Metabolomics Revealed the Tolerance and Growth Dynamics of Arbuscular Mycorrhizal Fungi (AMF) to Soil Salinity in Licorice
Abstract
:1. Introduction
2. Results
2.1. Licorice Growth Dynamics and Root AMF Inoculation Index Changes
2.2. Root System Architecture Analysis
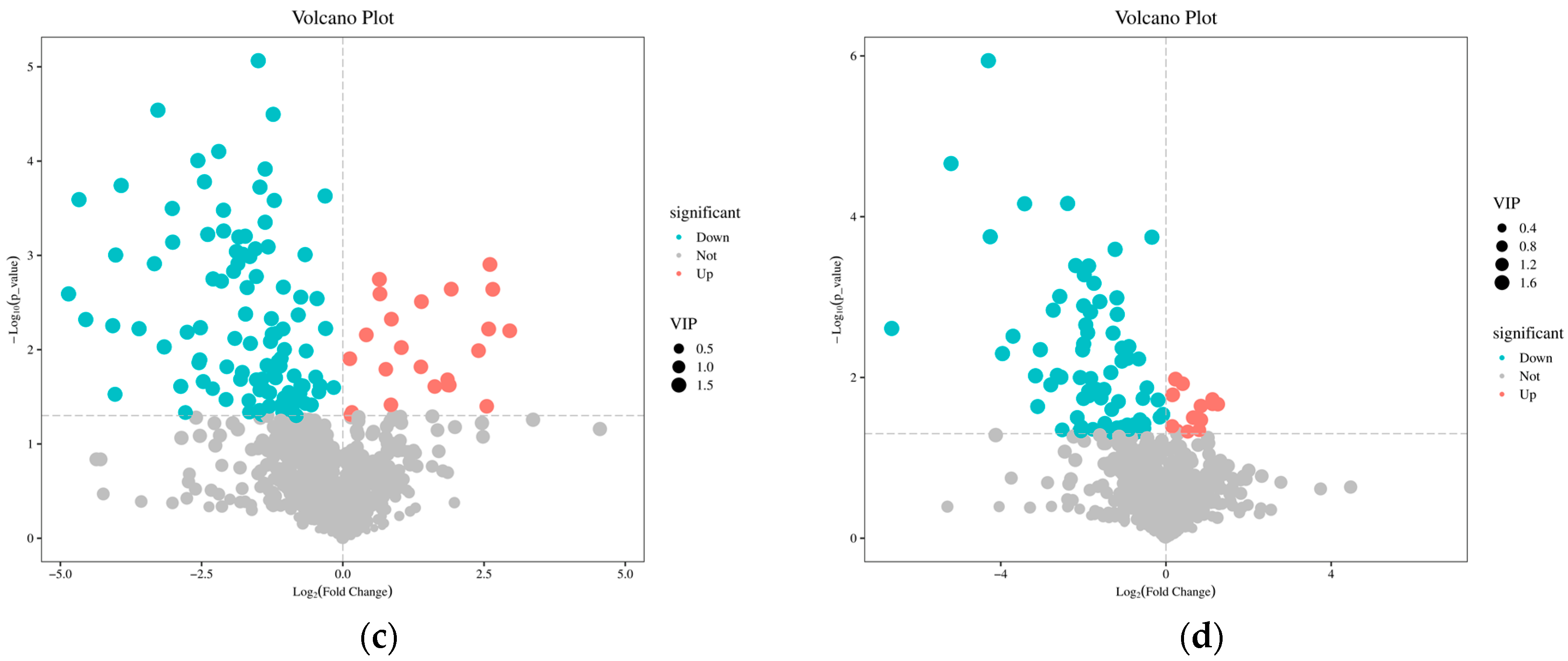
2.3. Metabolite Detection
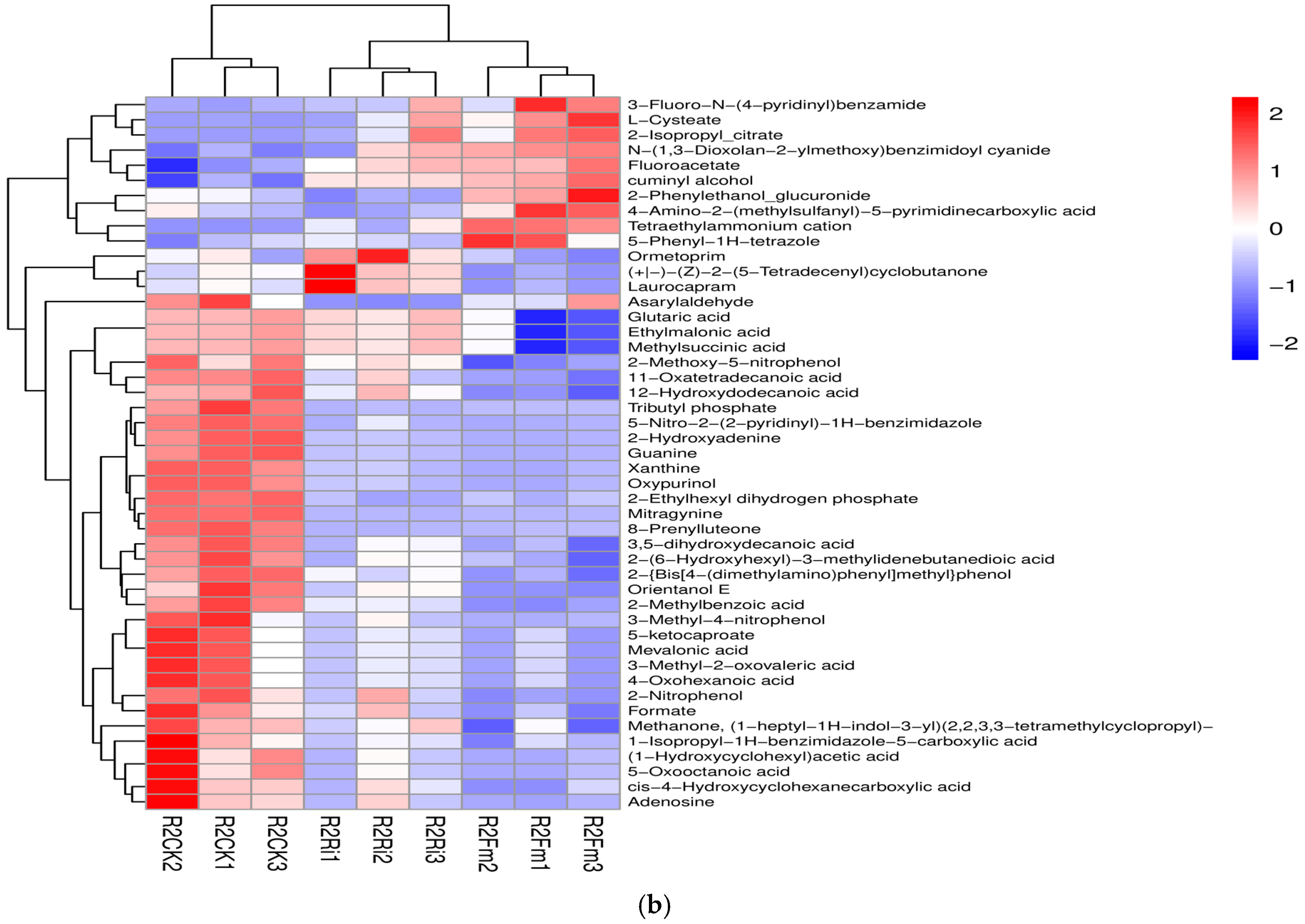
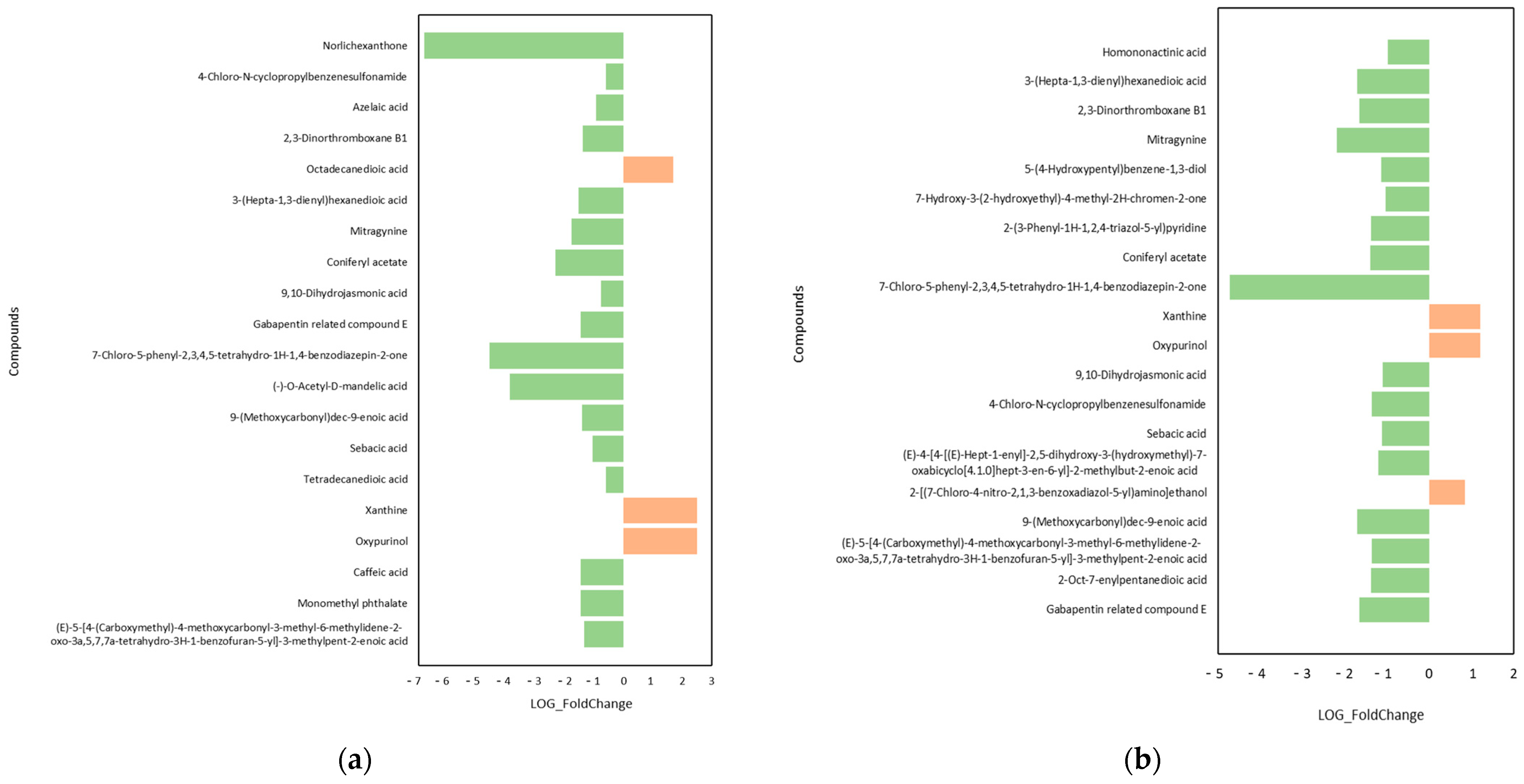
2.4. Changes in Root Differential Metabolites
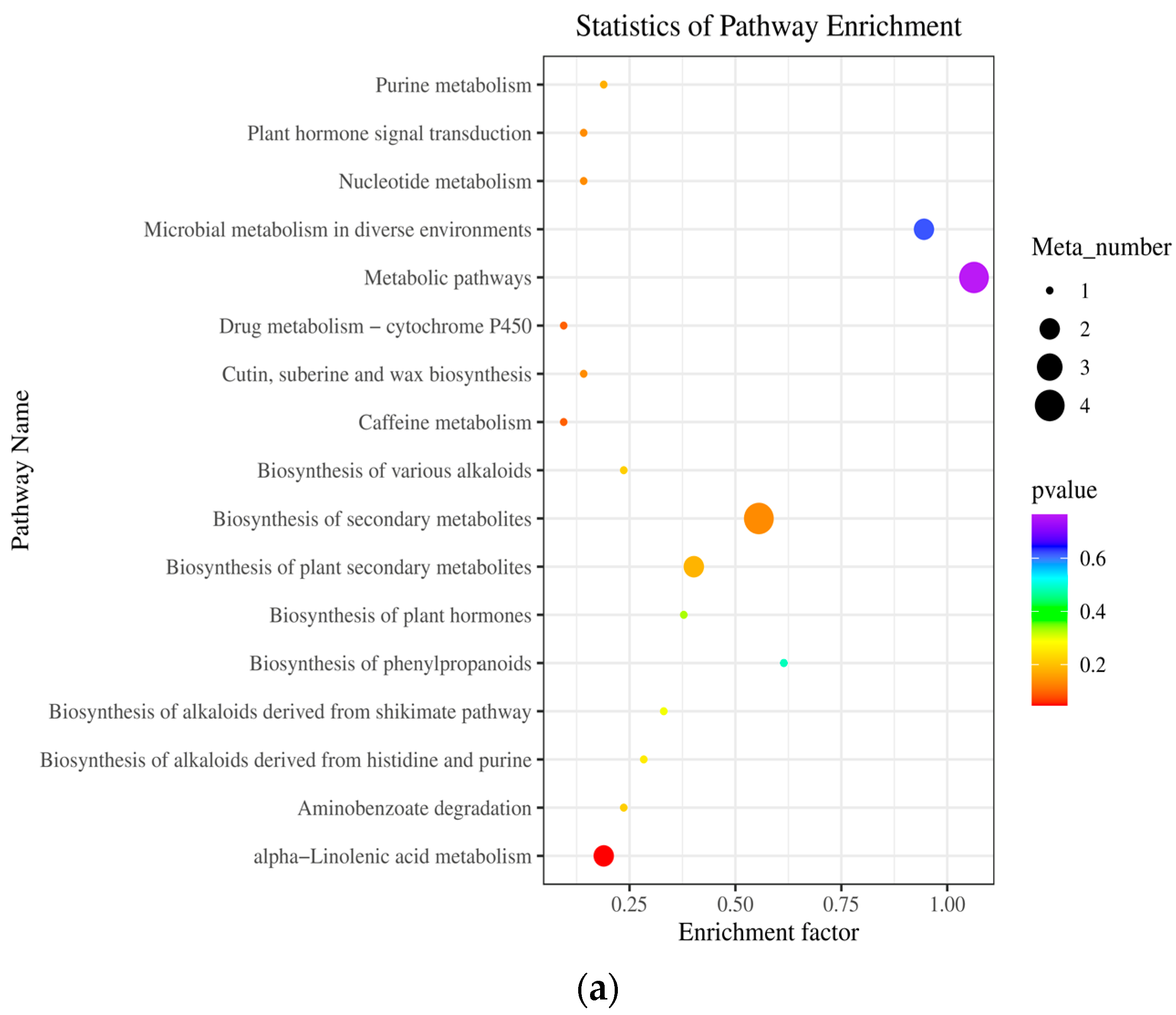
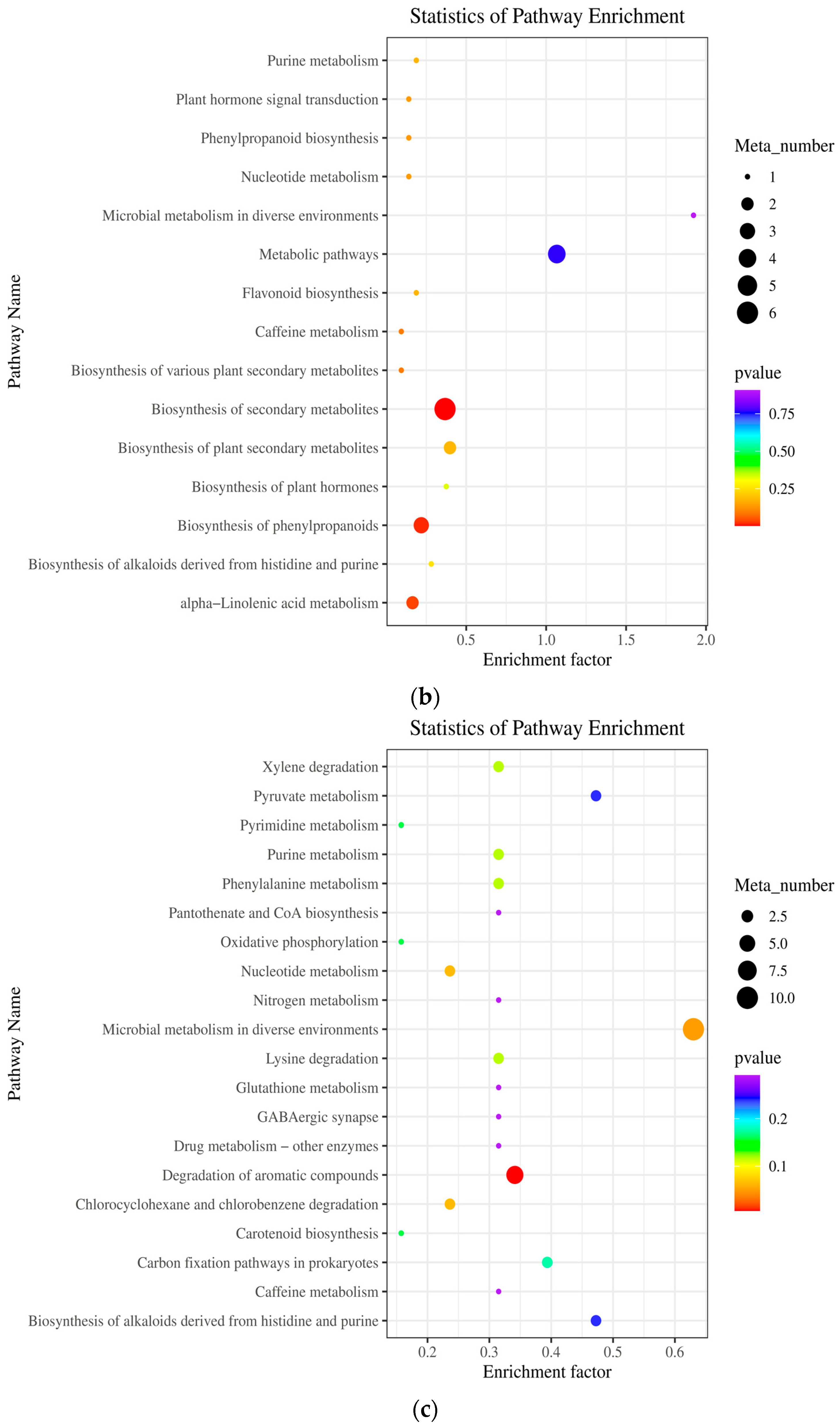
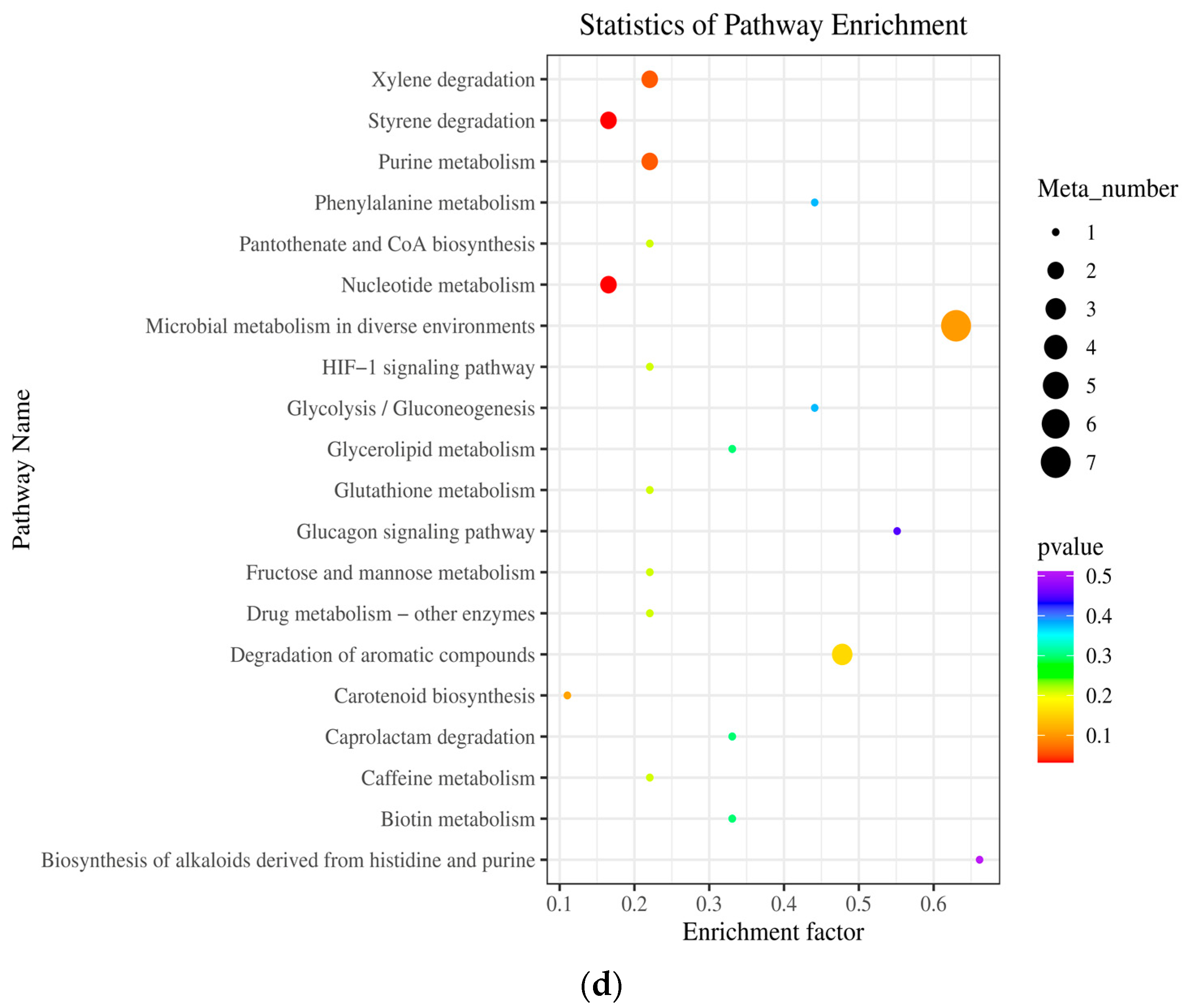
2.5. Metabolic Pathway Analysis (KEGG) of Differential Metabolites
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Plant Culture
4.3. Determinations of Root AMF Colonization Rate, Biomass Production, and Root System Architecture
4.4. Sample Extraction for Metabolomics Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mao, Q.L.; Wang, S. Brief analysis on the trend of improve saliane alkali soil in China. Hubei Agric. Sci. 2020, 59, 302–306. [Google Scholar] [CrossRef]
- Wang, J.L.; Han, N.N.; Feng, W.Z.; Jiang, L.M.; Zhao, C.K. Research on technology and equipment of soda-saline-alkali land remediation in Northeast China. Agric. Technol. 2018, 38, 14. [Google Scholar]
