Abiotic Stress Effect on Agastache mexicana subsp. mexicana Yield: Cultivated in Two Contrasting Environments with Organic Nutrition and Artificial Shading
Abstract
:1. Introduction
- (a)
- Percentage of cations;
- (b)
- Ratios of Ca/Mg, Ca/K, and Mg/K;
- (c)
- A relatively high CEC;
- (d)
- Naturally high soil pH (above 6).
2. Materials and Methods
2.1. Experiment Location and Soil Characteristics
2.2. Plant Material
- The seeds underwent chemical priming pre-treatment for germination (Citomax®), involving a solution with a dose of 1 mL per 1 L of distilled water, and were soaked for 24 h. The Supplementary Materials (see Table S1) provides further details regarding the hormonal components of the germination pre-treatment;
- The seeds were placed inside a laboratory in sterilized box Petri dishes on a double layer of paper towel moistened with distilled water;
- Seeds were subsequently watered on their surface by sprinkling until they were covered with a solution of GA3 at 200 ppm (or 200 mg) per liter of water for 4 days until their germination and transformation into seedlings;
- Once the seedlings had cotyledons (four leaves), they were sown in a tray with 200 cavities in a substrate of Canadian peat and vermiculite mixed in a 3/2 ratio, respectively, until they transformed into plants. During this process, the maximum and minimum temperature and RH were recorded as 33.9 and 28.4 °C and 61.4 and 51.2%, respectively;
- When the plants reached a height of 8 to 12 cm, they were immersed in a solution of water and rooting agent (at a proportion of 7 g/L) for 5 min. See Table S2 in the Supplementary Materials for further details about the chemical components of rooter treatment.
- 6.
- They were then transplanted into 15 L polyethylene bags (40 × 40 cm). The substrate was a mixture in a ratio of 50/50 v/v of humus of leaves and mountain clay loam soil of Taxco de Alarcón municipality. The analysis in the Supplementary Materials (see Table S3) indicates that the soil is loamy;
- 7.
- Soil analysis was performed. Refer to the Supplementary Materials for more information;
- 8.
- The Supplementary Materials, Figure S7, includes pictures of the seeds, seedlings, and plants.
2.3. Experimental Design
2.3.1. Input Factors and Irrigation
- (a)
- The plants were placed with Artificial Shading (AS) as follows:
- (b)
- Four treatments were carried out, two in every environment; where: = treatment one, = treatment two, = treatment three, = treatment four, as follows:
- (c)
- The Temperature and RH were measured every hour during the crop time with a programmed datalogger instrument in E1 and E2. See the results in Figure 1;
- (d)
- ON = Organic Nutrition or nutrition organic solution. A volumetric solution based on humic and fulvic acids was applied (2 mL/L of water). Note: According to the results of the analysis of the ingredient used for ON (commercial trade Hortihumus®), it was considered as mature compost due to: (a) C/N (Carbon/Nitrogen ratio) exceeding 15, (b) the quantity of organic matter, (c) the liquid state, (d) high fulvic and humic acids (12%). See Table S6 in the Supplementary Materials;
- (e)
- I = Irrigation, see Table 1.
2.3.2. Output Factors Measure
- (a)
- Plant height (HPl) from soil surface until the main stem’s apical meristem with a flexometer;
- (b)
- Leaf number (LN);
- (c)
- Internodes number (IN);
- (d)
- Branches number (BN);
- (e)
- The diameter of the stem (DT) was measured with a digital caliper (Vernier trademark SURTEK©, Minimum measuring range—Maximum measuring range = 1 mm–150 mm, Resolution = 0.01 mm) every 10 cm of length until the beginning of the inflorescence, based on the substrate surface. The measurements were made once only for every height in the vegetative development measurement throughout the time;
- (f)
- The Secondary Stem number (SSN) emerged from rhizomes
- (a)
- Inflorescence length (IL) was measured with a Truper© FH-8M, Flexmeter Gripper from the first whorl of the inflorescence until the last one (cm);
- (b)
- Chlorophyll content (ChC) (Quantity of chlorophyll content in a leaf). The reading was taken with a chlorophyll meter (Model SPAD-502, Trademark Konica Minolta©, SPAD value: Relative chlorophyll content index; −9.9 to 199.9, difference in optical density at two wavelengths: 650 nm and 940 nm), which quantitatively evaluates the intensity of leaf green always at 01:00 PM, obtaining measurements in three strata of the plant: low, middle and high stratum, in the vegetative and reproductive stages of the plant. The Chlorophyll 1 and 2 content (ChC1, ChC2, respectively) refer to two different measurement dates during the Crops time. Both environments were measured in February (ChC1) and April (ChC2). The reported value in this research corresponds to the middle stratum;
- (c)
- Fresh Weight (WF) (biomass of all plants, including roots, before drying);
- (d)
- Dry weight (WD) (biomass of all plants, including roots, after the dry process) were weighed on a granite scale model BASE-5EP, Trademark Truper©, Capacity 5 kg, Minimum division 1 g/0.01 oz, Minimum weight 10 g/0.1 oz, measurement units Grams (g)/Ounces (oz).
2.3.3. Statistical Methodology
2.3.4. Association Analysis of Input and Output Factors Using MATLAB Graphics
2.3.5. Mathematical Principle Applied in the Plot by Means of MATLAB
3. Results and Discussion
3.1. Statistical Results
3.2. Environmental Inputs and Agronomic Results Analysis by Means of MATLAB Plots

- (a)
- On the “y” axis, it is important to differentiate the difference between final and initial values as ΔY for each point on the “x” axis. Similarly, on the “x” axis, the ΔX should be considered as they represent the behavior of the population, the impact of nutrition, and the environment;
- (b)
- While analyzing ΔX and ΔY, it is important to pay attention to color change (LN values);
- (c)
- The length of the slope can be analyzed using the criteria and equations explained in Figure 2;
- (d)
- Pearson’s linear correlation coefficient has been calculated globally for the output factors of all treatments.
