Genetic Diversity of Tulipa alberti and T. greigii Populations from Kazakhstan Based on Application of Expressed Sequence Tag Simple Sequence Repeat Markers
Abstract
:1. Introduction
2. Results
2.1. Polymorphism of Tested EST-SSR Markers
2.2. Genetic Diversity in the Collected Populations of Tulipa alberti and Tulipa greigii
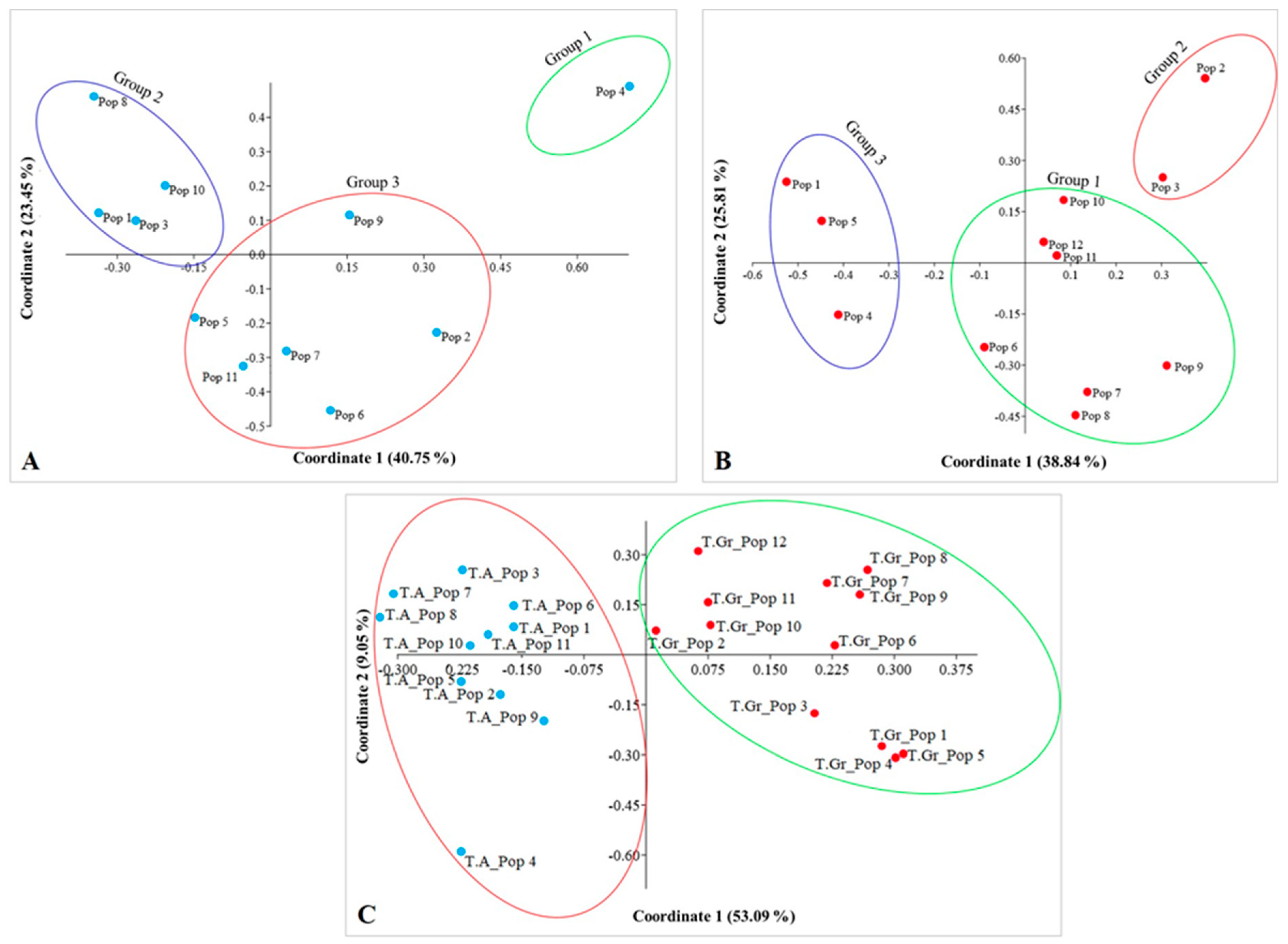
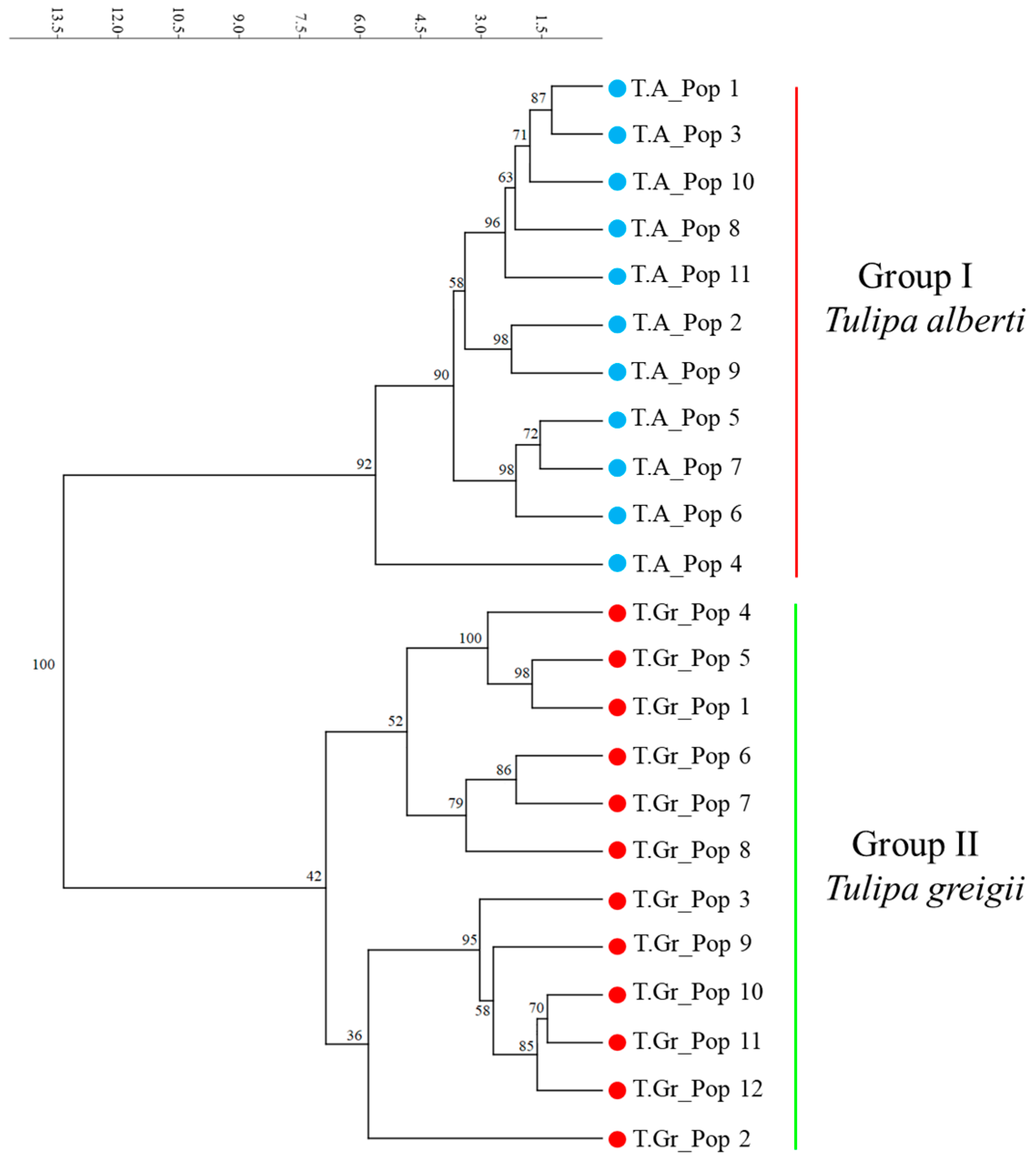
