Chemical Composition, Antioxidant and Anti-Enzymatic Activities, and In Vitro Insecticidal Potential of Origanum compactum (Benth.) Essential Oils
Abstract
:1. Introduction
2. Results
2.1. Mineral Composition
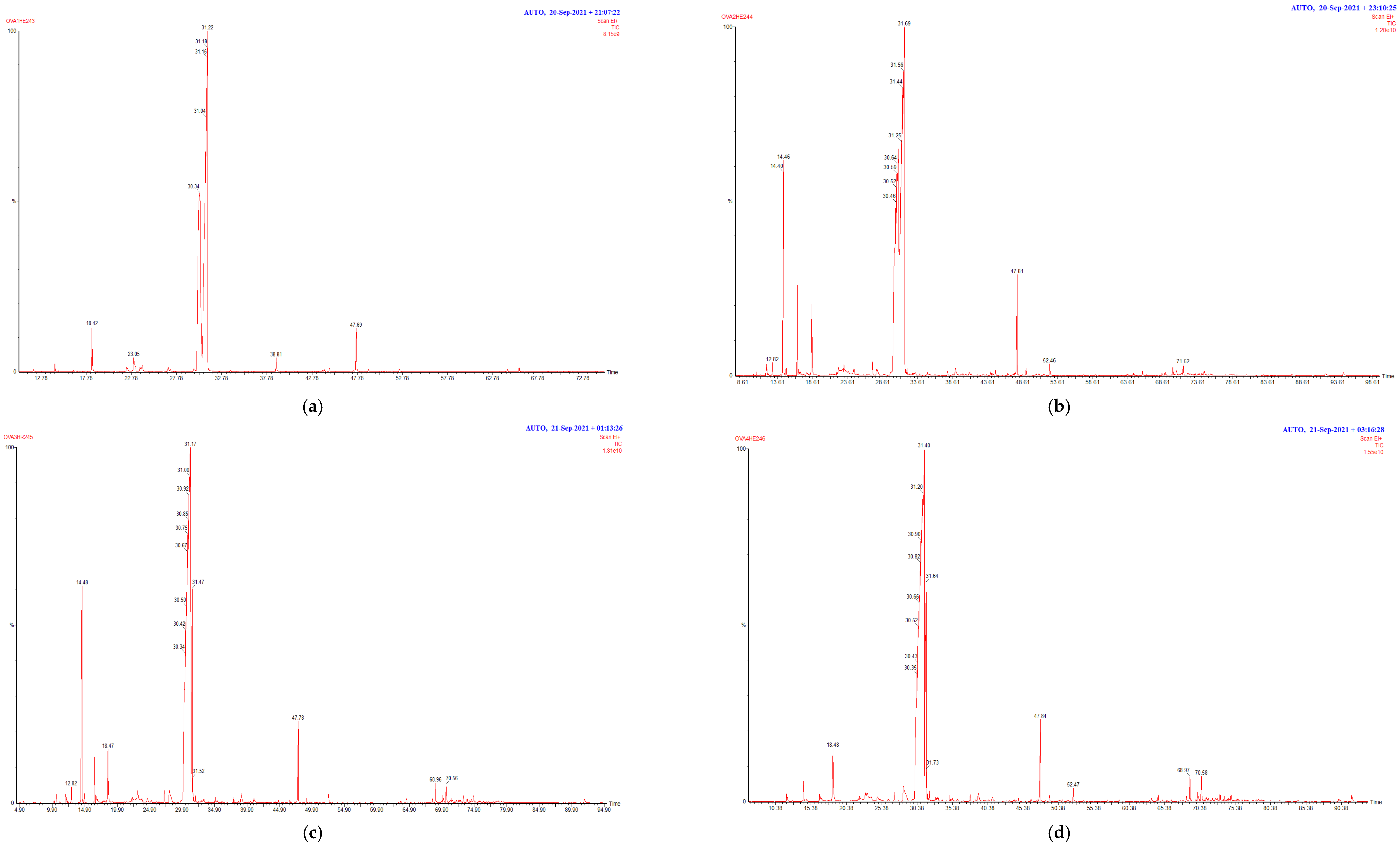
2.2. Chemical Composition of Essential Oils
2.3. Toxicity Assessment
2.4. Antioxidant Properties
2.5. Enzyme Inhibitory Effects Activities
2.6. Correlation Analysis
3. Discussion
4. Materials and Methods
4.1. Insect Rearing
4.2. Plant Material
4.3. Plant Mineral Analysis
4.4. Essential Oil Isolation and GC-MS Analysis
4.5. Contact Toxicity Assessment
4.6. Antioxidant Activities
4.6.1. DPPH Assay
4.6.2. Reducing Power Determination (FRAP)
4.6.3. β-Carotene Bleaching Test
4.7. Enzyme Inhibitory Activities
4.7.1. AChE Inhibition
4.7.2. Tyrosinase Inhibition
4.7.3. α-Glucosidase Inhibition
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, X.; He, T.; Wang, X.; Shen, M.; Yan, X.; Fan, S.; Le, W.; Xiaoping, W.; Xiao, X.; Hong, S.; et al. Traditional uses, chemical constituents and biological activities of plants from the genus Thymus. Chem. Biodivers. 2019, 16, e1900254. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Setzer, W.N. Aromatic herbs, medicinal plant-derived essential oils, and phytochemical extracts as potential therapies for coronaviruses: Future perspectives. Plants 2020, 9, 800. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, M.; Rebiai, A.; Sawicka, B.; Atanassova, M.; Ouakouak, H.; Larkem, I.; Egbuna, C.; Awuchi, C.G.; Boubekeur, S.; Ferhat, M.A.; et al. Effect of extraction methods on polyphenols, flavonoids, mineral elements, and biological activities of essential oil and extracts of Mentha pulegium L. Molecules 2021, 27, 11. [Google Scholar] [CrossRef] [PubMed]
- Bouzid, H.A.; Ibourki, M.; Hamdouch, A.; Oubannin, S.; Asbbane, A.; Hallouch, O.; Bijla, L.; Koubachi, J.; Majourhat, K.; Gharby, S. Moroccan Aromatic and Medicinal Plants: A review of Economy, Ethnobotany, Chemical composition, and Biological Activities of Commonly Used Plants. Food Humanit. 2024, 2, 100259. [Google Scholar] [CrossRef]
- Aboukhalid, K.; Machon, N.; Lambourdière, J.; Abdelkrim, J.; Bakha, M.; Douaik, A.; Korbecka-Glinka, G.; Gaboun, F.; Tomi, F.; Lamiri, A.; et al. Analysis of genetic diversity and population structure of the endangered Origanum compactum from Morocco, using SSR markers: Implication for conservation. Biol. Conserv. 2017, 212, 172–182. [Google Scholar] [CrossRef]
- Zejli, H.; Metouekel, A.; Zouirech, O.; Maliki, I.; El Moussaoui, A.; Lfitat, A.; Bousseraf, F.Z.; Almaary, K.S.; Nafidi, H.A.; Khallouki, F.; et al. Phytochemical Analysis, Antioxidant, Analgesic, Anti-Inflammatory, Hemagglutinin and Hemolytic Activities of Chemically Characterized Extracts from Origanum grosii (L.) and Thymus pallidus (L.). Plants 2024, 13, 385. [Google Scholar] [CrossRef]
- Et-Tazy, L.; Lamiri, A.; Satia, L.; Essahli, M.; Krimi Bencheqroun, S. In vitro antioxidant and antifungal activities of four essential oils and their major compounds against post-harvest fungi associated with chickpea in storage. Plants 2023, 12, 3587. [Google Scholar] [CrossRef]
- Loukili, E.H.; Ouahabi, S.; Elbouzidi, A.; Taibi, M.; Yahyaoui, M.I.; Asehraou, A.; Azougay, A.; Saleh, A.; Al Kamaly, O.; Parvez, M.K.; et al. Phytochemical composition and pharmacological activities of three essential oils collected from eastern Morocco (Origanum compactum, Salvia officinalis, and Syzygium aromaticum): A comparative study. Plants 2023, 12, 3376. [Google Scholar] [CrossRef]
- Plata-Rueda, A.; Zanuncio, J.C.; Serrão, J.E.; Martínez, L.C. Origanum vulgare essential oil against Tenebrio molitor (Coleoptera: Tenebrionidae): Composition, insecticidal activity, and behavioral response. Plants 2021, 10, 2513. [Google Scholar] [CrossRef]
- Bouhdid, S.; Skali, S.N.; Idaomar, M.; Zhiri, A.; Baudoux, D.; Amensour, M.; Abrini, J. Antibacterial and antioxidant activities of Origanum compactum essential oil. Afr. J. Biotechnol. 2008, 7, 1563–1570. [Google Scholar]