- Wei, X.B. Technique of Planting Alfalfa in Saline-Alkali Land in the West of Songnen Plains. Master’s Dissertation, Chinese Academy of Agricultural Sciences Dissertation, Beijing, China, 2013. [Google Scholar]
- Pan, Y.; Wu, L.J.; Yu, Z.L. Effect of salt and drought stress on antioxidant enzymes activities and SOD isoenzymes of liquorice (Glycyrrhiza uralensis Fisch). Plant Growth Regul. 2006, 49, 157–165. [Google Scholar] [CrossRef]
- Xu, K.P.; Liu, X.B. Summary and exploration of saline and alkaline land improvement programme in Hebao irrigation area. Gansu Sci. Technol. 2021, 37, 41–42+24. [Google Scholar]
- Brundrett, M.C.; Tedersoo, L. Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 2018, 220, 1108–1115. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Li, T.; Hu, Y.J.; Hao, Z.P.; Li, H.; Wang, Y.S.; Chen, B.D. First cloning and characterization of two functional aquaporin genes from an arbuscular mycorrhizal fungus Glomus intraradices. New Phytol. 2013, 197, 617–630. [Google Scholar] [CrossRef]
- Guerrero-Galán, C.; Calvo-Polanco, M.; Zimmermann, S.D. Ectomycorrhizal symbiosis helps plants to challenge salt stress conditions. Mycorrhiza 2019, 29, 291–301. [Google Scholar] [CrossRef]