2.3. Genetic Differentiation and Gene Flow among Populations of Tulipa alberti and Tulipa greigii Species
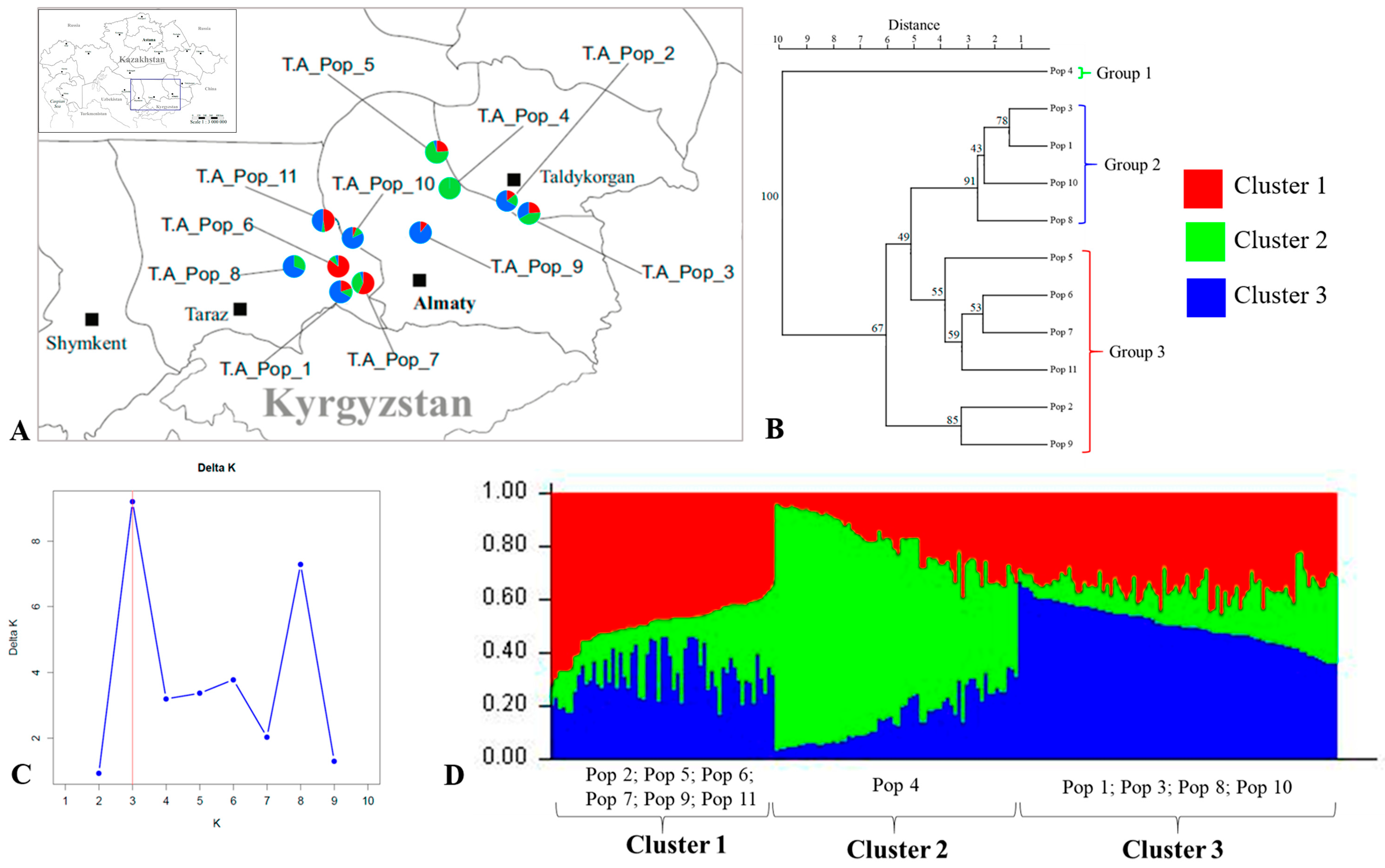
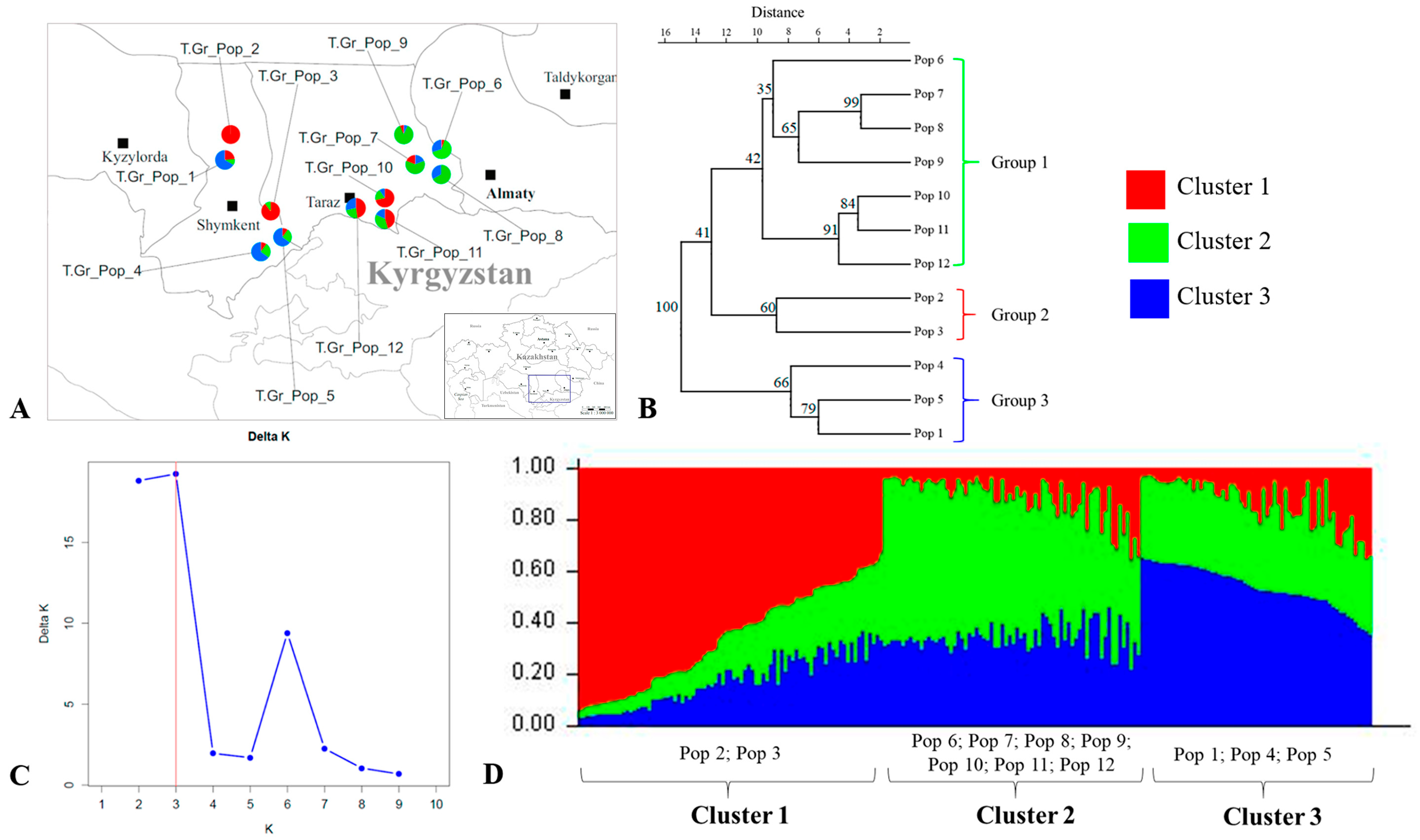
2.4. Genetic Structure of Tulipa alberti and Tulipa greigii Populations
3. Discussion
4. Materials and Methods
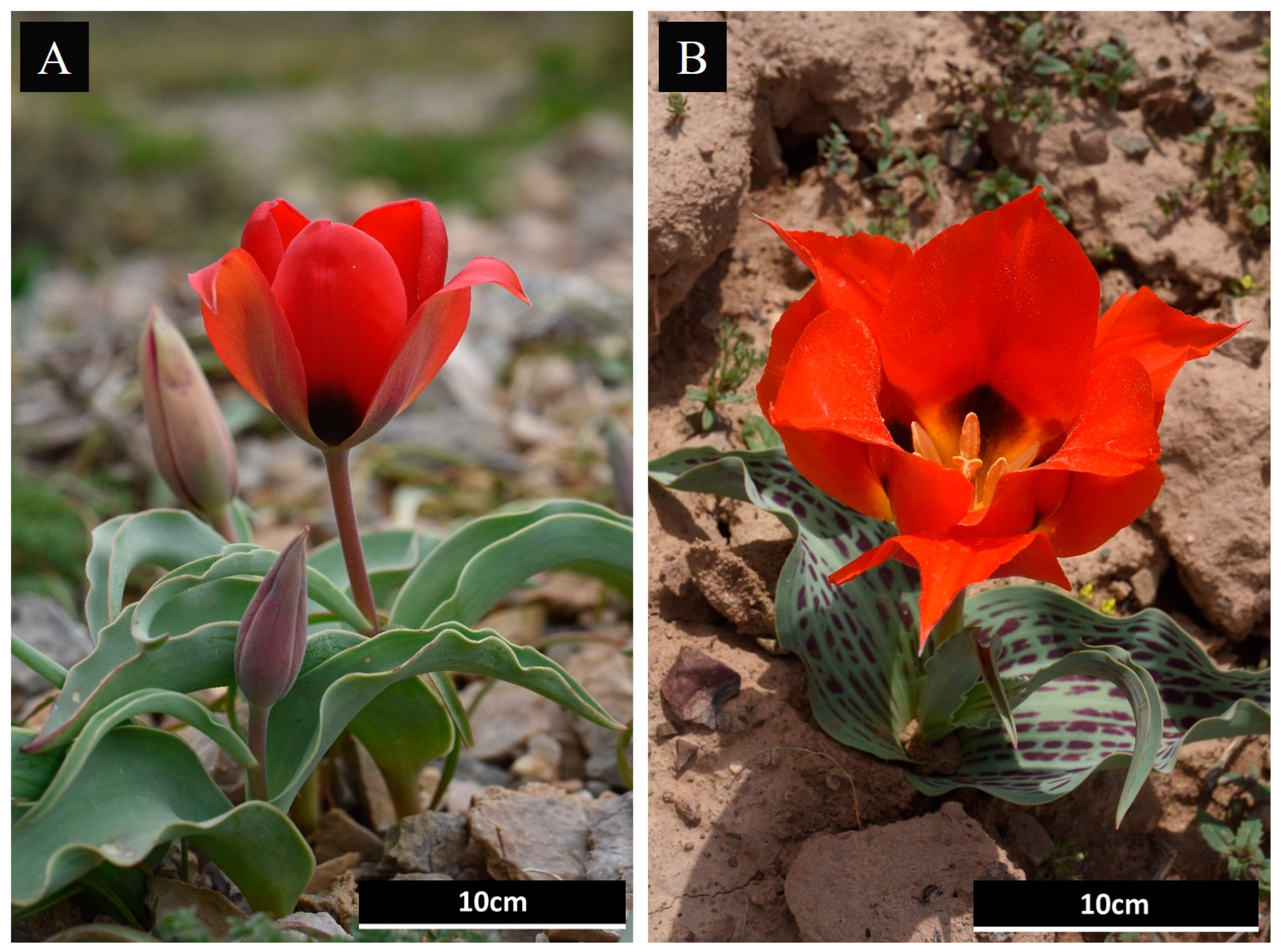
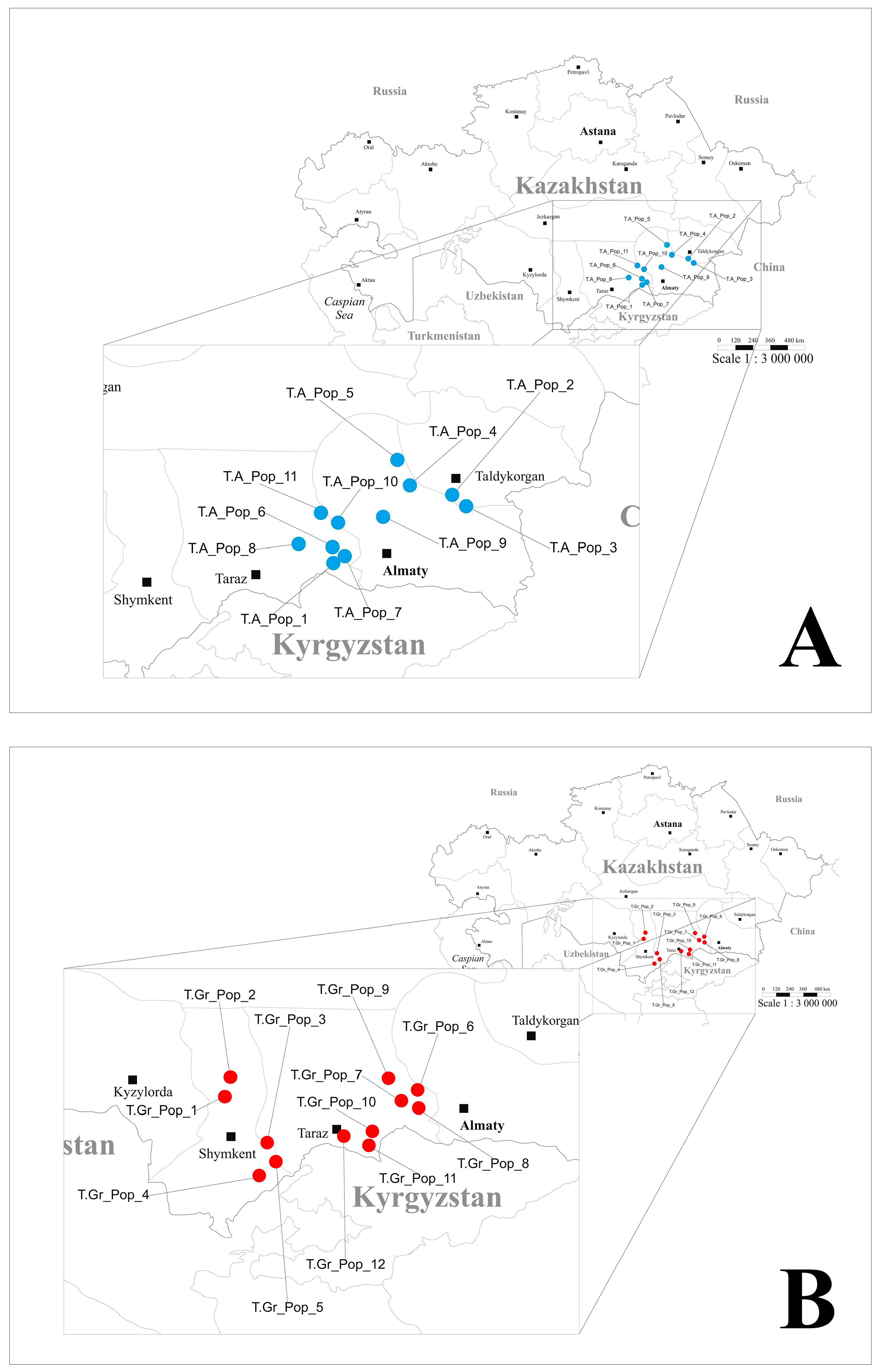
4.1. Plant Material and DNA Extraction
4.2. EST-SSR Analysis