- Aboukhalid, K.; Al Faiz, C.; Douaik, A.; Bakha, M.; Kursa, K.; Agacka-Mołdoch, M.; Machon, N.; Lamiri, A. Influence of environmental factors on essential oil variability in Origanum compactum Benth. growing wild in Morocco. Chem. Biodivers. 2017, 14, e1700158. [Google Scholar] [CrossRef] [PubMed]
- Bagamboula, C.F.; Uyttendaele, M.; Debevere, J. Inhibitory effect of thyme and basil essential oils, carvacrol, thymol, estragol, linalool and p-cymene towards Shigella sonnei and S. flexneri. Food Microbiol. 2004, 21, 33–42. [Google Scholar] [CrossRef]
- Oyedemi, S.O.; Okoh, A.I.; Mabinya, L.V.; Pirochenva, G.; Afolayan, A.J. The proposed mechanism of bactericidal action of eugenol,∝-terpineol and g-terpinene against Listeria monocytogenes, Streptococcus pyogenes, Proteus vulgaris and Escherichia coli. Afr. J. Biotechnol. 2009, 8, 1280–1286. [Google Scholar]
- Bouyahya, A.; Abrini, J.; El-Baabou, A.; Bakri, Y.; Dakka, N. Determination of phenol content and antibacterial activity of five medicinal plants ethanolic extracts from North-West of Morocco. J. Plant. Pathol. Microbiol. 2016, 7, 2. [Google Scholar] [CrossRef]
- Aimad, A.; Youness, E.A.; Sanae, R.; El Moussaoui, A.; Bourhia, M.; Salamatullah, A.M.; Alzahrani, A.; Alyahya, H.K.; Albadr, N.A.; Nafidi, H.A.; et al. Chemical composition and antifungal, insecticidal and repellent activity of essential oils from Origanum compactum Benth. used in the mediterranean diet. Front. Plant Sci. 2022, 13, 798259. [Google Scholar] [CrossRef]
- Baghouz, A.; Bouchelta, Y.; Es-Safi, I.; Bourhia, M.; Abdelfattah, E.M.; Alarfaj, A.A.; Hirad, A.H.; Nafidi, H.A.; Guemmouh, R. Identification of volatile compounds and insecticidal activity of essential oils from Origanum compactum Benth. and Rosmarinus officinalis L. against Callosobruchus maculatus (fab.). J. Chem. 2022, 1, 7840409. [Google Scholar] [CrossRef]
- Ali, M.; Muhammad, A.; Lin, Z.; He, H.; Zhang, Y. Exploring Lamiaceae-derived bioactive compounds as nature’s arsenal for sustainable pest management. Phytochem. Rev. 2024, 1–25. [Google Scholar] [CrossRef]
- Ghalbane, I.; Alahyane, H.; Aboussaid, H.; Chouikh, N.E.; Costa, J.; Romane, A.; El Messoussi, S. Chemical composition and insecticidal properties of Moroccan Lavandula dentata and Lavandula stoechas essential oils against Mediterranean fruit fly, Ceratitis capitata. Neotrop. Entomol. 2022, 51, 628–636. [Google Scholar] [CrossRef]
- Benelli, G.; Canale, A.; Flamini, G.; Cioni, P.L.; Demi, F.; Ceccarini, L.; Macchia, M.; Conti, B. Biotoxicity of Melaleuca alternifolia (Myrtaceae) essential oil against the Mediterranean fruit fly, Ceratitis capitata (Diptera: Tephritidae), and its parasitoid Psyttalia concolor (Hymenoptera: Braconidae). Ind. Crops Prod. 2013, 50, 596–603. [Google Scholar] [CrossRef]
- El-Guendouz, S.; Aazza, S.; Lyoussi, B.; Antunes, M.D.; Faleiro, M.L.; Miguel, M.G. Anti-acetylcholinesterase, antidiabetic, anti-inflammatory, antityrosinase and antixanthine oxidase activities of Moroccan propolis. Int. J. Food Sci. Technol. 2016, 51, 1762–1773. [Google Scholar] [CrossRef]
- Ouknin, M.; Aghraz, A.; Chibane, M.; Boumezzourh, A.; Costa, J.; Majidi, L. Enzyme inhibitory, antioxidant activity and phytochemical analysis of essential oil from cultivated Rosmarinus officinalis. J. Food Meas. Charact. 2021, 15, 3782–3790. [Google Scholar] [CrossRef]
- Chen, J.; Yu, X.; Huang, Y. Inhibitory mechanisms of glabridin on tyrosinase. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 168, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Balogun, F.O.; Ashafa, A.O.T. Aqueous root extracts of Dicoma anomala (Sond.) extenuates postprandial hyperglycaemia in vitro and its modulation on the activities of carbohydrate-metabolizing enzymes in streptozotocin-induced diabetic Wistar rats. S. Afr. J. Bot. 2017, 112, 102–111. [Google Scholar] [CrossRef]
- Siahbalaei, R.; Kavoosi, G. Chemical composition and evaluation of anti-diabetic activity of oil extracts from Oliveria decumbens, Thymus kotschyanus, Trachyspermum ammi and Zataria multiflora. Int. J. Food Sci. Technol. 2021, 15, 276–287. [Google Scholar] [CrossRef]