- Kakouridis, A.; Hagen, J.A.; Kan, M.P.; Mambelli, S.; Feldman, L.J.; Herman, D.J.; Weber, P.K.; Pett-Ridge, J.; Firestone, M.K. Routes to roots: Direct evidence of water transport by arbuscular mycorrhizal fungi to host plants. New Phytol. 2022, 236, 210–221. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Zobel, M. How mycorrhizal associations drive plant population and community biology. Science 2020, 367, e1223. [Google Scholar] [CrossRef]
- Deng, M.; Hu, S.; Guo, L.; Jiang, L.; Huang, Y.; Schmid, B.; Liu, C.; Chang, P.; Li, S.; Liu, X.; et al. Tree mycorrhizal association types control biodiversity-productivity relationship in a subtropical forest. Sci. Adv. 2023, 9, e4468. [Google Scholar] [CrossRef]
- Luo, S.; Phillips, R.P.; Jo, I.; Fei, S.; Liang, J.; Schmid, B.; Eisenhauer, N. Higher productivity in forests with mixed mycorrhizal strategies. Nat. Commun. 2023, 14, 1377. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, P.; Ferlian, O.; Huang, Y.; Luo, S.; Quosh, J.; Eisenhauer, N. Tree diversity effects on productivity depend on mycorrhizae and life strategies in a temperate forest experiment. Ecology 2023, 104, e3896. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.B.; Xiong, R.C. Basics and Practice of Liquorice Cultivation; China Agricultural Science and Technology Press: Beijing, China, 2015; Volume 12. [Google Scholar]
