Combined Application of Myo-Inositol and Corn Steep Liquor from Agricultural Waste Alleviate Salt Stress in Brassica rapa
Abstract
:1. Introduction
2. Results
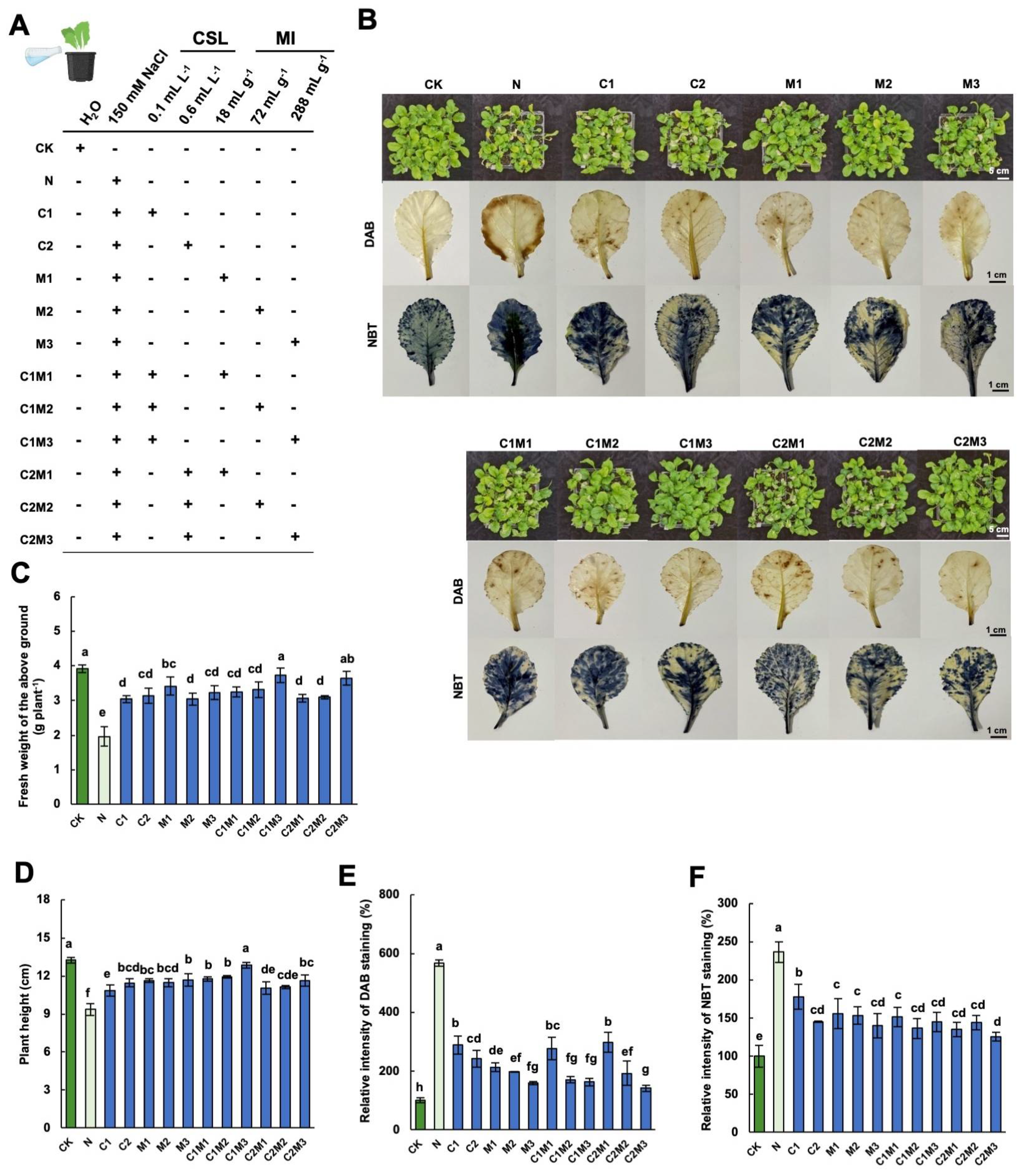
2.1. Analysis of the Effects of CSL, MI, and Their Combination on Cabbage Growth and Salt Tolerance
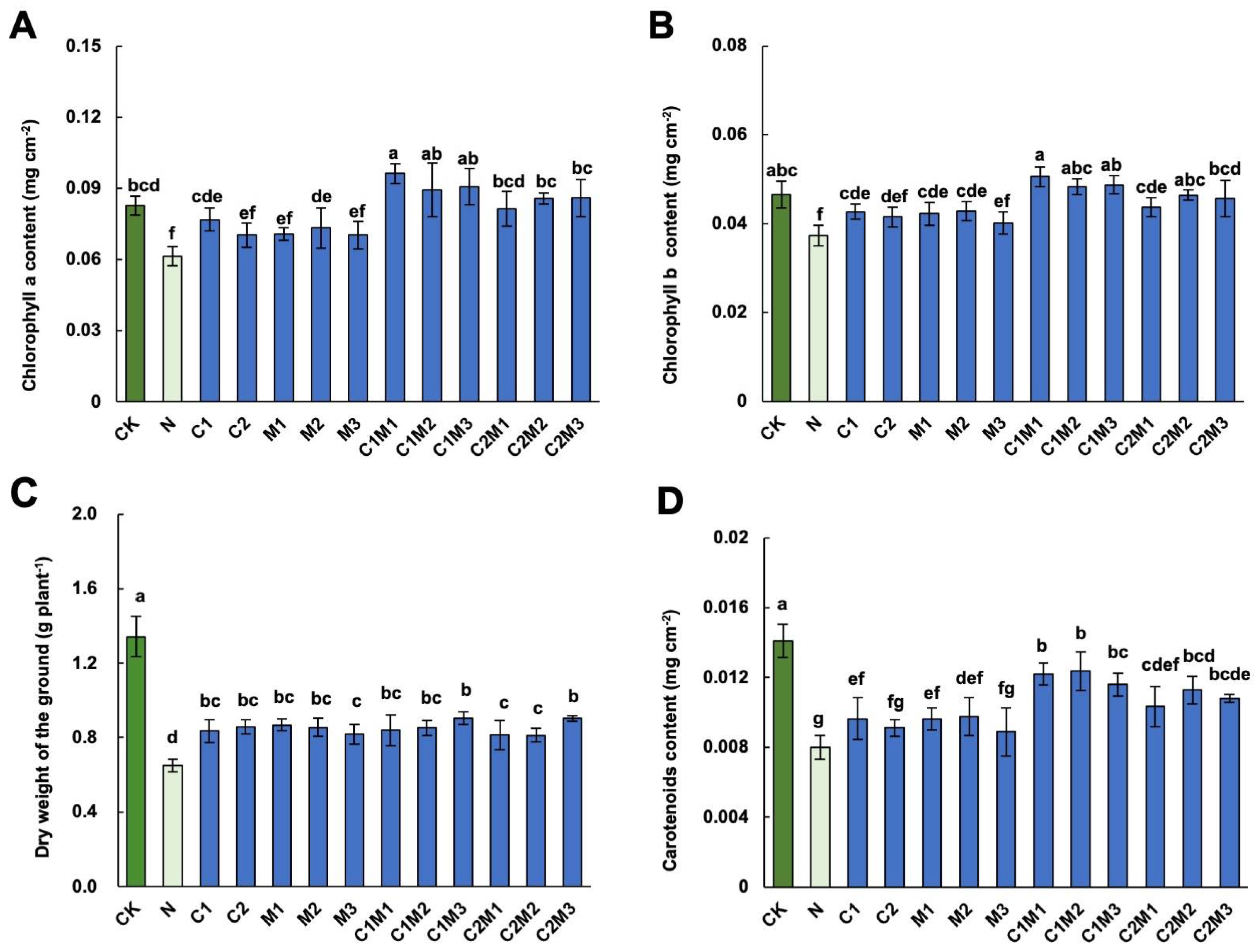
2.2. Analysis of the Effects of CSL, MI, and Their Combination on Cabbage Leaf Photosynthetic Pigments
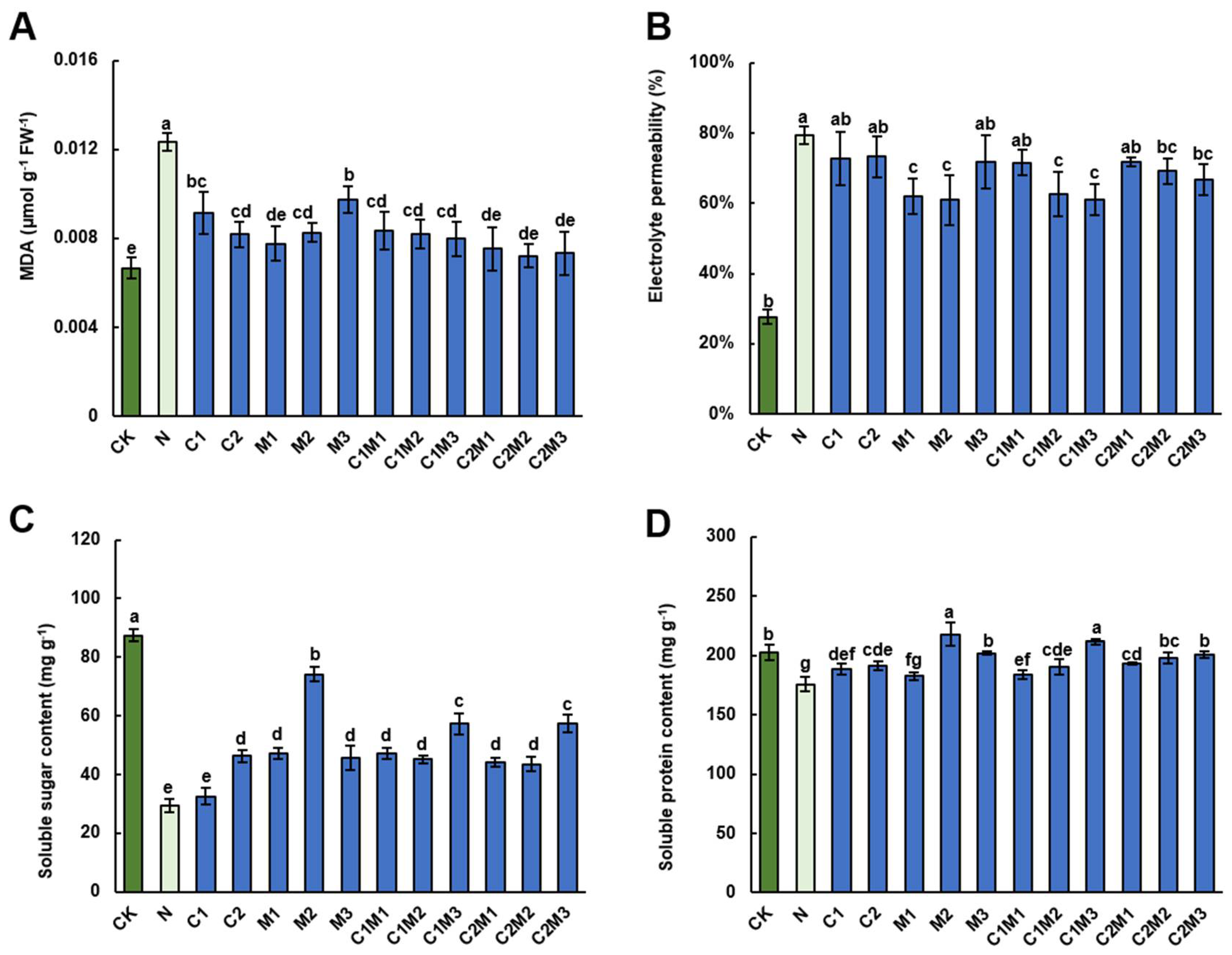
2.3. Analysis of the Effects of CSL, MI, and Their Combination on Physiological Characteristics of Cabbage Leaves under Salt Stress
2.3.1. Malondialdehyde (MDA) Content
2.3.2. Electrolyte Leakage (EL)
2.4. Analysis of Changes in the Content of Soluble Sugars and Soluble Proteins
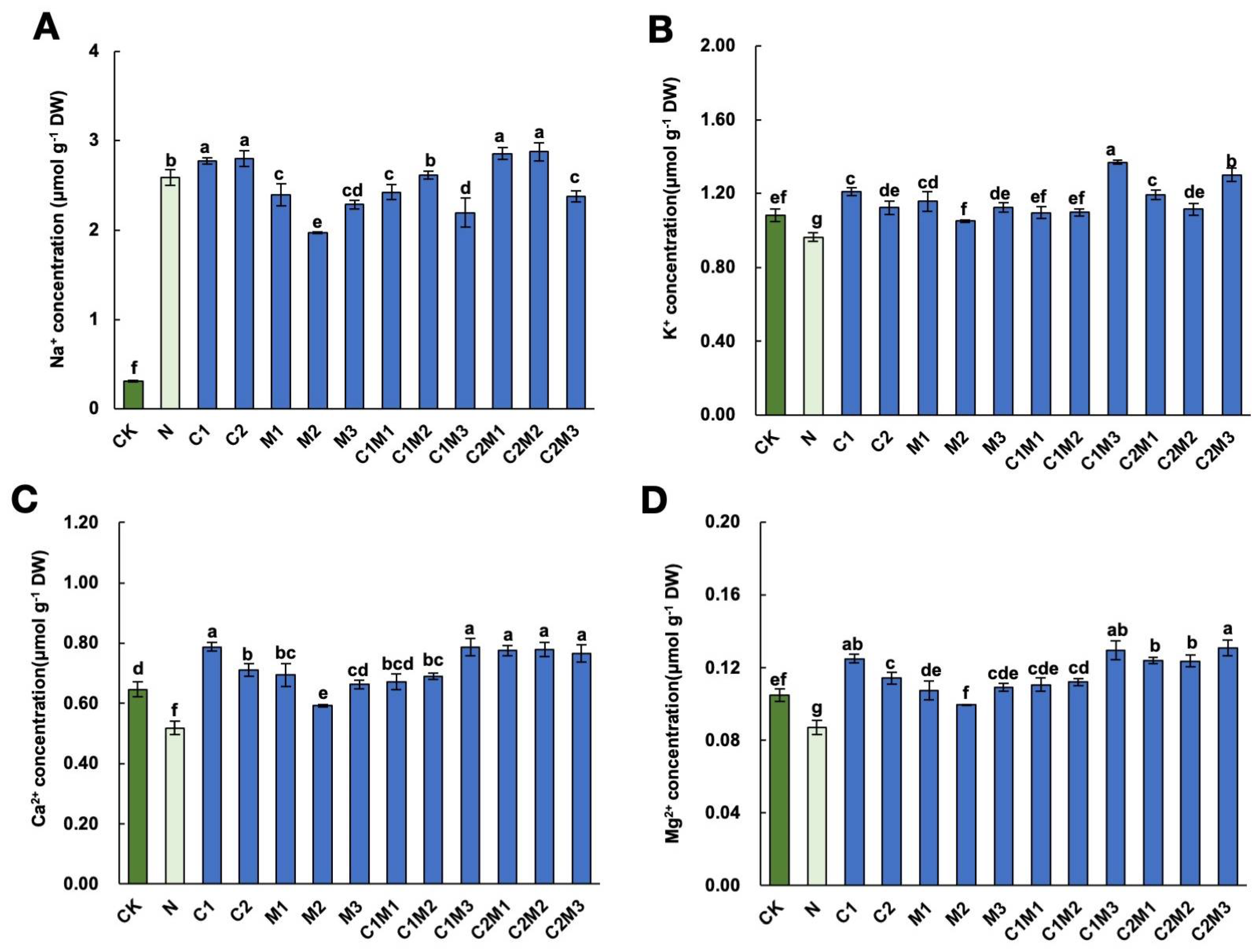
2.5. Analysis of Ionic Homeostasis Changes
2.5.1. Changes in Na+, K+, Ca2+, and Mg2+ Ion Contents
2.5.2. Analysis of Changes in Ion Ratios
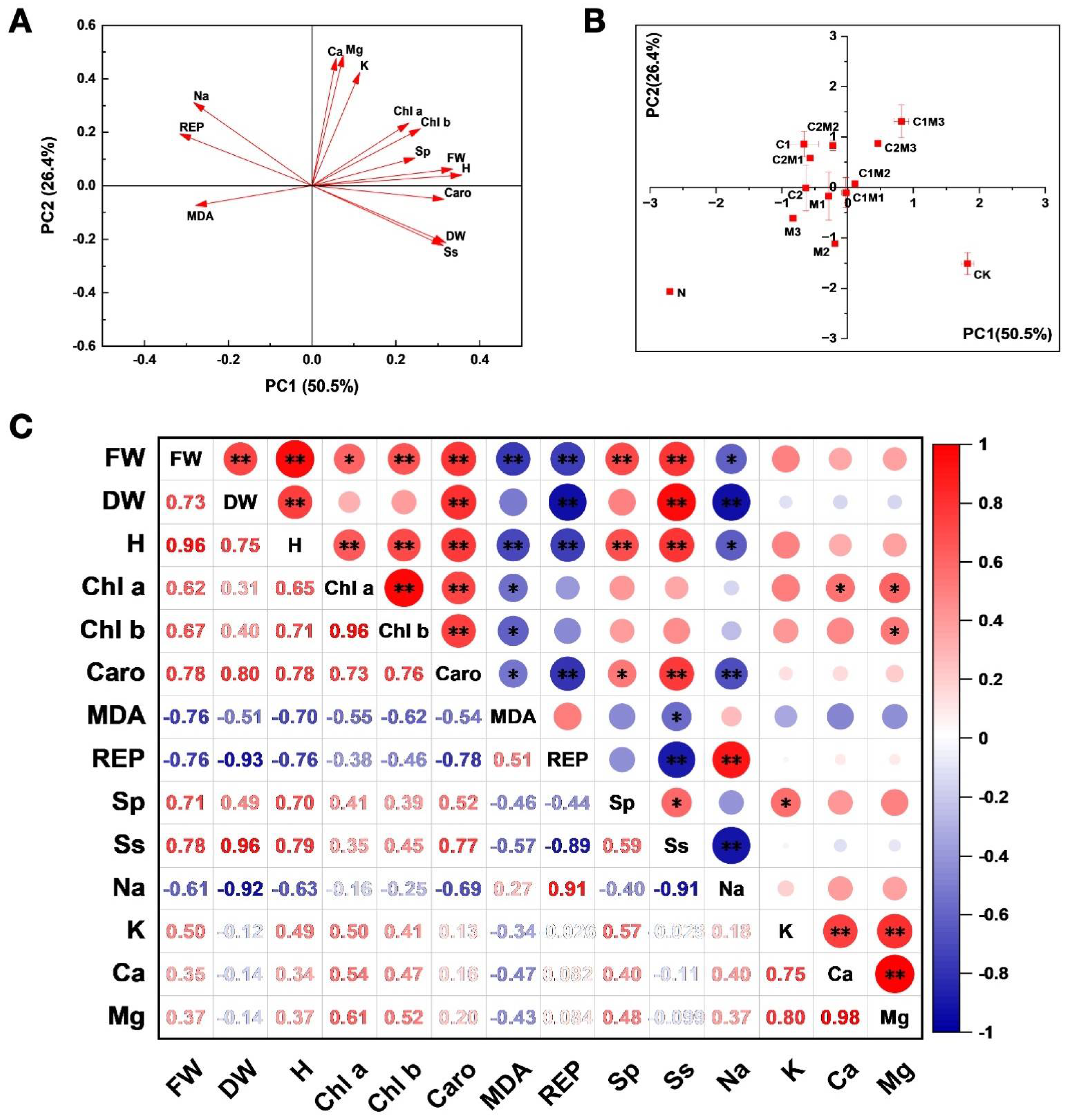
2.6. Principal Component and Correlation Analysis of the Effects on the Growth and Physiological Characteristics of Cabbage
2.6.1. Principal Component Analysis
2.6.2. Correlation Analysis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Plant Growth Condition and Experimental Design
4.3. Determinations and Measurements
4.4. Principal Component Analysis (PCA) and Correlation Analysis