- Miller, D.D. Minerals. In Fennema’s Food Chemistry; CRC Press: Boca Raton, FL, USA, 2017; pp. 627–679. [Google Scholar]
- Watts, D.L. Nutrient interrelationships: Minerals, vitamins, endocrines. J. Orthomol. Med. 1990, 5, 11–19. [Google Scholar]
- Viegas, C.; Araújo, N.; Marreiros, C.; Simes, D. The interplay between mineral metabolism, vascular calcification and inflammation in Chronic Kidney Disease (CKD): Challenging old concepts with new facts. Aging 2019, 11, 4274. [Google Scholar] [CrossRef]
- Macrae, R.; Robinson, R.K.; Sadler, M.J. Encyclopaedia of Food Science, Food Technology and Nutrition; Academic Press: London, UK, 1993. [Google Scholar]
- El Ansari, Z.N.; Boussaoudi, I.; Benkaddour, R.; Hamdoun, O.; Lemrini, M.; Martin, P.; Badoc, A.; Lamarti, A. Conservation of Thymus pallidus Cosson ex Batt. by shoot tip and axillary bud in vitro culture. J. Plant. Biotechnol. 2020, 47, 53–65. [Google Scholar] [CrossRef]
- Sanchez-Castillo, C.P.; Dewey, P.J.; Aguirre, A.; Lara, J.J.; Vaca, R.; de la Barra, P.L.; Ortiz, M.; Escamilla, I.; James, W.P.T. The mineral content of Mexican fruits and vegetables. J. Food Compos. Anal. 1998, 11, 340–356. [Google Scholar] [CrossRef]
- Custódio, L.; Silvestre, L.; Rocha, M.I.; Rodrigues, M.; Vizetto-Duarte, C.; Pereira, H.; Barreira, L.; Varela, J. Methanol extracts from Cystoseira tamariscifolia and Cystoseira nodicaulis are able to inhibit cholinesterases and protect a human dopaminergic cell line from hydrogen peroxide-induced cytotoxicity. Pharm. Biol. 2016, 54, 1687–1696. [Google Scholar] [CrossRef]
- Ghanmi, M.; Strani, B.; Aberchane, M.; Ismaili, M.R.; Aafi, A.; El Abid, A. Plantes aromatiques et médicinales du Maroc: Les mille et une vertus, Edition Maroc-Nature; Centre Nationale de la Recherche Forestière: Rabat, Morocco, 2011; pp. 46–108. [Google Scholar]
- Belkamel, A.; Bammi, J.; Belkamel, A. Origanum compactum (benth.). J. Anim. Plant Sci. 2013, 19, 2880–2887. [Google Scholar]
- Benazzouz, M.A. Essential Oils, Importance and Potential: Bibliographic Update of the Latest Research on Their Uses and Toxicity, and Analysis of the Composition of Essential Oils from Fifteen of the Most Consumed Plants in Morocco. Ph.D. Thesis, Faculté de Médecine et de Pharmacie , Université Mohamed V, Rabat, Morocco, 2011. [Google Scholar]
- Chrysargyris, A.; Evangelides, E.; Tzortzakis, N. Seasonal variation of antioxidant capacity, phenols, minerals and essential oil components of sage, spearmint and sideritis plants grown at different altitudes. Agronomy 2021, 11, 1766. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Mikallou, M.; Petropoulos, S.; Tzortzakis, N. Profiling of essential oils components and polyphenols for their antioxidant activity of medicinal and aromatic plants grown in different environmental conditions. Agronomy 2020, 10, 727. [Google Scholar] [CrossRef]
- Najar, B.; Pistelli, L.; Ferri, B.; Angelini, L.G.; Tavarini, S. Crop yield and essential oil composition of two Thymus vulgaris chemotypes along three years of organic cultivation in a hilly area of central Italy. Molecules 2021, 26, 5109. [Google Scholar] [CrossRef]
- Ouknin, M.; Romane, A.; Costa, J.; Majidi, L. Comparative study of the chemical profiling, antioxidant and antimicrobial activities of essential oils of different parts of Thymus willdenowii Boiss & Reut. Nat. Prod. Res. 2019, 33, 2398–2401. [Google Scholar] [CrossRef] [PubMed]
- El Abdali, Y.; Mahraz, A.M.; Beniaich, G.; Mssillou, I.; Chebaibi, M.; Bin Jardan, Y.A.; Lahkimi, A.; Nafidi, H.A.; Aboul-Soud, M.A.; Bourhia, M.; et al. Essential oils of Origanum compactum Benth: Chemical characterization, in vitro, in silico, antioxidant, and antibacterial activities. Open Chem. 2023, 21, 20220282. [Google Scholar] [CrossRef]