- Lee, H.H.; Lee, S.; Shin, Y.S.; Cho, M.; Kang, H.; Cho, H. Anti-Cancer effect of quercetin in xenograft models with EBV-associated human gastric carcinoma. Molecules 2016, 21, 1286. [Google Scholar] [CrossRef] [PubMed]
- Huan, C.; Xu, Y.; Zhang, W.; Guo, T.; Pan, H.; Gao, S. Research progress on the antiviral activity of glycyrrhizin and its Derivatives in liquorice. Front. Pharmacol. 2021, 12, 680674. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Lu, J.H. Taxonomy and Experimental Biology of the Genus glycyrrhiza L.; Fudan Press: Shanghai, China, 2015. [Google Scholar]
- Zhao, K.F.; Fan, H.; Ungar, I.A. Survey of halophyte species in China. Plant Sci. 2002, 163, 491–498. [Google Scholar] [CrossRef]
- Shen, Z.; Pu, X.; Wang, S.; Dong, X.; Cheng, X.; Cheng, M. Silicon improves ion homeostasis and growth of liquorice under salt stress by reducing plant Na+ uptake. Sci. Rep. 2022, 12, 5089. [Google Scholar] [CrossRef]
- Zhang, S.B.; Chen, L.; Wang, Q.; Wang, Y.-S.; Jing, W.-M. Effects of AM fungi on introduction and cultivation of seven rare and endangered plants in arid region. Arid Land Geogr. 2017, 40, 780–786. [Google Scholar] [CrossRef]