4.5. Data Processing and Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Machado, R.M.A.; Serralheiro, R.P. Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Taiz, L.; Møller, I.M.; Murphy, A.; Zeiger, E. (Eds.) Plant Physiology and Development; Sinauer Associates Incorporated: Sunderland, MA, USA, 2015. [Google Scholar]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of plant responses to salt stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Cao, B.; Chen, Z.; Xu, K. Root morphology ion absorption and antioxidative defense system of two Chinese cabbage cultivars (Brassica rapa L.) reveal the different adaptation mechanisms to salt and alkali stress. Protoplasma 2022, 259, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Linić, I.; Šamec, D.; Grúz, J.; Vujčić Bok, V.; Strnad, M.; Salopek Sondi, B. Involvement of phenolic acids in short-term adaptation to salinity stress is species-specific among Brassicaceae. Plants 2019, 8, 155. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Shi, Q.; Li, X.; Gao, P.; Feng, D.; Zhang, X.; Lu, Y.; Yan, J.; Shen, S.; Zhao, J.; et al. Synergistic effects of carbon cycle metabolism and photosynthesis in Chinese cabbage under salt stress. Hortic. Plant J. 2022; in press. [Google Scholar]
- Pavlović, I.; Mlinarić, S.; Tarkowská, D.; Oklestkova, J.; Novák, O.; Lepeduš, H.; Bok, V.V.; Brkanac, S.R.; Strnad, M.; Salopek-Sondi, B. Early Brassica crops responses to salinity stress: A comparative analysis between Chinese cabbage, white cabbage, and kale. Front. Plant Sci. 2019, 10, 450. [Google Scholar] [CrossRef]
- Xi, T.; Yang, L.; Yuan, M.; Li, M.; Zhang, J.; Zhou, A.; Gao, X.; Wen, H. Effects of NaCl stress on seed germination of cabbage, cabbage, and rapeseed. Seed 2016, 35, 32–35. [Google Scholar]