- Bakhy, K.; Benlhabib, O.; Bighelli, A.; Casanova, J.; Tomi, F.; Al Faiz, C. Yield and chemical variability of the essential oil isolated from aerial parts of wild Origanum compactum Benth. From Moroccan Western Rif. Am. J. Essent. Oils Nat. Prod. 2014, 1, 9–17. [Google Scholar]
- Laghmouchi, Y.; Belmehdi, O.; Senhaji, N.S.; Abrini, J. Chemical composition and antibacterial activity of Origanum compactum Benth. essential oils from different areas at northern Morocco. S. Afr. J. Bot. 2018, 115, 120–125. [Google Scholar] [CrossRef]
- Horwath, A.B.; Grayer, R.J.; Keith-Lucas, D.M.; Simmonds, M.S. Chemical characterisation of wild populations of Thymus from different climatic regions in southeast Spain. Biochem. Syst. Ecol. 2008, 36, 117–133. [Google Scholar] [CrossRef]
- Ložienė, K.; Venskutonis, P.R. Influence of environmental and genetic factors on the stability of essential oil composition of Thymus pulegioides. Biochem. Syst. Ecol. 2005, 33, 517–525. [Google Scholar] [CrossRef]
- Muñoz-Bertomeu, J.; Arrillaga, I.; Segura, J. Essential oil variation within and among natural populations of Lavandula latifolia and its relation to their ecological areas. Biochem. Syst. Ecol. 2007, 35, 479–488. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Quality Control Methods for Medicinal Plant Materials; WHO: Geneva, Switzerland, 1998. [Google Scholar]
- El Babili, F.; Bouajila, J.; Souchard, J.P.; Bertrand, C.; Bellvert, F.; Fouraste, I.; Moulis, C.; Valentin, A. Oregano: Chemical analysis and evaluation of its antimalarial, antioxidant, and cytotoxic activities. J. Food Sci. 2011, 76, C512–C518. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, P.R.; Riveros, C.G.; Zygadlo, J.A.; Grosso, N.R.; Nepote, V. Antioxidant activity of essential oil of oregano species from Argentina in relation to their chemical composition. Int. J. Food Sci. Technol. 2011, 46, 2648–2655. [Google Scholar] [CrossRef]
- Asensio, C.M.; Grosso, N.R.; Juliani, H.R. Quality characters, chemical composition and biological activities of oregano (Origanum spp.) Essential oils from Central and Southern Argentina. Ind. Crops Prod. 2015, 63, 203–213. [Google Scholar] [CrossRef]
- Assaggaf, H.; El Hachlafi, N.; El Fadili, M.; Elbouzidi, A.; Ouassou, H.; Jeddi, M.; Alnasser, S.M.; Qasem, A.; Attar, A.; Al-Farga, A.; et al. GC/MS Profiling, In Vitro Antidiabetic Efficacy of Origanum compactum Benth. Essential Oil and In Silico Molecular Docking of Its Major Bioactive Compounds. Catalysts 2023, 13, 1429. [Google Scholar] [CrossRef]
- Salazar, M.O.; Osella, M.I.; Arcusin, D.E.; Lescano, L.E.; Furlan, R.L. New α-glucosidase inhibitors from a chemically engineered essential oil of Origanum vulgare L. Ind. Crops Prod. 2020, 156, 112855. [Google Scholar] [CrossRef]
- Lima, R.K.; Cardoso, M.G.; Moraes, J.C.; Carvalho, S.M.; Rodrigues, V.G.; Guimarães, L.G.L. Chemical composition and fumigant effect of essential oil of Lippia sidoides Cham. and monoterpenes against Tenebrio molitor (L.) (coleoptera: Tenebrio-nidae). Ciênc. Agrotec. 2011, 35, 664–671. [Google Scholar] [CrossRef]
- Benchouikh, A.; Allam, T.; Kribii, A.; Ounine, K. The Study of the Insecticidal Effect of Nigella Sativa Essential Oil Against Tuta Absoluta Larvae. Int. J. Sci. Technol. Res. 2015, 4, 88–90. [Google Scholar]
- Yakhlef, G.; Hambaba, L.; Pinto, D.C.G.A.; Silva, A.M.S. Chemical composition and insecticidal, repellent and antifungal activities of essential oil of Mentha rotundifolia (L.) from Algeria. Ind. Crops Prod. 2020, 158, 112988. [Google Scholar] [CrossRef]
- Erler, F.; Tunc, I. Monoterpenoids as fumigants against greenhouse pests: Toxic, development and reproduction-inhibiting effects. J. Plant Dis. Prot. 2005, 112, 181–192. [Google Scholar]
- Park, J.H.; Jeon, Y.J.; Lee, C.H.; Chung, N.; Lee, H.S. Insecticidal toxicities of carvacrol and thymol derived from Thymus vulgaris Lin. against Pochazia shantungensis Chou & lu., newly recorded pest. Sci. Rep. 2017, 7, 40902. [Google Scholar] [CrossRef]
- Khanavi, M.; Laghaei, P.; Isman, M.B. Essential oil composition of three native Persian plants and their inhibitory effects in the cabbage looper, Trichoplusia ni. J. Asia-Pac. Entomol. 2017, 20, 1234–1240. [Google Scholar] [CrossRef]