- Parihar, M.; Rakshit, A.; Rana, K.; Tiwari, G.; Jatav, S.S. The Effect of Arbuscular Mycorrhizal Fungi Inoculation in Mitigating Salt Stress of Pea (Pisum sativum L.). Commun. Soil Sci. Plant Anal. 2020, 51, 1545–1559. [Google Scholar] [CrossRef]
- Liu, X.S.; Sun, Y.L.; An, X.X. Effects of phosphorus application and inoculation with arbuscular mycorrhizal fungi and phosphorus-solubilizing bacteria on the photosynthetic characteristics and biomass of alfalfa. Acta Pratacult. Sin. 2023, 32, 189–199. [Google Scholar] [CrossRef]
- Wang, Y.H.; Dong, F.X.; Tang, M. Transcriptome analysis of arbuscular mycorrhizal Casuarina glauca in damage mitigation of roots on NaCl stress. Microorganisms 2022, 10, 15. [Google Scholar] [CrossRef]
- Ma, S.; Zhu, L.; Wang, J.; Liu, X.; Jia, Z.; Li, C.; Liu, J.; Zeng, J.; Zhang, J. Arbuscular mycorrhizal fungi promote Gleditsia sinensis Lam. root growth under salt stress by regulating nutrient uptake and physiology. Forests 2022, 13, 688. [Google Scholar] [CrossRef]
- Caruso, T.; Mafrica, R.; Bruno, M.; Vescio, R.; Sorgonà, A. Root architectural traits of rooted cuttings of two fig cultivars: Treatments with arbuscular mycorrhizal fungi formulation. Sci. Hortic. 2021, 283, 110083. [Google Scholar] [CrossRef]