- Xu, L.; Geelen, D. Developing biostimulants from agro-food and industrial by-products. Front. Plant Sci. 2018, 9, 1567. [Google Scholar] [CrossRef]
- Martínez-Arcos, A.; Moldes, A.B.; Vecino, X. Adding value to secondary streams of corn wet milling industry. CyTA-J. Food 2021, 19, 675–681. [Google Scholar] [CrossRef]
- Navarro-Morillo, I.; Navarro-Perez, V.; Perez-Millan, R.; Navarro-León, E.; Blasco, B.; Cámara-Zapata, J.M.; Garcia-Sanchez, F. Effects of Root and Foliar Application of Corn Steep Liquor on Pepper Plants: A Physiological, Nutritional, and Morphological Study. Horticulturae 2023, 9, 221. [Google Scholar] [CrossRef]
- Hull, S.R.; Yang, B.Y.; Venzke, D.; Kulhavy, K.; Montgomery, R. Composition of corn steep water during steeping. J. Agric. Food Chem. 1996, 44, 1857–1863. [Google Scholar] [CrossRef]
- Niwa, T.; Doi, U.; Kato, Y.; Osawa, T. Antioxidative properties of phenolic antioxidants isolated from corn steep liquor. J. Agric. Food Chem. 2001, 49, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.M.; Liu, E.Q.; Bao, Y.; Duan, S.L.; She, J.; Liu, H.; Wu, T.T.; Cao, X.Q.; Zhang, J.; Li, B.; et al. Low concentration of corn steep liquor promotes seed germination, plant growth, biomass production and flowering in soybean. Plant Growth Regul. 2019, 87, 29–37. [Google Scholar] [CrossRef]
- Obayori, O.S.; Adebusoye, S.A.; Ilori, M.O.; Oyetibo, G.O.; Omotayo, A.E.; Amund, O.O. Effects of corn steep liquor on growth rate and pyrene degradation by Pseudomonas strains. Curr. Microbiol. 