- Bisrat, D.; Jung, C. Insecticidal Toxicities of Three Main Constituents Derived from Trachyspermum ammi (L.) Sprague ex Turrill Fruits against the Small Hive Beetles, Aethina tumida Murray. Molecules 2020, 25, 1100. [Google Scholar] [CrossRef]
- Youssefi, M.R.; Tabari, M.A.; Esfandiari, A.; Kazemi, S.; Moghadamnia, A.A.; Sut, S.; Dall’Acqua, S.; Benelli, G.; Maggi, F. Efficacy of Two Monoterpenoids, Carvacrol and Thymol, and Their Combinations against Eggs and Larvae of the West Nile Vector Culex pipiens. Mol. Artic. 2019, 24, 1867. [Google Scholar] [CrossRef] [PubMed]
- Alahyane, H.; Ouknin, M.; Aboussaid, H.; El Messoussi, S.; Costa, J.; Majidi, L. Biological activities of essential oils from Moroccan plants against the honey bee ectoparasitic mite, Varroa destructor. Int. J. Acarol. 2022, 48, 50–56. [Google Scholar] [CrossRef]
- Jukic, M.; Politeo, O.; Maksimovic, M.; Milos, M.; Milos, M. In vitro acetylcholinesterase inhibitory properties of thymol, carvacrol and their derivatives thymoquinone and thymohydroquinone. Phytother. Res. 2007, 21, 259–261. [Google Scholar] [CrossRef]
- Aazza, S.; Lyoussi, B.; Miguel, M.G. Antioxidant and antiacetylcholinesterase activities of some commercial essential oils and their major compounds. Molecules 2011, 16, 7672–7690. [Google Scholar] [CrossRef]
- López, V.; Cascella, M.; Benelli, G.; Maggi, F.; Gómez-Rincón, C. Green drugs in the fight against Anisakis simplex—Larvicidal activity and acetylcholinesterase inhibition of Origanum compactum essential oil. Parasitol. Res. 2018, 117, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Taherkhani, M. Tyrosinase inhibition, in vitro antimicrobial, antioxidant, cytotoxicity and anticancer activities of the essential oil from the leaves of Artemisia turanica, growing wild in Iran. J. Essent. Oil-Bear. Plants 2016, 19, 1141–1154. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz Navajas, Y.; Sánchez Zapata, E.; Fernández-López, J.; Pérez-Álvarez, J.A. Antioxidant activity of essential oils of five spice plants widely used in a Mediterranean diet. Flavour Fragr. J. 2010, 25, 13–19. [Google Scholar] [CrossRef]
- Daneshzadeh, M.S.; Abbaspour, H.; Amjad, L.; Nafchi, A.M. An investigation on phytochemical, antioxidant and antibacterial properties of extract from Eryngium billardieri F. Delaroche. J. Food Meas. Charact. 2020, 14, 708–715. [Google Scholar] [CrossRef]
- Mousavian, D.; Mohammadi Nafchi, A.; Nouri, L.; Abedinia, A. Physicomechanical properties, release kinetics, and antimicrobial activity of activated low-density polyethylene and orientated polypropylene films by Thyme essential oil active component. J. Food Meas. Charact. 2021, 15, 883–891. [Google Scholar] [CrossRef]
- Blenau, W.; Rademacher, E.; Baumann, A. Plant essential oils and formamidines as insecticides/acaricides: What are the molecular targets? Apidologie 2012, 43, 334–347. [Google Scholar] [CrossRef]
- Zarrad, K.; Laarif, A.; Ben, H.A.; Chaieb, I.; Mediouni, B.J.J. Anticholinesterase potential of monoterpenoids on the whitefly Bemisia tabaci and their kinetic studies. J. Agric. Sci. Technol. 2017, 19, 643–652. [Google Scholar]
- Lee, S.E.; Lee, B.H.; Choi, W.S.; Park, B.S.; Kim, J.G.; Campbell, B.C. Fumigant toxicity of volatile natural products from Korean spices and medicinal plants towards the rice weevil, Sitophilus oryzae (L). Pest Manag. Sci. 2001, 57, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.M.; Kim, J.; Kang, J.; Koh, S.H.; Ahn, Y.J.; Kang, K.S.; Park, I.K. Fumigant toxicity and acetylcholinesterase inhibitory activity of 4 Asteraceae plant essential oils and their constituents against Japanese termite (Reticulitermes speratus Kolbe). Pestic. Biochem. Physiol. 2014, 113, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Park, C.G.; Jang, M.; Yoon, K.A.; Kim, J. Insecticidal and acetylcholinesterase inhibitory activities of Lamiaceae plant essential oils and their major components against Drosophila suzukii (Diptera: Drosophilidae). Ind. Crops Prod. 2016, 89, 507–513. [Google Scholar] [CrossRef]