- Zixuan, W.; Tiantian, X.; Yaru, W.; Jieyan, Y.; Xiuqing, Y. Growth promotion and mechanism of arbuscular mycorrhizal fungi (AMF) on Ammopiptanthus mongolicus seedlings. Arid Zone Res. 2023, 40, 78–89. [Google Scholar] [CrossRef]
- Nie, H.; Chen, H.; Li, G.; Su, K.; Song, M.; Duan, Z.; Li, X.; Cao, X.; Huang, J.; Huang, S.; et al. Comparison of flavonoids and phenylpropanoids compounds in Chinese water chestnut processed with different methods. Food Chem. 2021, 335, 127662. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, W.; Wang, Y.; Zhang, L.; Huang, S.; Lin, J. Metabolomics analysis reveals the alkali tolerance mechanism in Puccinellia tenuiflora plants inoculated with arbuscular mycorrhizal fungi. Microorganisms 2020, 8, 327. [Google Scholar] [CrossRef]
- Liang, S.M.; Zhang, F.; Zou, Y.N.; Kuča, K.; Wu, Q.S. Metabolomics analysis reveals drought responses of trifoliate orange by arbuscular mycorrhizal fungi with a focus on terpenoid profile. Front. Plant Sci. 2021, 12, 740524. [Google Scholar] [CrossRef]