2010, 60, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Chinta, Y.D.; Kano, K.; Widiastuti, A.; Fukahori, M.; Kawasaki, S.; Eguchi, Y.; Misu, H.; Odani, H.; Zhou, S.; Narisawa, K.; et al. Effect of corn steep liquor on lettuce root rot (Fusarium oxysporum f. sp. lactucae) in hydroponic cultures. J. Sci. Food Agric. 2014, 94, 2317–2323. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Dou, S.; Wang, D.; Zheng, S.; Li, S.; Zhang, Y.; Bai, Y. Improvement effect of corn stalk and other organic materials on sodic alkaline soil and changes in humus characteristics. J. Agro-Environ. Sci. 2019, 40, 2159–2166. [Google Scholar]
- Klages, K.; Boldingh, H.; Smith, G.S. Accumulation of myo-inositol in Actinidia seedlings subjected to salt stress. Ann. Bot. 1999, 84, 521–527. [Google Scholar] [CrossRef]
- Case, K.C.; Salsaa, M.; Yu, W.; Greenberg, M.L. Regulation of inositol biosynthesis: Balancing health and pathophysiology. In Lipid Signaling in Human Diseases; Springer: Berlin/Heidelberg, Germany, 2020; pp. 221–260. [Google Scholar]
- Kanter, U.; Usadel, B.; Guerineau, F.; Li, Y.; Pauly, M.; Tenhaken, R. The inositol oxygenase gene family of Arabidopsis is involved in the biosynthesis of nucleotide sugar precursors for cell-wall matrix polysaccharides. Planta 2005, 221, 243–254. [Google Scholar] [CrossRef]
- Loewus, F.A.; Murthy PP, N. myo-Inositol metabolism in plants. Plant Sci. 2000, 150, 1–19. [Google Scholar] [CrossRef]
- Mitsuhashi, N.; Kondo, M.; Nakaune, S.; Ohnishi, M.; Hayashi, M.; Hara-Nishimura, I.; Richardson, A.; Fukaki, H.; Nishimura, M.; Mimura, T. Localization of myo-inositol-1-phosphate synthase to the endosperm in developing seeds of Arabidopsis. J. Exp. Bot. 2008, 59, 3069–3076. [Google Scholar] [CrossRef]