- Tong, F.; Gross, A.D.; Dolan, M.C.; Coats, J.R. Phenolic monoterpenoid carvacrol inhibits the binding of nicotine to the housefly nicotinic acetylcholine receptor. Pest Manag. Sci. 2013, 69, 775–780. [Google Scholar] [CrossRef]
- Miyazawa, K.; Noguchi, T. Distribution and origin of tetrodoxin. J. Crops Prot. 2001, 20, 11–33. [Google Scholar] [CrossRef]
- Srivastava, N.J.; Singh, B.; Chanda, D.; Shanker, K. Chemical composition and acetylcholinesterase inhibitory activity of Artemisia maderaspatana essential oil. J. Agric. Food Chem. 2015, 53, 1677–1683. [Google Scholar] [CrossRef]
- Msaad-Guerfali, M.; Djobbi, W.; Charaabi, K.; Hamden, H.; Fadhl, S.; Marzouki, W.; Dhaouadi, F.; Chevrier, C. Evaluation of Providencia rettgeri pathogenicity against laboratory Mediterranean fruit fly strain (Ceratitis capitata). PLoS ONE 2018, 13, e0196343. [Google Scholar] [CrossRef]
- Fennane, M.; Ibn Tattou, M.; Mathez, J. Practical fora of Morocco: Manual of Determination of Vascular Plants. Pteridophyta, Gymnospermae, Angiospermae (Lauraceae-Neuradaceae); Scientific Institute, Mohamed V University: Rabat, Morocco, 1999; pp. 1–560. [Google Scholar]
- Ouknin, M.; Romane, A.; Arjouni, M.Y.; Majidi, L. Mineral Composition, multivariate analysis of some oligo-elements and heavy metals in some species of genus Thymus. J. Mater. Environ. Sci. 2018, 9, 980–985. [Google Scholar] [CrossRef]
- European Pharmacopoeia Convention Inc. European Pharmacopoeia, 3rd ed.; Council of Europe: Strasbourg, France, 1997; pp. 121–122. [Google Scholar]
- König, W.A.; Joulain, D.; Hochmuth, D.H. Terpenoids and Related Constituents of Essential Oils, Library of Mass Finder 2.1; Institute of Organic Chemistry, University of Hamburg: Hamburg, Germany, 2011. [Google Scholar]
- Oyaizu, M. Studies on products of browning reactions: Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Koleva, I.I.; Van Beek, T.A.; Linssen, J.P.; Groot, A.D.; Evstatieva, L.N. Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem. Anal. 2002, 13, 8–17. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Yamashita, D.; Takeda, Y.; Yonemori, S. Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitors from Garcinia subelliptica. Biosci. Biotechnol. Biochem. 2005, 69, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.I.; Apostolidis, E.; Shetty, K.J.B.T. In vitro studies of eggplant (Solanum melongena) phenolics as inhibitors of key enzymes relevant for type 2 diabetes and hypertension. Bioresour. Technol. 2008, 99, 2981–2988. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis, 3rd ed.Cambridge University Press: Cambridge, UK, 1971; 333p. [Google Scholar]
- Adak, T.; Barik, N.; Patil, N.B.; Govindharaj, G.P.P.; Gadratagi, B.G.; Annamalai, M.; Mukherjee, A.K.; Rath, P.C. Nanoemulsion of eucalyptus oil: An alternative to synthetic pesticides against two major storage insects (Sitophilus oryzae (L.) and Tribolium castaneum (Herbst)) of rice. Ind. Crops Prod. 2020, 143, 111849. [Google Scholar] [CrossRef]
- Garrido-Miranda, K.A.; Giraldo, J.D.; Schoebitz, M. Essential oils and their formulations for the control of curculionidae pests. Front. Agron. 2022, 4, 876687. [Google Scholar] [CrossRef]
| OC1 | OC2 | OC3 | OC4 | |||||
|---|---|---|---|---|---|---|---|---|
| Aerial Part | Aerial Part | Aerial Part | Aerial Part | |||||
| Mean | S.D | Mean | S.D | Mean | S.D | Mean | S.D | |
| Al | 27.33 | 1.49 | 4.86 | 0.2 | 19.45 | 2.45 | 22.30 | 1.64 |
| As | 0.03 | 0.01 | 0.04 | 0.01 | 0.07 | 0.02 | 0.03 | 0.01 |
| B | 0.25 | 0.01 | 0.28 | 0.04 | 0.62 | 0.08 | 0.67 | 0.01 |
| Ba | 0.19 | 0.02 | 0.39 | 0.11 | 0.82 | 0.19 | 0.69 | 0.01 |
| Ca | 512.20 | 5.50 | 220.67 | 3.25 | 325.50 | 10.20 | 295.50 | 8.44 |
| Cr | 0.11 | 0.01 | 0.20 | 0.12 | 0.67 | 0.15 | 0.06 | 0.01 |
| Cu | 0.23 | 0.01 | 0.08 | 0.03 | 0.25 | 0.07 | 0.03 | 0.01 |
| Fe | 112.60 | 2.20 | 79.50 | 1.80 | 59.70 | 5.40 | 43.55 | 1.22 |