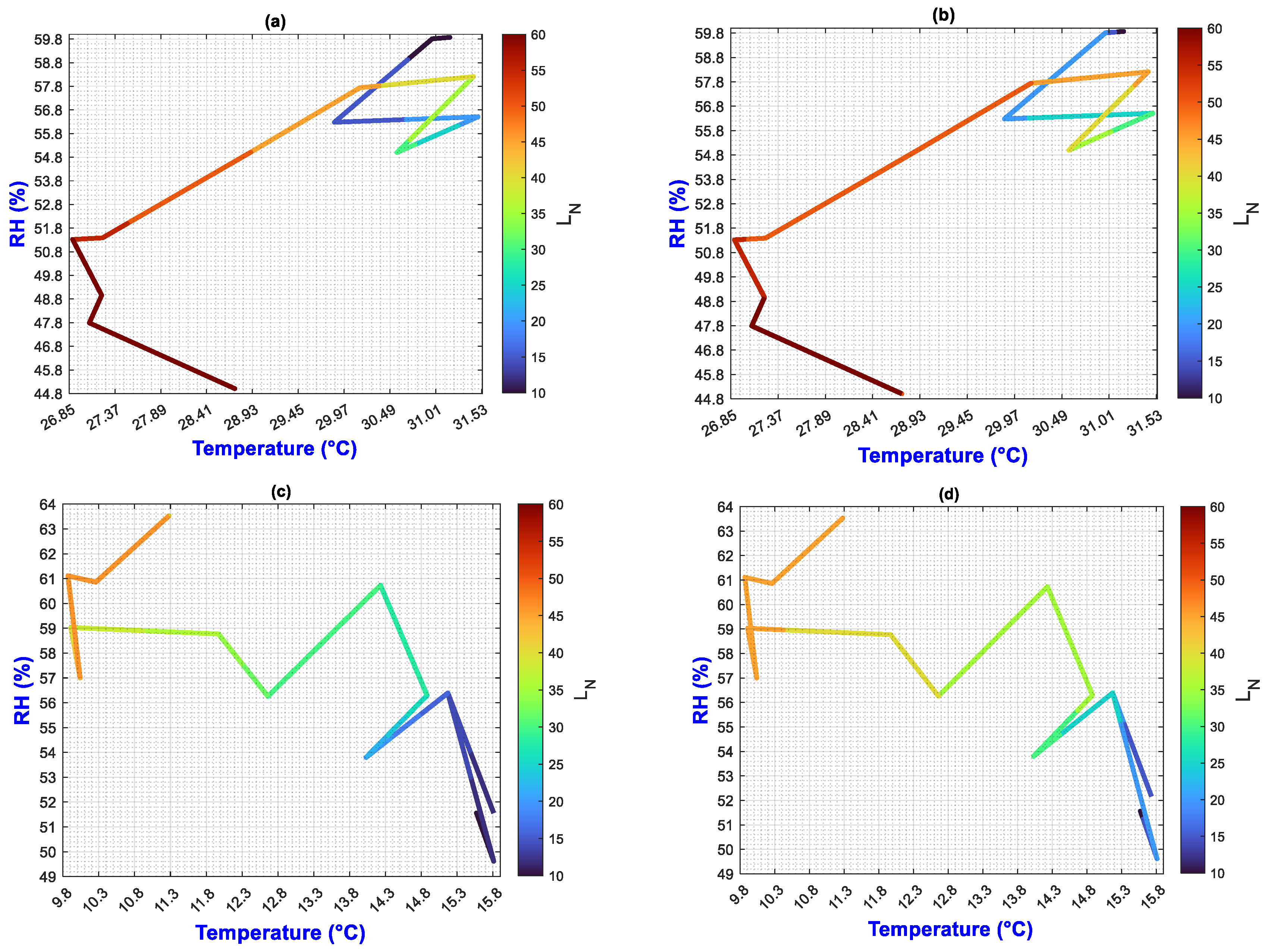
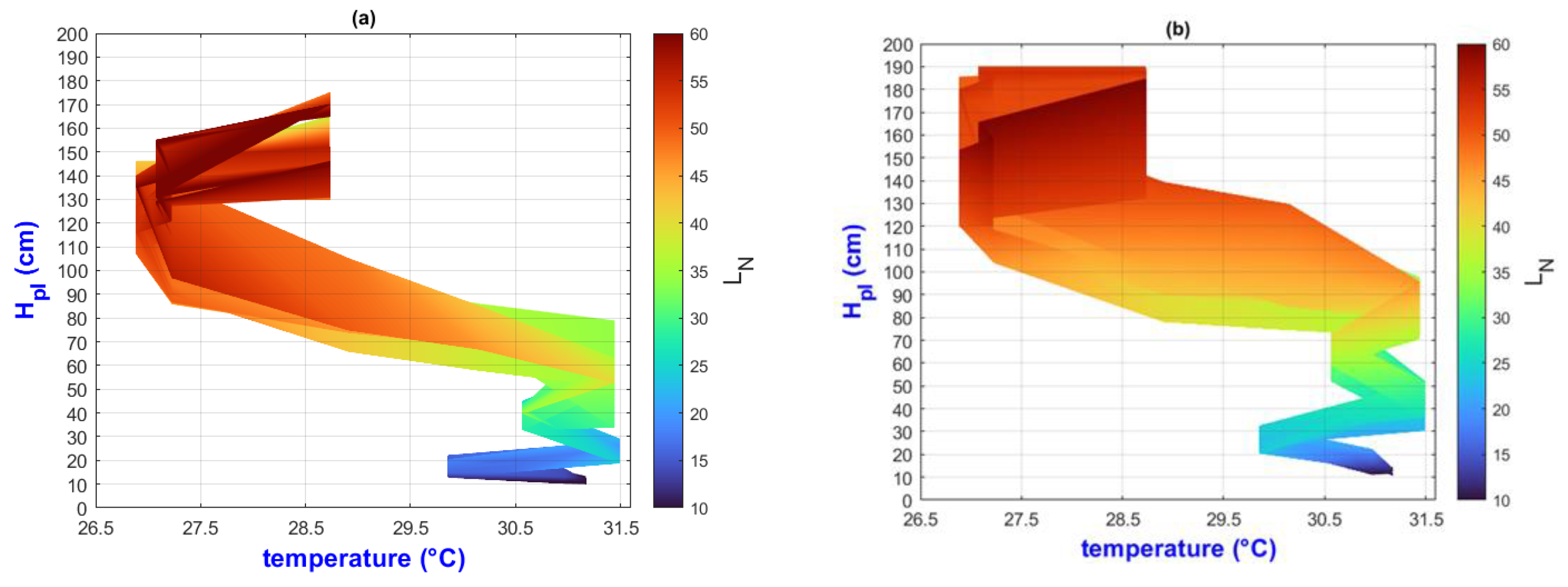
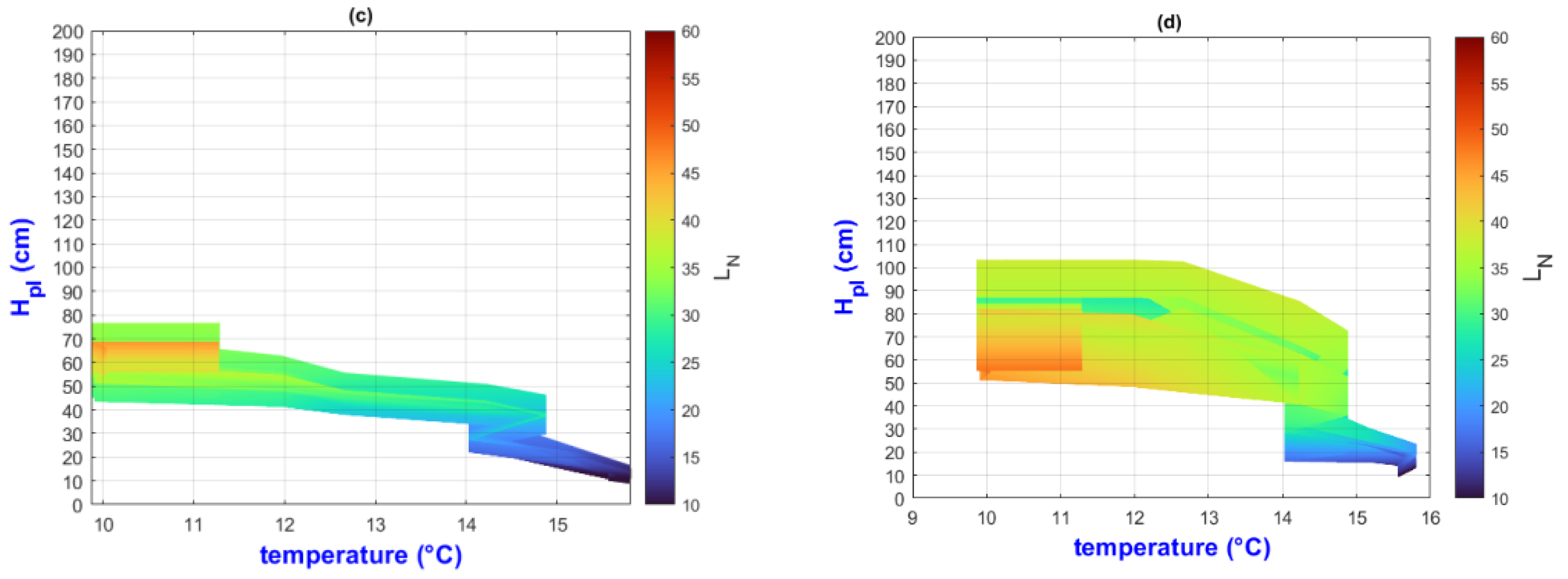
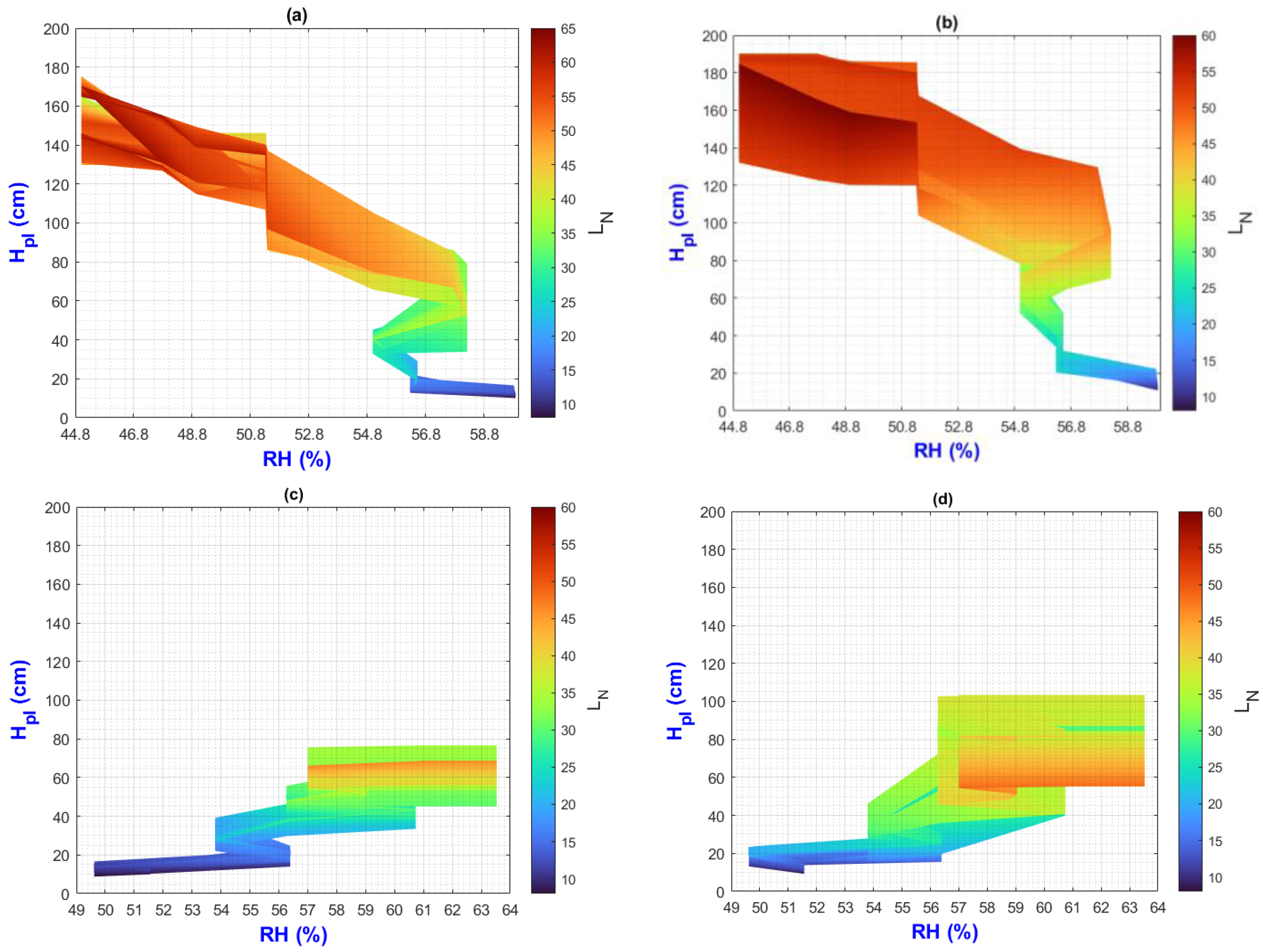
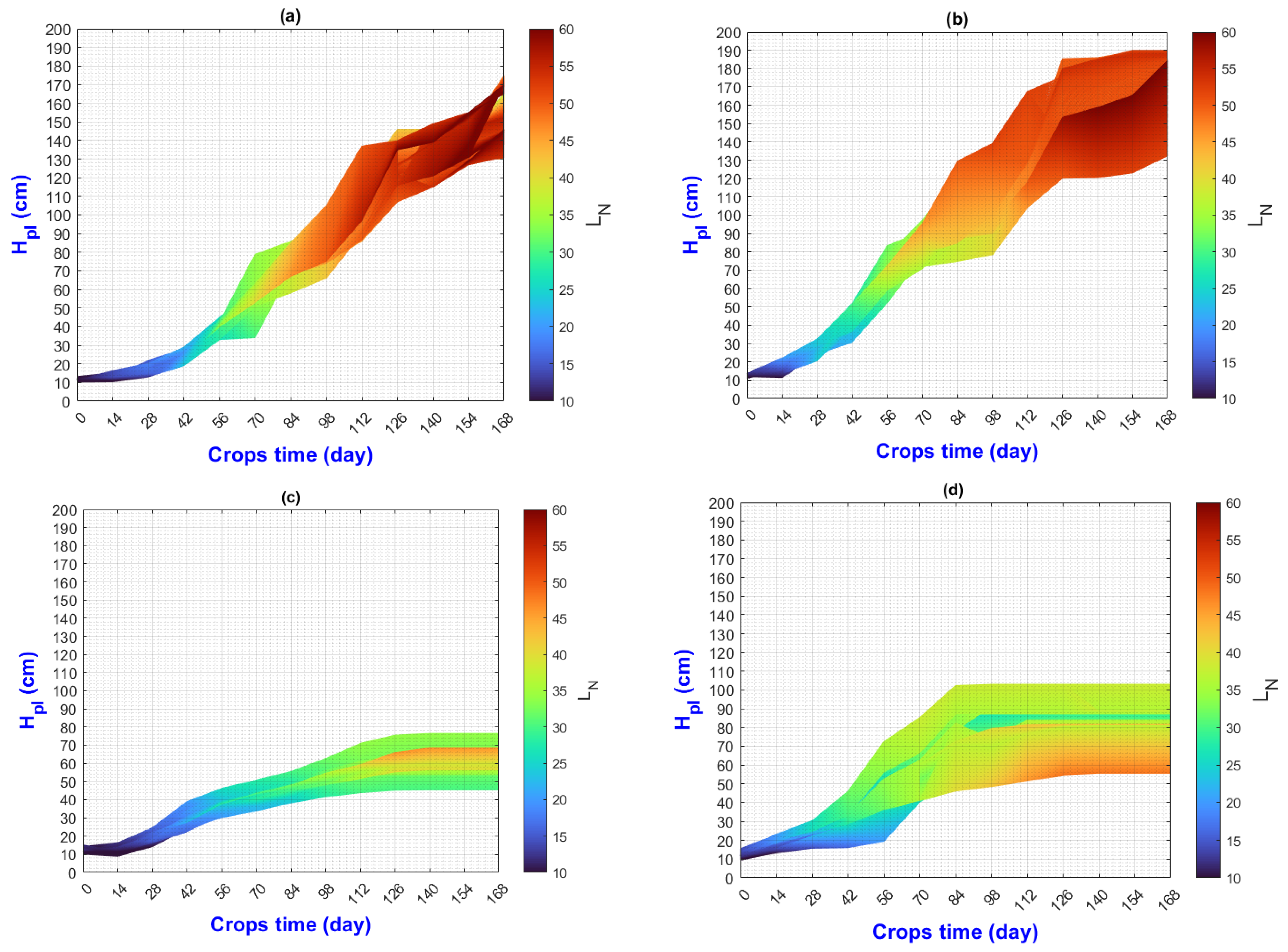
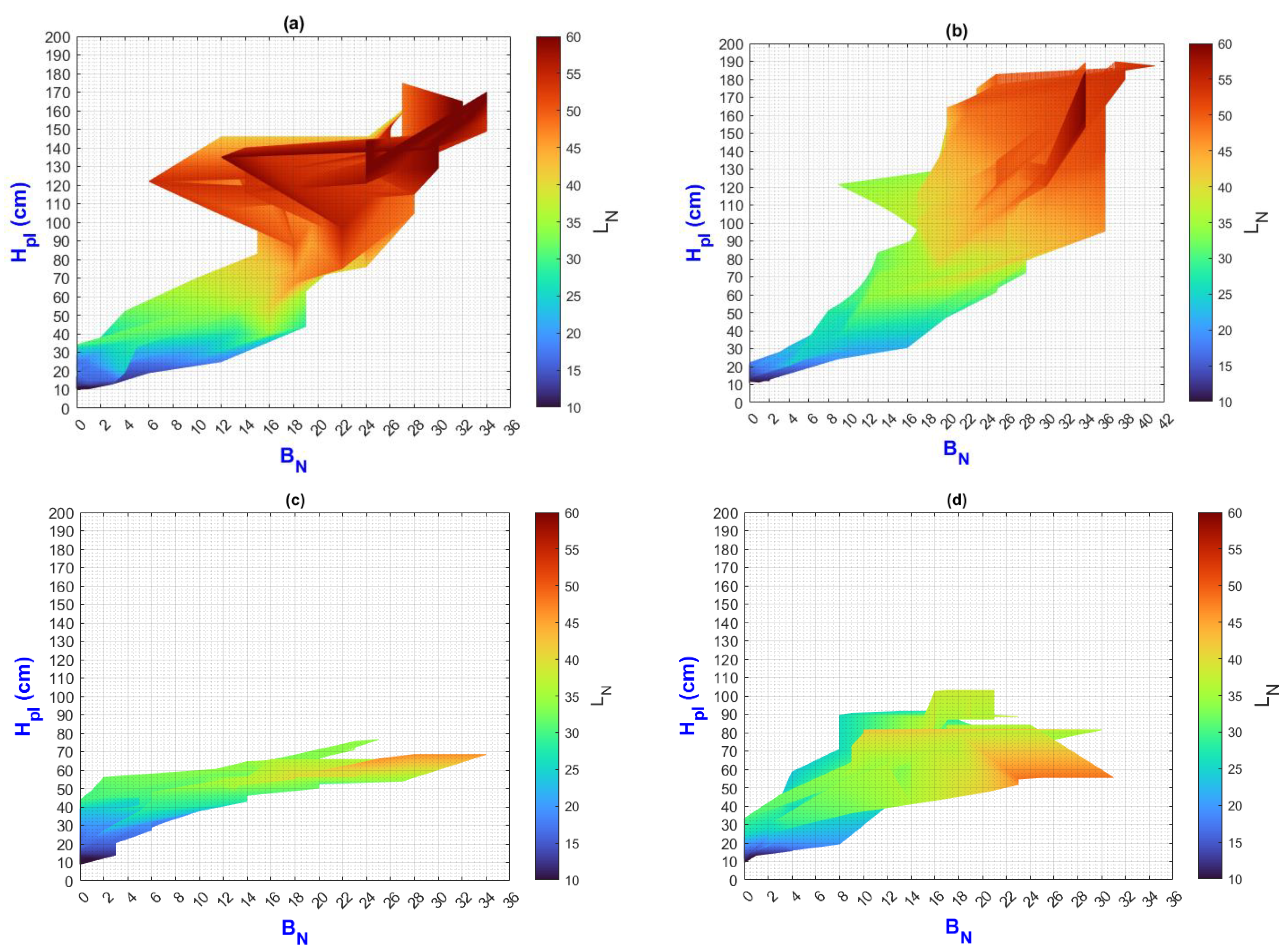
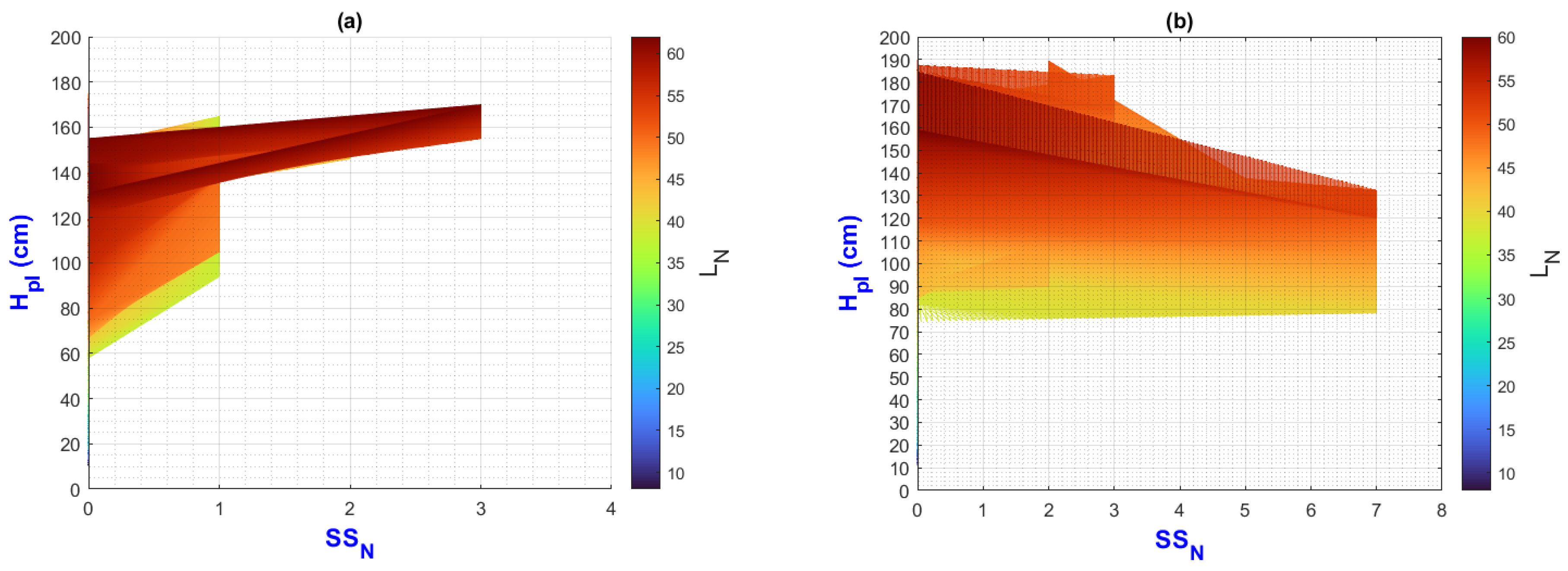
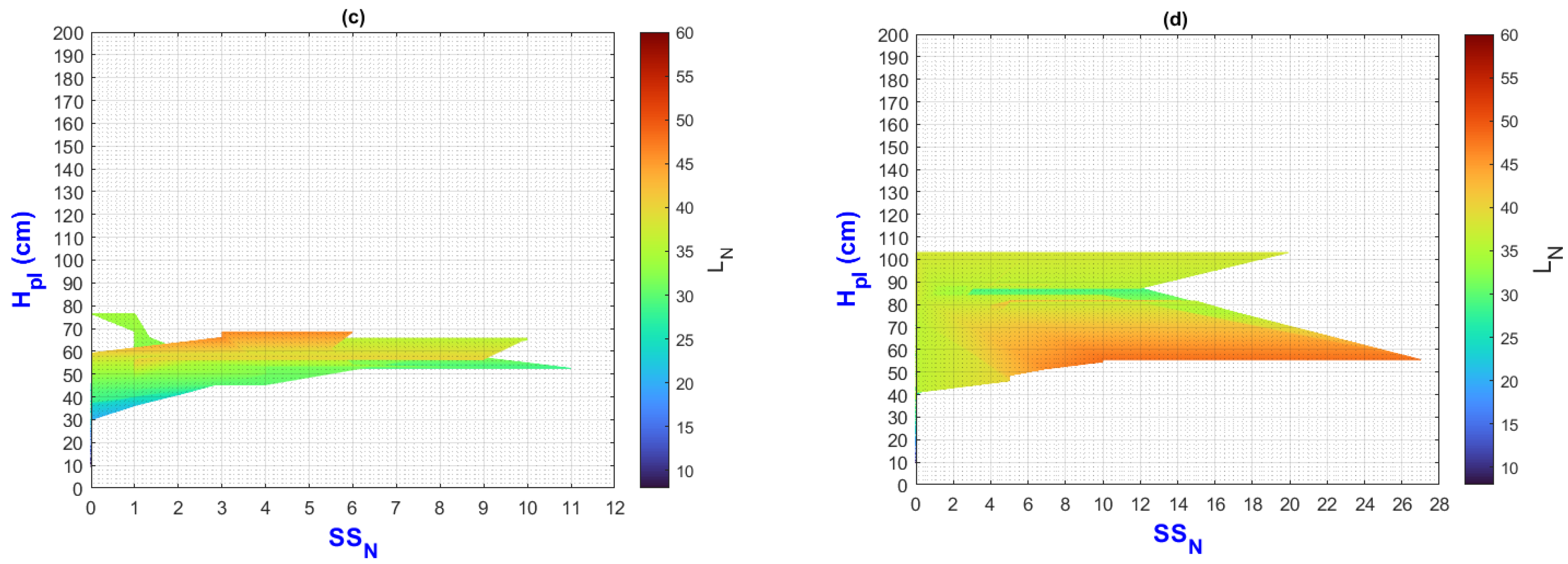

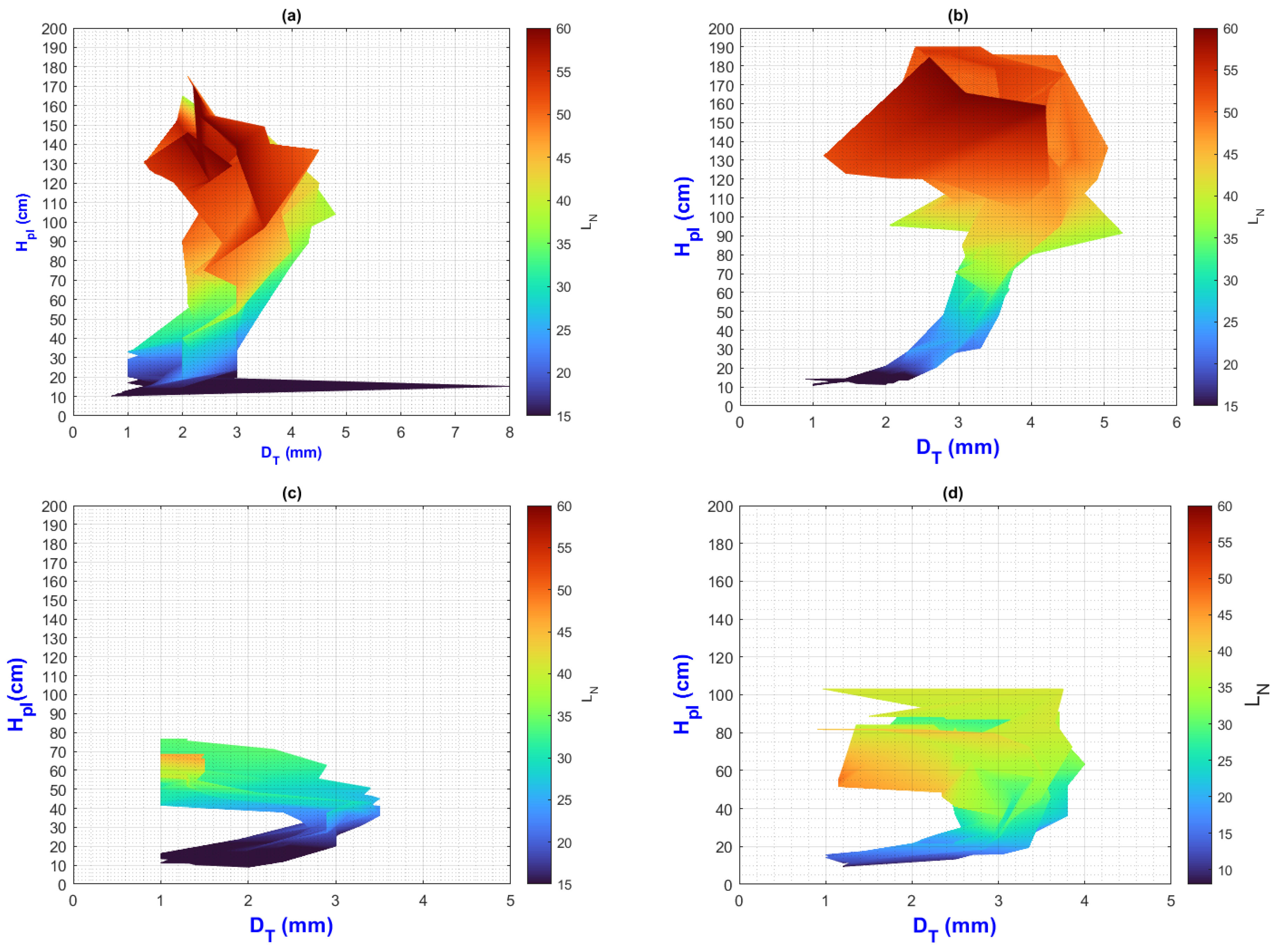
3.3. General Discussion
3.3.1. Germination and Comparison of Yields between Environments
3.3.2. Impact of Heat Stress in E1
3.3.3. Impact of Cold Stress in E2
3.3.4. Impact of BCSR Approach-ON in Yield and Soil Health
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martínez, M.A.; Montechiarini, N.H.; Gosparini, C.O.; Arango, M.R.; Gallo CD, V.; Craviotto, R.M. Viabilidad, vigor y germinación de semillas verdes de soja. Para Mejor. Prod. 2019, 58, 15–22. [Google Scholar]
- Tiwari, P.; Kumar, R. Effects of pre-sowing seed treatments on germination and seedling growth performance of Ocimum basilicum L. J. Pharmacogn. Phytochem. 2020, 9, 1401–1405. [Google Scholar]
- Mwase, W.F.; Mvula, T. Effect of seed size and pre-treatment methods of Bauhinia thonningii Schum. on germination and seedling growth. Afr. J. Biotechnol. 2011, 10, 5143–5148. [Google Scholar]
- Raei, Y.; Alami-Milani, M. Organic cultivation of medicinal plants: A review. J. Biodivers. Environ. Sci. 2014, 4, 6–18. [Google Scholar]