- Chen, H.; Xiong, L. myo-Inositol-1-phosphate synthase is required for polar auxin transport and organ development. J. Biol. Chem. 2010, 285, 24238–24247. [Google Scholar] [CrossRef]
- Netondo, G.W.; Onyango, J.C.; Beck, E. Sorghum and salinity: II. Gas exchange and chlorophyll fluorescence of sorghum under salt stress. Crop Sci. 2004, 44, 806–811. [Google Scholar] [CrossRef]
- Hu, L.; Zhou, K.; Li, Y.; Chen, X.; Liu, B.; Li, C.; Gong, X.; Ma, F. Exogenous myo-inositol alleviates salinity-induced stress in Malus hupehensis Rehd. Plant Physiol. Biochem. 2018, 133, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Wang, L.; Yang, S.; Zheng, W.; Chen, H. Regulatory effects and physiological mechanisms of inositol on salt tolerance during wheat germination. J. Henan Agric. Univ. 2019, 53, 331–336+364. [Google Scholar]
- Yuan, H.; Guo, W.; Zhao, L.; Wu, J.; Huang, W.; Song, X.; Yu, Y.; Yao, Y.; Cheng, L.; Zhao, D. Effects of exogenous inositol on salt tolerance in maize. Heilongjiang Agric. Sci. 2014, 1, 17–20. [Google Scholar]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Dell’aversana, E.; Hessini, K.; Ferchichi, S.; Fusco, G.M.; Woodrow, P.; Ciarmiello, L.F.; Abdelly, C.; Carillo, P. Salinity duration differently modulates physiological parameters and metabolites profile in roots of two contrasting barley genotypes. Plants 2021, 10, 307. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Ma, L.; Fan, B.; Wang, G. Effects of Different Corn Cob Concentrations on the Growth and Yield of Flammulina velutipes. Rural Sci. Technol. 2021, 12, 50–52. [Google Scholar]
