrus L.: Comparative Phytochemical Composition and Anti-Proliferative and Antioxidant Activities" Plants 11, no. 8: 1025. https://doi.org/10.3390/plants11081025
APA StyleMeringolo, L., Bonesi, M., Sicari, V., Rovito, S., Passalacqua, N. G., Loizzo, M. R., & Tundis, R. (2022). Essential Oils and Extracts of Juniperus macrocarpa Sm. and Juniperus oxycedrus L.: Comparative Phytochemical Composition and Anti-Proliferative and Antioxidant Activities. Plants, 11(8), 1025. https://doi.org/10.3390/plants11081025