- Carrubba, A. Sustainable fertilization in medicinal and aromatic plants. In Medicinal and Aromatic Plants of the World: Scientific, Production, Commercial and Utilization Aspects; Springer: Dordrecht, The Netherlands, 2015; pp. 187–203. [Google Scholar]
- Karlen, D.L.; Mausbach, M.J.; Doran, J.W.; Cline, R.G.; Harris, R.F.; Schuman, G.E. Soil quality: A concept, definition, and framework for evaluation (a guest editorial). Soil Sci. Soc. Am. J. 1997, 61, 4–10. [Google Scholar] [CrossRef]
- Ferrante, A.; Mariani, L. Agronomic management for enhancing plant tolerance to abiotic stresses: High and low values of temperature, light intensity, and relative humidity. Horticulturae 2018, 4, 21. [Google Scholar] [CrossRef]
- Husen, A.; Iqbal, M. (Eds.) Medicinal Plants: Their Response to Abiotic Stress; Springer Nature: Singapore, 2023. [Google Scholar]
- Yadav, S.; Modi, P.; Dave, A.; Vijapura, A.; Patel, D.; Patel, M. Effect of abiotic stress on crops. Sustain. Crop Prod. 2020, 3, 5–16. [Google Scholar]
- Radulov, I.; Berbecea, A.; Sala, F.; Crista, F.; Lato, A. Mineral fertilization influence on soil pH, cationic exchange capacity and nutrient content. Res. J. Agric. Sci. 2011, 43, 160–165. [Google Scholar]
- Hodges, S.C. Soil Fertility Basics; Soil Science Extension North Carolina State University; North Carolina State University: Raleigh, NC, USA, 2010. [Google Scholar]
- Pernes-Debuyser, A.; Tessier, D. Soil physical properties affected by long-term fertilization. Eur. J. Soil Sci. 2004, 55, 505–512. [Google Scholar] [CrossRef]