- Hnilickova, H.; Kraus, K.; Vachova, P.; Hnilicka, F. Salinity stress affects photosynthesis, malondialdehyde formation, and proline content in Portulaca oleracea L. Plants 2021, 10, 845. [Google Scholar] [CrossRef]
- Smirnoff, N. Botanical briefing: The function and metabolism of ascorbic acid in plants. Ann. Bot. 1996, 78, 661–669. [Google Scholar] [CrossRef]
- Santos, C.V. Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves. Sci. Hortic. 2004, 103, 93–99. [Google Scholar] [CrossRef]
- Verma, S.; Mishra, S.N. Putrescine alleviation of growth in salt stressed Brassica juncea by inducing antioxidative defense system. J. Plant Physiol. 2005, 162, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Singh, D.; Pandey, H.; Jatav, R.B.; Singh, V.; Pandey, D. An overview of roles of enzymatic and nonenzymatic antioxidants in plant. In Antioxidant Defense in Plants, 1st ed.; Aftab, T., Hakeem, K.R., Eds.; Springer: Singapore, 2022; pp. 1–13. [Google Scholar]
- Khan, M.H.; Panda, S.K. Alterations in root lipid peroxidation and antioxidative responses in two rice cultivars under NaCl-salinity stress. Acta Physiol. Plant. 2008, 30, 81–89. [Google Scholar] [CrossRef]
- Al-Mushhin, A.A.; Qari, S.H.; Fakhr, M.A.; Alnusairi, G.S.; Alnusaire, T.S.; ALrashidi, A.A.; Latef, A.A.H.A.; Ali, O.M.; Khan, A.A.; Soliman, M.H. Exogenous Myo-Inositol Alleviates Salt Stress by Enhancing Antioxidants and Membrane Stability via the Upregulation of Stress Responsive Genes in Chenopodium quinoa L. Plants 2021, 10, 2416. [Google Scholar] [CrossRef]