- Cui, L.; Zhu, Y.; Zhao, T.; Yang, J.; Wu, J. Soil ion components and soil salts transport in frozen layer in seasonal freezing-thawing areas. Trans. Chin. Soc. Agric. Eng. 2019, 35, 75–82. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Guo, W.J.; Zhang, Z.L.; Liu, Q.; Yin, H.J. Research progress of root exudates collection technology. Chin. J. Appl. Ecol. 2019, 30, 3951–3962. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0—The Human Metabolome Database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef]
- Hattori, M.; Tanaka, N.; Kanehisa, M.; Goto, S. SIMCOMP/SUBCOMP: Chemical structure search servers for network analyses. Nucleic Acids Res 2010, 38, W652–W656. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Chong, J.; Li, S.; Xia, J. MetaboAnalystR 3.0: Toward an Optimized Workflow for Global Metabolomics. Metabolites 2020, 10, 186. [Google Scholar] [CrossRef] [PubMed]

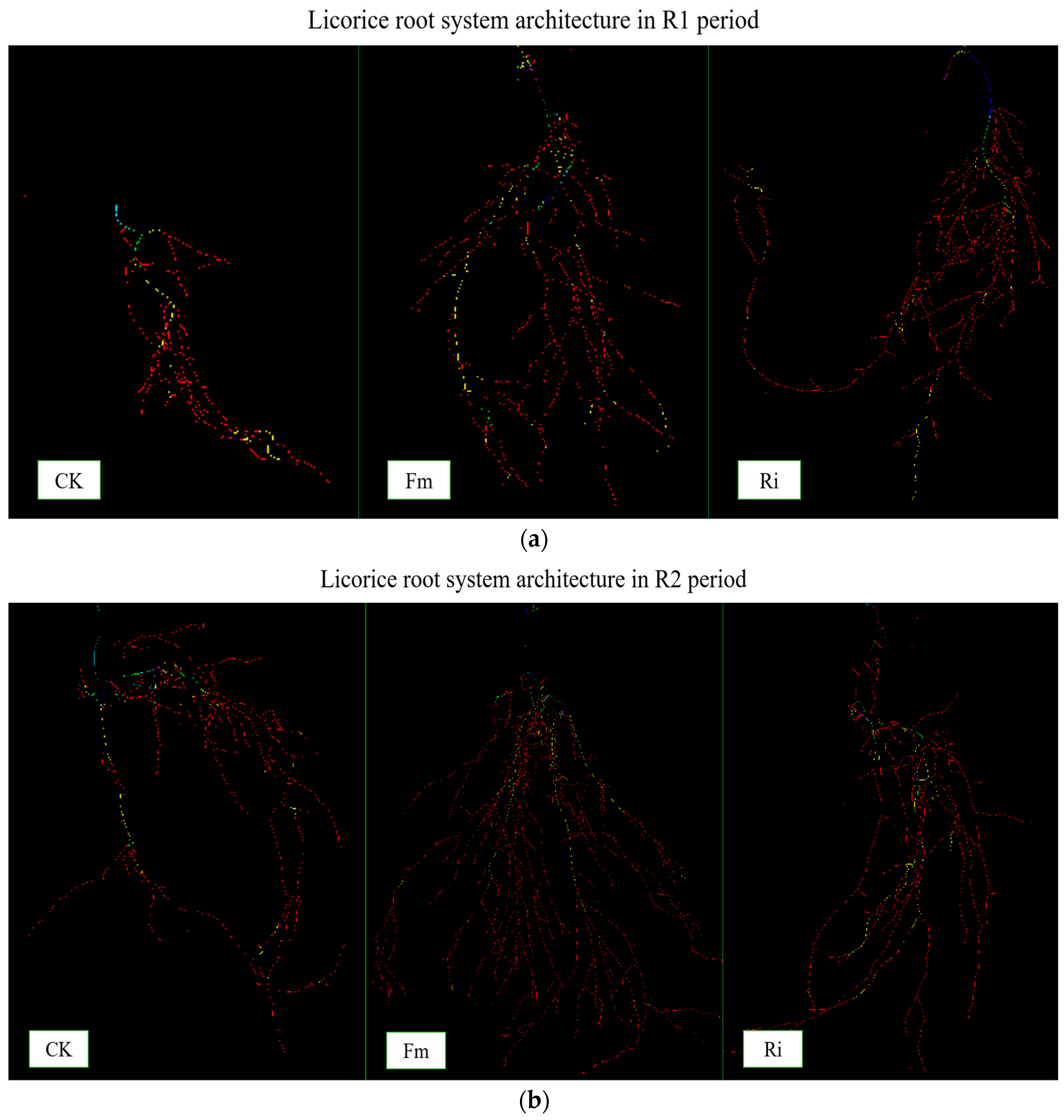




| Parameters | Treatments | Treatment Time/Period | |
|---|---|---|---|
| R1 | R2 | ||
| Root total length (cm) | CK | 24.52 ± 2.39 c | 86.35 ± 2.92 c |
| Fm | 47.35 ± 5.61 a | 177.46 ± 18.51 a | |
| Ri | 35.93 ± 1.86 b | 120.85 ± 16.30 b | |
| Root average diameter (mm) | CK | 0.37 ± 0.04 b | 0.57 ± 0.07 b |
| Fm | 0.50 ± 0.08 a | 0.84 ± 0.05 a | |
| Ri | 0.46 ± 0.05 ab | 0.72 ± 0.07 a | |