- Cruz-Macías, W.O.; Rodríguez-Larramendi, L.A.; Salas-Marina M, Á.; Hernández-García, V.; Campos-Saldaña, R.A.; Chávez-Hernández, M.H.; Gordillo-Curiel, A. Efecto de la materia orgánica y la capacidad de intercambio catiónico en la acidez de suelos cultivados con maíz en dos regiones de Chiapas, México. Terra Latinoam. 2020, 38, 475–480. [Google Scholar] [CrossRef]
- Saha, N.; Mandal, B. Soil testing protocols for organic farming—Concept and approach. Commun. Soil Sci. Plant Anal. 2011, 42, 1422–1433. [Google Scholar] [CrossRef]
- Linder, K.J. The Effect of Soil Cation Balancing on Soil Properties and Weed Communities in an Organic Rotation. Master’s Thesis, The Ohio State University, Columbus, OH, USA, 2015. [Google Scholar]
- Kopittke, P.M.; Menzies, N.W. A review of the use of the basic cation saturation ratio and the “ideal” soil. Soil Sci. Soc. Am. J. 2007, 71, 259–265. [Google Scholar] [CrossRef]
- Chaganti, V.N.; Culman, S.W.; Herms, C.; Sprunger, C.D.; Brock, C.; Leiva Soto, A.; Doohan, D. Base cation saturation ratios, soil health, and yield in organic field crops. Agron. J. 2021, 113, 4190–4200. [Google Scholar] [CrossRef]
- Zalewska, M.; Nogalska, A.; Wierzbowska, J. Effect of basic cation saturation ratios in soil on yield of annual ryegrass (Lolium multiflorum L.). J. Elem. 2018, 23, 95–105. [Google Scholar] [CrossRef]
- Brock, C.; Jackson-Smith, D.; Culman, S.; Doohan, D.; Herms, C. Soil balancing within organic farming: Negotiating meanings and boundaries in an alternative agricultural community of practice. Agric. Hum. Values 2021, 38, 449–465. [Google Scholar] [CrossRef]