| K | 213.45 | 9.50 | 195.99 | 13.60 | 398.45 | 11.50 | 322.25 | 10.50 |
| Mg | 98.45 | 2.20 | 65.50 | 4.20 | 77.60 | 6.37 | 59.70 | 3.50 |
| Mn | 0.50 | 0.02 | 0.33 | 0.1 | 1.01 | 0.09 | 1.65 | 0.10 |
| Na | 11.45 | 0.08 | 9.30 | 0.70 | 7.60 | 0.3 | 16.32 | 0.15 |
| Ni | 0.21 | 0.03 | 0.15 | 0.07 | 0.92 | 0.04 | 0.22 | 0.01 |
| P | 69.77 | 1.46 | 45.75 | 4.10 | 66.90 | 2.50 | 56.45 | 1.50 |
| Pb | 0.01 | 0.01 | 0.21 | 0.01 | 0.25 | 0.03 | 0.06 | 0.01 |
| Si | 0.13 | 0.03 | 3.95 | 0.33 | 2.79 | 0.25 | 1.13 | 0.01 |
| Sr | 0.16 | 0.02 | 0.30 | 0.02 | 0.79 | 0.04 | 0.7 | 0.01 |
| Zn | 0.10 | 0.03 | 0.39 | 0.05 | 0.55 | 0.02 | 0.63 | 0.01 |
| No. a | Compound Name | RI l b | RIa c | OC1 d | OC2 d | OC3 d | OC4 d |
|---|---|---|---|---|---|---|---|
| 1 | methyl isovalerate | 721 | 720 | _ | _ | 0.02 | _ |
| 2 | 2-hexenal | 830 | 825 | _ | 0.02 | 0.03 | _ |
| 3 | (Z)-3-hexen-1-ol | 831 | 835 | _ | 0.01 | 0.01 | 0.19 |
| 4 | 3-heptanone | 865 | 863 | _ | 0.01 | 0.01 | _ |
| 5 | α-thujene | 932 | 924 | _ | 0.03 | 0.06 | _ |
| 6 | α-pinene | 936 | 933 | _ | 0.08 | 0.17 | _ |
| 7 | camphene | 950 | 947 | _ | 0.02 | 0.03 | _ |
| 8 | 1-octen-3-ol | 963 | 961 | 0.11 | 0.31 | 0.23 | 0.19 |
| 9 | 3-octanone | 964 | 965 | 0.04 | 0.18 | 0.12 | 0.09 |
| 10 | β-pinene | 978 | 974 | _ | 0.03 | 0.05 | |
| 11 | 3-octanol | 981 | 980 | _ | _ | _ | 0.01 |
| 12 | β-myrcene | 987 | 982 | 0.01 | 0.29 | 0.34 | 0.02 |
| 13 | δ-3-carene | 1010 | 1008 | _ | 0.02 | 0.03 | _ |
| 14 | α-terpinene | 1013 | 1011 | _ | 0.01 | 0.02 | 0.01 |
| 15 | p-cymene | 1015 | 1014 | 0.27 | 7.23 | 8.64 | 0.34 |
| 16 | eucalyptol | 1024 | 1023 | 0.05 | _ | _ | 0.05 |
| 17 | limonene | 1025 | 1024 | _ | 0.14 | 0.16 | _ |
| 18 | γ-terpinene | 1051 | 1050 | 0.04 | 1.54 | 0.75 | 0.02 |
| 19 | trans-sabinene hydrate | 1051 | 1055 | 0.05 | 0.19 | 0.3 | 0.28 |
| 20 | cis-linalol oxide | 1072 | 1059 | 0.04 | 0.09 | 0.11 | 0.04 |
| 21 | 1-nonen-3-ol | 1058 | 1063 | 0.02 | 0.03 | 0.04 | _ |
| 22 | fenchone | 1076 | 1072 | 0.01 | _ | _ | _ |
| 23 | trans-linalol oxide | 1072 | 1074 | 0.07 | _ | _ | _ |
| 24 | meta-cymenene | 1073 | 1075 | _ | 0.16 | 0.14 | _ |
| 25 | cis-sabinene hydrate | 1083 | 1081 | _ | 0.01 | _ | _ |
| 26 | linalool | 1086 | 1085 | 1.96 | 1.78 | 1.44 | 1.54 |
| 27 | cis-p-mentha-2-en-1-ol | 1108 | 1110 | _ | 0.01 | 0.01 | _ |
| 28 | verbenol | 1139 | 1132 | _ | 0.03 | 0.02 | _ |
| 29 | endo-borneol | 1150 | 1153 | 0.33 | _ | _ | _ |
| 30 | terpinen-4-ol | 1164 | 1165 | 1.1 | 0.52 | 0.16 | 0.17 |
| 31 | α-terpineol | 1179 | 1176 | 0.66 | 0.42 | 0.14 | 0.23 |
| 32 | trans-piperitol | 1192 | 1195 | 0.01 | _ | _ | _ |
| 33 | pulegone | 1213 | 1221 | 0.3 | 0.33 | 0.76 | 1.07 |
| 34 | carvacrol methyl ether | 1226 | 1227 | 0.08 | 0.19 | 0.17 | 0.13 |
| 35 | thymol | 1267 | 1273 | 28.72 | 31.89 | 72.89 | 80.39 |
| 36 | carvacrol | 1278 | 1285 | 61.84 | 47.37 | 6.54 | 7.44 |
| 37 | piperitenone | 1315 | 1317 | 0.02 | 0.08 | 0.1 | 0.11 |
| 38 | eugenol | 1331 | 1331 | 0.06 | 0.02 | 0.02 | 0.02 |
| 39 | caryophyllene | 1421 | 1420 | 0.5 | _ | _ | _ |
| 40 | aromadendrene | 1443 | 1447 | 0.02 | 0.02 | _ | _ |
| 41 | α-humulene | 1456 | 1452 | 0.04 | _ | _ | _ |
| 42 | γ-muurolene | 1471 | 1472 | 0.01 | 0.02 | 0.01 | 0.01 |
| 43 | α-muurolene | 1496 | 1492 | 0.02 | 0.01 | 0.01 | _ |
| 44 | β-bisabolene | 1483 | 1501 | 0.06 | 0.04 | 0.01 | 0.01 |
| 45 | γ-cadinene | 1507 | 1508 | 0.07 | 0.04 | 0.02 | 0.03 |
| 46 | calamenene | 1517 | 1511 | 0.03 | 0.01 | 0.01 | 0.01 |
| 47 | δ-cadiene | 1520 | 1516 | 0.12 | 0.06 | 0.03 | 0.04 |
| 48 | spathulenol | 1572 | 1566 | 0.05 | 0.02 | _ | 0.04 |
| 49 | caryophyllene oxide | 1578 | 1572 | 1.56 | 1.82 | 1.34 | 1.74 |
| 50 | humulene epoxide II | 1601 | 1597 | _ | 0.10 | 0.07 | _ |
| Monoterpene hydrocarbons | 0.37 | 9.39 | 10.08 | 0.44 | |||
| Oxygenated monoterpenes | 93.81 | 83.60 | 83.42 | 91.91 | |||
| Sesquiterpene hydrocarbons | 2.48 | 0.20 | 0.09 | 0.09 | |||
| Oxygenated sesquiterpenes | 1.61 | 1.94 | 1.41 | 1.78 | |||
| Total identified compounds | 98.27 | 95.13 | 95.00 | 94.22 | |||