- El-Badri, A.M.; Batool, M.; Mohamed, I.A.; Wang, Z.; Wang, C.; Tabl, K.M.; Khatab, A.; Kuai, J.; Wang, J.; Wang, B.; et al. Mitigation of the salinity stress in rapeseed (Brassica napus L.) productivity by exogenous applications of bio-selenium nanoparticles during the early seedling stage. Environ. Pollut. 2022, 310, 119815. [Google Scholar] [CrossRef]
- Ali, M.; Afzal, S.; Parveen, A.; Kamran, M.; Javed, M.R.; Abbasi, G.H.; Malik, Z.; Riaz, M.; Ahmad, S.; Chattha, M.S.; et al. Silicon mediated improvement in the growth and ion homeostasis by decreasing Na+ uptake in maize (Zea mays L.) cultivars exposed to salinity stress. Plant Physiol. Biochem. 2021, 158, 208–218. [Google Scholar] [CrossRef]
- Sharma, N.; Chaudhary, C.; Khurana, P. Role of myo-inositol during skotomorphogenesis in Arabidopsis. Sci. Rep. 2020, 10, 17329. [Google Scholar] [CrossRef]
- Valluru, R.; Van den Ende, W. Myo-inositol and beyond–emerging networks under stress. Plant Sci. 2011, 181, 387–400. [Google Scholar] [CrossRef]
- Jia, Q.; Kong, D.; Li, Q.; Sun, S.; Song, J.; Zhu, Y.; Liang, K.; Ke, Q.; Lin, W.; Huang, J. The function of inositol phosphatases in plant tolerance to abiotic stress. Int. J. Mol. Sci. 2019, 20, 3999. [Google Scholar] [CrossRef]
- Li, H. Principles and Techniques of Plant Physiological and Biochemical Experiments, 1st ed.; Higher Education Press: Beijing, China, 2000; pp. 134–137. [Google Scholar]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Wang, S. Experimental Course of Plant Physiology, 1st ed.; Science Press: Beijing, China, 2017; pp. 207–212. [Google Scholar]