- Badalingappanavar, R.; Hanumanthappa, M.; Veeranna, H.K.; Kolakar, S.; Khidrapure, G. Organic fertilizer management in cultivation of medicinal and aromatic crops: A review. J. Pharmacogn. Phytochem. 2018, 7, 126–129. [Google Scholar]
- Hendawy, S.F.; Khalid, K.A. Effect of chemical and organic fertilizers on yield and essential oil of chamomile flower heads. Med. Aromat. Plant Sci. Biotechnol. 2011, 5, 43–48. [Google Scholar]
- Tan, K.H. Humic Matter in Soil and the Environment: Principles and Controversies; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Melo López, L. Análisis y caracterización de ácidos fúlvicos y su interacción con algunos metales pesados. 2006.
- Cooper, L.; Abi-Ghanem, R. El Valor de las Sustancias Húmicas en el Ciclo de Vida del Carbón de los Cultivos: Ácidos Húmicos, ácidos Fúlvicos; Huma Gro: Gilbert, AZ, USA, 2017; pp. 1–8. [Google Scholar]
- Veobides-Amador, H.; Guridi-Izquierdo, F.; Vázquez-Padrón, V. Las sustancias húmicas como bioestimulantes de plantas bajo condiciones de estrés ambiental. Cultiv. Trop. 2018, 39, 102–109. [Google Scholar]
- Haghighi, M.; Kafi, M.; Colmillo, P. Actividad fotosintética y metabolismo de N de la lechuga afectados por el ácido húmico. Rev. Int. Cienc. Veg. 2012, 18, 182–189. [Google Scholar]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- García, E. Modificaciones al Sistema de Clasificación Climática de Köppen; Universidad Nacional Autónoma de México: Mexico City, Mexico, 2005. [Google Scholar]