| Essential Oil * | T(h) | LC50 (µL/mL) (95% LD) | LC90 (µL/mL) (95% LD) | χ2 | DF |
|---|---|---|---|---|---|
| OC1 | 24 | 13.979 (9.929–23.891) | 26.412 (17.814–47.183) | 12.667 | 23 |
| 48 | 2.515 (1.846–3.202) | 5.502 (4.187–7.324) | 11.164 | 23 | |
| OC2 | 24 | 21.559 (13.971–44.689) | 61.227 (32.668–190.732) | 18.853 | 23 |
| 48 | 2.853 (2.022–3.738) | 6.515 (4.879–9.084) | 11.487 | 23 | |
| OC3 | 24 | 58.907 (30.391–204.549) | 104.979 (49.68–431.667) | 9.786 | 23 |
| 48 | 5.213 (3.778–7.256) | 10.721 (7.837–16.831) | 10.981 | 23 | |
| OC4 | 24 | 91.29 (42.355–407.43) | 219.189 (79.387–1901.429) | 11.674 | 23 |
| 48 | 7.445 (5.407–11.164) | 16.088 (10.712–32.996) | 13.481 | 23 |
| DPPH (IC50 µg/mL) | FRAP (IC50 µg/mL) | β-Carotene (IC50 µg/mL) | |
|---|---|---|---|
| OC1 | 55.96 ± 1.07 b | 105.78 ± 2.14 e | 30.96 ± 1.27 a |
| OC2 | 109.15 ± 2.01 d | 59.69 ± 2.06 c | 83.91 ± 1.30 d |
| OC3 | 158.54 ± 4.50 e | 92.48 ± 1.77 d | 121.03 ± 0.70 f |
| OC4 | 87.89 ± 1.05 c | 42.50 ± 0.54 b | 52.74 ± 1.16 b |
| Gallic acid | 41.77 ± 0.90 a | 10.45 ± 0.25 a | 98.21 ± 0.51 e |
| BHT | 185.96 ± 1.24 f | 36.51 ± 0.60 b | 75.15 ± 1.33 c |
| Plants Code | Essential Oil Doses (mg/mL) | AChE (%) | Tyrosinase (%) | α-Glucosidase (%) |
|---|---|---|---|---|
| OC1 | 0.25 | 45.00 ± 0.77 a | 42.45 ± 0.43 a | 47.66 ± 0.12 a |
| 0.5 | 61.12 ± 0.53 b | 57.66 ± 0.70 b | 57.50 ± 0.50 b | |
| 0.75 | 79.20 ± 0.50 c | 70.45 ± 0.30 c | 67.20 ± 0.60 c | |
| 1 | 94.01 ± 0.70 d | 83.20 ± 0.60 d | 85.50 ± 0.30 d | |
| Positive control | 77.30 ± 0.40 c | 83.44 ± 0.73 e | 87.33 ± 0.50 d | |
| OC2 | 0.25 | 52.45 ± 0.44 a | 53.12 ± 0.23 a | 54.11 ± 0.40 a |
| 0.5 | 67.13 ± 0.50 b | 66.79 ± 0.50 b | 59.20 ± 0.33 b | |
| 0.75 | 78.40 ± 0.90 c | 76.11 ± 0.20 c | 68.48 ± 0.40 c | |
| 1 | 85.89 ± 0.40 d | 83.02 ± 0.10 d | 87.80 ± 0.60 d | |
| Positive control | 78.70 ± 0.50 c | 84.80 ± 0.33 d | 87.50 ± 0.23 d | |
| OC3 | 0.25 | 54.60 ± 0.70 a | 48.75 ± 0.70 a | 44.20 ± 0.40 a |
| 0.5 | 70.10 ± 0.20 b | 57.90 ± 0.50 b | 57.30 ± 0.13 b | |
| 0.75 | 84.33 ± 0.90 d | 66.10 ± 0.55 c | 65.10 ± 0.05 c | |
| 1 | 93.33 ± 0.50 d | 76.40 ± 0.66 d | 79.90 ± 0.22 d | |
| Positive control | 78.23 ± 0.43 c | 84.40 ± 0.30 e | 87.90 ± 0.08 e | |
| OC4 | 0.25 | 41.80 ± 0.65 a | 54.80 ± 0.40 a | 52.50 ± 0.80 a |
| 0.5 | 51.33 ± 0.44 b | 68.30 ± 0.54 b | 59.80 ± 0.20 b | |
| 0.75 | 68.11 ± 0.67 c | 77.70 ± 0.43 c | 69.53 ± 0.50 c | |
| 1 | 84.75 ± 0.66 e | 87.40 ± 0.60 d | 80.70 ± 0.70 d | |
| Positive control | 78.89 ± 0.67 d | 84.70 ± 0.23 d | 87.80 ± 0.30 e |
| Plant Species | Species Code | Local Name | Harvesting Site | Collection Time | Voucher Specimen | GPS Coordinates | Oil Yield (% (w/w)) |
|---|---|---|---|---|---|---|---|
| Origanum compactum Benth. (OC) | OC1 | Izzikni | Gouman | June 2021 | ER-22-10 | 31°49′43.9″ N, 6°06′16.4″ W | 4.50 ± 0.15 |
| OC2 | Izzikni | Tisslmit | June 2021 | ER-22-11 | 31°49′31.4″ N, 6°06′22.2″ W | 3.90 ± 0.20 | |
| OC3 | Izzikni | Amzray | June 2021 | ER-22-12 | 31°50′01.7″ N, 6°07′27.9″ W | 4.20 ± 0.30 | |
| OC4 | Izzikni | Imi ntisslmit | June 2021 | ER-20-13 | 31°50′18.3″ N, 6°06′07.7″ W | 3.45 ± 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouknin, M.; Alahyane, H.; Costa, J.; Majidi, L. Chemical Composition, Antioxidant and Anti-Enzymatic Activities, and In Vitro Insecticidal Potential of Origanum compactum (Benth.) Essential Oils. Plants 2024, 13, 2424. https://doi.org/10.3390/plants13172424
Ouknin M, Alahyane H, Costa J, Majidi L. Chemical Composition, Antioxidant and Anti-Enzymatic Activities, and In Vitro Insecticidal Potential of Origanum compactum (Benth.) Essential Oils. Plants. 2024; 13(17):2424. https://doi.org/10.3390/plants13172424
Chicago/Turabian StyleOuknin, Mohamed, Hassan Alahyane, Jean Costa, and Lhou Majidi. 2024. "Chemical Composition, Antioxidant and Anti-Enzymatic Activities, and In Vitro Insecticidal Potential of Origanum compactum (Benth.) Essential Oils" Plants 13, no. 17: 2424. https://doi.org/10.3390/plants13172424