- Cui, J.; Jiang, R. Experimental Course of Soil, Plant, and Environmental Analysis, 1st ed.; China Agricultural University Press: Beijing, China, 2020; pp. 156–158. [Google Scholar]
- El-Badri, A.M.; Batool, M.; Mohamed, I.A.A.; Wang, Z.; Khatab, A.; Sherif, A.; Ahmad, H.; Khan, M.N.; Hassan, H.M.; Elrewainy, I.M.; et al. Antioxidative and metabolic contribution to salinity stress responses in two rapeseed cultivars during the early seedling stage. Antioxidants 2021, 10, 1227. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Wang, X.; Zhang, W.; Chen, Q. Combined Application of Myo-Inositol and Corn Steep Liquor from Agricultural Waste Alleviate Salt Stress in Brassica rapa. Plants 2023, 12, 4110. https://doi.org/10.3390/plants12244110
Zhang X, Wang X, Zhang W, Chen Q. Combined Application of Myo-Inositol and Corn Steep Liquor from Agricultural Waste Alleviate Salt Stress in Brassica rapa. Plants. 2023; 12(24):4110. https://doi.org/10.3390/plants12244110
Chicago/Turabian StyleZhang, Xinjun, Xian Wang, Wenna Zhang, and Qing Chen. 2023. "Combined Application of Myo-Inositol and Corn Steep Liquor from Agricultural Waste Alleviate Salt Stress in Brassica rapa" Plants 12, no. 24: 4110. https://doi.org/10.3390/plants12244110
APA StyleZhang, X., Wang, X., Zhang, W., & Chen, Q. (2023). Combined Application of Myo-Inositol and Corn Steep Liquor from Agricultural Waste Alleviate Salt Stress in Brassica rapa. Plants, 12(24), 4110. https://doi.org/10.3390/plants12244110