- Alcántara Jiménez, J.Á.; Hernandez Castro, E.; Ayvar Serna, S.; Nava, A.D.; Brito Guadarrama, T. Phenotypic and agronomic characteristics of six genotypes of papaya (Carica papaya L.) from Tuxpan, Guerrero, Mexico. Rev. Venez. Cienc. Tecnol. Aliment. 2010, 1, 35–46. [Google Scholar]
- Damián-Nava, A.; González-Hernández, V.A.; Sánchez-García, P.; Peña-Valdivia, C.B.; Livera-Munoz, M.; Brito-Guadarrama, T. Crecimiento y fenología del guayabo (Psidium guajava L.) cv.“Media China” en Iguala, Guerrero. Rev. Fitotec. Mex. 2004, 27, 349. [Google Scholar] [CrossRef]
- García, E. Modificaciones al Sistema de Clasificación Climática de Köppen; Universidad Nacional Autónoma de México: Mexico City, Mexico, 2004. [Google Scholar]
- Chávez-Hernández, C.G.; Barrera Aguilar, C.C.; Téllez Espinosa, G.J.; Chimal-Sánchez, E.; García-Sánchez, R. Colonización micorrízica y comunidades de hongos micorrizógenos arbusculares en plantas medicinales del bosque templado “Agua Escondida”, Taxco, Guerrero, México. Sci. Fungorum 2021, 51, e1325. [Google Scholar] [CrossRef]
- Gross, J.; Ligges, U. Nortest: Tests for Normality. R Package Version 1.0-4. 2015. Available online: https://cran.r-project.org/web/packages/nortest/ (accessed on 20 September 2023).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019; Available online: https://cran.r-project.org/web/packages/car/index.html (accessed on 20 September 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 20 September 2023).