| Root total surface area (cm2) | CK | 3.84 ± 0.22 c | 14.85 ± 2.00 b |
| Fm | 13.01 ± 2.28 a | 28.61 ± 4.86 a | |
| Ri | 7.10 ± 1.33 b | 16.82 ± 1.05 b | |
| Root volume (cm3) | CK | 0.06 ± 0.01 c | 0.25 ± 0.03 c |
| Fm | 0.21 ± 0.02 a | 0.54 ± 0.08 a | |
| Ri | 0.14 ± 0.03 b | 0.38 ± 0.03 b | |
| Number of root branches (n.) | CK | 56.00 ± 14.53 b | 218.00 ± 35.00 c |
| Fm | 98.67 ± 21.55 a | 495.33 ± 30.73 a | |
| Ri | 81.33 ± 5.13 ab | 319.00 ± 31.00 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, L.; Zhang, C.; Li, J.; Zhao, Z.; Liu, Y. Metabolomics Revealed the Tolerance and Growth Dynamics of Arbuscular Mycorrhizal Fungi (AMF) to Soil Salinity in Licorice. Plants 2024, 13, 2652. https://doi.org/10.3390/plants13182652
Fan L, Zhang C, Li J, Zhao Z, Liu Y. Metabolomics Revealed the Tolerance and Growth Dynamics of Arbuscular Mycorrhizal Fungi (AMF) to Soil Salinity in Licorice. Plants. 2024; 13(18):2652. https://doi.org/10.3390/plants13182652
Chicago/Turabian StyleFan, Li, Chen Zhang, Jiafeng Li, Zhongtao Zhao, and Yan Liu. 2024. "Metabolomics Revealed the Tolerance and Growth Dynamics of Arbuscular Mycorrhizal Fungi (AMF) to Soil Salinity in Licorice" Plants 13, no. 18: 2652. https://doi.org/10.3390/plants13182652