- Felipe de Mendiburu. Agricolae: Statistical Procedures for Agricultural Research. R Package Version 1.3-5. 2021. Available online: https://cran.r-project.org/web/packages/agricolae/index.html (accessed on 20 September 2023).
- Longhi, D.A.; Dalcanton, F.; Aragão, G.M.F.D.; Carciofi, B.A.M.; Laurindo, J.B. Microbial growth models: A general mathematical approach to obtain μ max and λ parameters from sigmoidal empirical primary models. Braz. J. Chem. Eng. 2017, 34, 369–375. [Google Scholar] [CrossRef]
- Yuan, R.; Yang, B.; Liu, Y.; Huang, L. Modified Gompertz sigmoidal model removing fine-ending of grain-size distribution. Open Geosci. 2019, 11, 29–36. [Google Scholar] [CrossRef]
- Martiñón-Martínez, R.J.; Vargas-Hernández, J.; López-Upton, J.; Gómez-Guerrero, A.; Vaquera-Huerta, H. Respuesta de Pinus pinceana Gordon a estrés por sequía y altas temperaturas. Rev. Fitotec. Mex. 2010, 33, 239–248. [Google Scholar] [CrossRef]
- Saeedeh, R.; Mehrnaz, H.; Mansour, G. Silicon-nanoparticle mediated changes in seed germination and vigor index of marigold (Calendula officinalis L.) compared to silicate under PEG-induced drought stress. Gesunde Pflanz. 2021, 73, 575–589. [Google Scholar]
- Reséndiz-Muñoz, J.; Cruz-Lagunas, B.; Fernández-Muñoz, J.L.; de Jesús Adame-Zambrano, T.; Delgado-Núñez, E.J.; Zagaceta-Álvarez, M.T.; Aguilar-Cruz, K.A.; Urbieta-Parrazales, R.; Miranda-Viramontes, I.; Morales-Barrera, J.; et al. Influence of artificial shading and SiO2 on Agastache mexicana subsp. mexicana Ability to survive under water stress. Horticulturae 2023, 9, 995. [Google Scholar] [CrossRef]
- Retana-Cordero, M.; Fisher, P.R.; Gómez, C. Modeling the effect of temperature on ginger and turmeric rhizome sprouting. Agronomy 2021, 11, 1931. [Google Scholar] [CrossRef]
- Smit, M.A. Characterising the factors that affect germination and emergence in sugarcane. Int. Sugar J. 2011, 113, 65. [Google Scholar]
- Passioura, J.B. Roots and drought resistance. In Developments in Agricultural and Managed Forest Ecology; Elsevier: Amsterdam, The Netherlands, 1983; Volume 12, pp. 265–280. [Google Scholar]
- Dougherty, B. Regenerative Agriculture: The Path to Healing Agroecosystems and Feeding the World in the 21st Century; A Report for Nuffield International Farming Scholars, Nuffield International Project; Nuffield International: Moama, NSW, Australia, 2019; Volume 45. [Google Scholar]
- Garrido Valero, M. Interpretación de Análisis de Suelos. 1994. Available online: https://www.mapa.gob.es/ministerio/pags/biblioteca/hojas/hd_1993_05.pdf (accessed on 20 September 2023).
- FAO. Organización de las Naciones Unidas Para la Alimentación y Agricultura. Textura del Suelo. 2019. Available online: https://www.fao.org/fishery/docs/CDrom/FAO_Training/FAO_Training/General/x6706s/x6706s06.htm (accessed on 20 September 2023).
- Yildirim, E. Foliar and soil fertilization of humic acid affect productivity and quality of tomato. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2007, 57, 182–186. [Google Scholar] [CrossRef]
- Abdel-Baky, Y.R.; Abouziena, H.F.; Amin, A.A.; Rashad El-Sh, M.; Abd El-Sttar, A.M. Improve quality and productivity of some faba bean cultivars with foliar application of fulvic acid. Bull. Natl. Res. Cent. 2019, 43, 2. [Google Scholar] [CrossRef]
- Varas Abad, P.L. Evaluación de Dosis de Ácido Húmico Granulado de Leonardita y Ácidos Húmicos y Fúlvicos con Macro y Micro Nutrientes en el Cultivo de Cebollita China (Var. Roja chiclayana), Bajo Condiciones Agroecológicas en la Provincia de Lamas; Universidad Nacional de San Martín: Buenos Aires, Argentina, 2012. [Google Scholar]
- Available online: https://agrawdata.com/blog/suelos-francos/ (accessed on 20 September 2023).
- Blevins, R.L.; Frye, W.W. Conservation tillage: An ecological approach to soil management. Adv. Agron. 1993, 51, 33–78. [Google Scholar]
| Stage | Total Irrigation per Week | |||
|---|---|---|---|---|
| 1 | 3 L water | 2 L water + ON | 2 L water | 1 L water + ON |
| 2 | 5.5 L water | 4.5 L water + ON | 2 L water | 1 L water + ON |
| 3 | 7 L water | 6 L water + ON | 3 L water | 2 L water + ON |
| 4 | 7 L water | 6 L water + ON | 3.5 L water | 2.5 L water + ON |
| Concept | Output Factors p-Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| WF | WD | ChC1 | ChC2 | HPl | LN | IN | BN | DT | SSN | ||
| ANOVA Assumptions | N | 0.05 | 0.08 | 0.25 | 0.84 | 0.48 | 0.30 | 0.30 | 0.91 | 0.79 | 0.14 |
| H | 0.21 | 0.17 | 0.67 | 0.95 | 0.90 | 0.19 | 0.19 | 0.39 | 0.27 | 0.57 | |
| Box-Cox Parameter | 0 | 0.50 | −2.40 | 7.60 | 0 | -- | -- | -- | −0.30 | 0.39 | |
| R-squared | % | 75% | 83% | 65% | 5% | 89% | 65% | 66% | 29% | 50% | 71% |
| Treatment ANOVA | SSTr | 9.55 | 293.96 | 1.08 × 10−8 | 1.26 × 102 | 7.61 | 2692 | 673.0 | 414.88 | 2.57 | 172.26 |
| TMS | 3.18 | 97.99 | 3.60 × 10−9 | 4.19 × 10−2 | 2.54 | 897.3 | 224.3 | 138.29 | 0.86 | 57.42 | |
| 36.74 | 57.55 | 21.8 | 0.61 | 96.05 | 22.9 | 22.9 | 4.84 | 12.12 | 29.5 | ||
| P | 4.7 × 10−11 | 8.2 × 10−14 | 3.1 × 10−8 | 0.61 | <2.2 × 10−16 | 1.8 × 10−8 | 1.8 × 10−8 | 6.2 × 10−3 | 1.2 × 10−5 | 8.3 × 10−10 | |
| Residuals ANOVA | RSS | 3.12 | 61.30 | 5.93 × 10−9 | 2.50 × 10−4 | 0.95 | 1410.4 | 352.6 | 1028.9 | 2.55 | 70.07 |
| RMS | 0.09 | 1.70 | 1.65 × 10−10 | 6.83 × 10−2 | 0.026 | 39.18 | 9.8 | 28.58 | 0.07 | 1.95 | |
| T | Box-Cox Transformation | ||||||
|---|---|---|---|---|---|---|---|
| WF | WD | HPl | Chc1 | ChC2 | DS | SSN | |
| Ec | |||||||
| Sample Mean | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WF | WD | ChC1 | ChC2 | HPl | LN | IN | BN | DT | SSN | |
| O | 67.05 | 20.63 | 41.7 | 45.7 | 118.1 | 43.8 | 21.9 | 25.8 | 2.0 | 6.2 |
| 83.1 a | 28.5 a | 37.3 b | 44.6 b | 157.6 a | 53.2 a | 26.6 a | 27.3 ab | 2.4 a | 1.0 c | |
| 103.0 a | 34.9 a | 35.7 b | 46.4 b | 170.3 a | 50.6 a | 25.3 a | 30.4 a | 2.8 a | 2.0 c | |
| 28.2 c | 6.3 c | 44.8 a | 46.1 a | 59.8c | 34.4 b | 17.2 b | 22.6 b | 1.3 b | 7.1 b | |
| 53.9 b | 12.8 b | 49.1 a | 45.9 a | 84.6b | 37.0 b | 18.5 b | 23.0 b | 1.5 b | 14.8 a | |
| #Isoline/Concept | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.21 | 3.7 | 1.65 | 1.77 | 3.23 | 1.45 | 7 | 0.62 | 2.45 | 1.18 | 3.23 | |
| 0.3 | 3.18 | 0.14 | 1.64 | 3.51 | 0.52 | 2.17 | 0.11 | 7.36 | 8.36 | 1.68 | |
| 1 | 1.6 | 2.6 | 3.2 | 3.8 | 3.9 | 4.8 | 4.8 | 4.8 | 4.8 | 4.8 | |
| 1.6 | 2.0 | 2.2 | 3 | 3.2 | 3.4 | 3.4 | 4.2 | 4.4 | 4.4 | 4.4 |
| #Isoline/Concept | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4.85 | 4.62 | 2.67 | 2.83 | 4.44 | 4.74 | 2.60 | 2.10 | 2.03 | 4.11 | 0.46 | 2.95 | |
| 12.07 | 7.16 | 1.12 | 2.27 | 4.62 | 2.85 | 3.64 | 0.13 | 15.61 | 24.17 | 0.64 | 2.71 | |
| 1.0 | 1.6 | 1.2 | 1.6 | 2.2 | 3.0 | 3.2 | 3.6 | 4.0 | 4.6 | 4.6 | 4.6 | |
| 1.3 | 1.1 | 1.3 | 1.4 | 2.2 | 2.7 | 2.8 | 3.0 | 3 | 3 | 3 | 3 |
| Yield and Productivity | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Environment | Tr | Hpl (cm) | LN (#) | WF (g) | WD (g) | Bloom (%) | IL (cm) | IL/Hpl | WD/WF |
| E1 | 157.6 | 53.2 | 83.1 | 28.5 | 90 | 24.5 | 0.155 | 0.343 | |
| 170.3 | 50.6 | 103.0 | 34.9 | 100 | 26 | 0.153 | 0.339 | ||
| E2 | 59.8 | 34.4 | 28.2 | 6.3 | 20 | 1 | 0.017 | 0.223 | |
| 84.62 | 37.0 | 53.9 | 12.8 | 100 | 11.3 | 0.134 | 0.237 | ||
| E1 | 319.46 | 103.8 | 186.1 | 63.4 | 95 | 50.5 | 0.308 | 0.681 | |
| E2 | 144.39 | 71.4 | 82.1 | 19.1 | 60 | 12.3 | 0.150 | 0.460 | |
| E2/E1 | 0.44 | 0.69 | 0.44 | 0.30 | 63 | 0.24 | 0.49 | 0.68 | |
| Ratios between Treatments | |||||||||
| Concept/Output | Ratio | Hpl (cm) | LN (#) | WF (g) | WD (g) | Bloom (%) | IL (cm) | IL/Hpl | WD/WF |
| E1 | 0.93 | 1.05 | 0.81 | 0.82 | 0.90 | 0.94 | 1.01 | 1.01 | |
| E2 | 0.71 | 0.93 | 0.52 | 0.49 | 0.20 | 0.09 | 0.13 | 0.94 | |
| ON | 0.50 | 0.73 | 0.52 | 0.37 | 1.00 | 0.43 | 0.88 | 0.70 | |
| BCSR | 0.38 | 0.65 | 0.34 | 0.22 | 0.22 | 0.04 | 0.11 | 0.65 | |
| Concept/ Treatment | MSS (#) | SSS (#) | TS (#) | WMS (mg) | WSS (mg) | TW (mg) | MSSW (mg) | SSSW (mg) | AWpS (mg) | Sp1gr (#) |
|---|---|---|---|---|---|---|---|---|---|---|
| 94 | 204 | 298 | 5.2 | 39.0 | 44.2 | 0.055 | 0.191 | 0.148 | 5621 | |
| 68 | 626 | 694 | 13.9 | 149.7 | 163.6 | 0.204 | 0.239 | 0.236 | 4242 | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 55 | 0 | 55 | 13.9 | 0 | 13.9 | 0.253 | 0 | 0.253 | 3957 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Barrera, J.; Reséndiz-Muñoz, J.; Cruz-Lagunas, B.; Fernández-Muñoz, J.L.; Godínez-Jaimes, F.; de Jesús Adame-Zambrano, T.; Vázquez-Villamar, M.; Romero-Rosales, T.; Zagaceta-Álvarez, M.T.; Aguilar-Cruz, K.A.; et al. Abiotic Stress Effect on Agastache mexicana subsp. mexicana Yield: Cultivated in Two Contrasting Environments with Organic Nutrition and Artificial Shading. Plants 2024, 13, 2661. https://doi.org/10.3390/plants13182661
Morales-Barrera J, Reséndiz-Muñoz J, Cruz-Lagunas B, Fernández-Muñoz JL, Godínez-Jaimes F, de Jesús Adame-Zambrano T, Vázquez-Villamar M, Romero-Rosales T, Zagaceta-Álvarez MT, Aguilar-Cruz KA, et al. Abiotic Stress Effect on Agastache mexicana subsp. mexicana Yield: Cultivated in Two Contrasting Environments with Organic Nutrition and Artificial Shading. Plants. 2024; 13(18):2661. https://doi.org/10.3390/plants13182661
Chicago/Turabian StyleMorales-Barrera, Judith, Juan Reséndiz-Muñoz, Blas Cruz-Lagunas, José Luis Fernández-Muñoz, Flaviano Godínez-Jaimes, Tania de Jesús Adame-Zambrano, Mirna Vázquez-Villamar, Teollincacihuatl Romero-Rosales, María Teresa Zagaceta-Álvarez, Karen Alicia Aguilar-Cruz, and et al. 2024. "Abiotic Stress Effect on Agastache mexicana subsp. mexicana Yield: Cultivated in Two Contrasting Environments with Organic Nutrition and Artificial Shading" Plants 13, no. 18: 2661. https://doi.org/10.3390/plants13182661