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turktas, M.; Metin, Ö.K.; Baştuğ, B.; Ertuğrul, F.; Sarac, Y.I.; Kaya, E. Molecular phylogenetic analysis of Tulipa (Liliaceae) based on noncoding plastid and nuclear DNA sequences with an emphasis on Turkey. Bot. J. Linn. Soc. 2013, 172, 270–279. [Google Scholar] [CrossRef]
- Hall, A.D. The Genus Tulipa; Royal Horticultural Society: London, UK, 1940. [Google Scholar]
- Wendelbo, P. Tulips and Irises of Iran and Their Relatives; Ariamehr Botanical Garden: Tehran, Iran, 1977. [Google Scholar]
- Botschantzeva, Z. Tulips: Taxonomy, Morphology, Cytology, Phytogeogr; CRC Press: Boca Raton, FL, USA, 1982. [Google Scholar]
- Stork, A. Tulipes Sauvages et Cultivees (Wild and Cultivated Tulips; Serie Documentaire 13; Conservatoire et Jardin Botaniques: Geneva, Switzerland, 1984. [Google Scholar]
- Rechinger, K.-H. Tulipa. In Flora Iranica; Cont. No. 165; Akademische Druck- und Verlagsanstalt: Graz, Austria, 1990. [Google Scholar]
- Matin, F. Tulips of Iran; Agricultural Research Education and Development: Tehran, Iran, 1998. [Google Scholar]
- Govaerts, R. World Checklist of Selected Plant Families. Royal Botanic Gardens, Kew. 2009. Available online: http://apps.kew.org/wcsp/qsearch.do (accessed on 3 June 2024).
- Zonneveld, B.J. The systematic value of nuclear genome size for “all” species of Tulipa L. (Liliaceae). Plant Syst. Evol. 2009, 281, 217–245. [Google Scholar] [CrossRef]
- Christenhusz, M.J.; Govaerts, R.; David, J.C.; Hall, T.; Borland, K.; Roberts, P.S.; Fay, M.F. Tiptoe through the tulips–cultural history, molecular phylogenetics and classification of Tulipa (Liliaceae). Bot. J. Linn. Soc. 2013, 172, 280–328. [Google Scholar] [CrossRef]
- Van Raamsdonk, L.W.D.; De Vries, T. Species relationships and taxonomy in Tulipa subgenus Tulipa (Liliaceae). Plant Syst. Evol. 1995, 195, 13–44. [Google Scholar] [CrossRef]
- Ivashchenko, A.A.; Belyalov, O.V. Kazakhstan Is the Birthplace of Tulips; Atamura: Almaty, Kazakhstan, 2019. [Google Scholar]
- Yang, J.B.; Tang, M.; Li, H.T.; Zhang, Z.R.; Li, D.Z. Complete chloroplast genome of the genus Cymbidium: Lights into the species identification, phylogenetic implications and population genetic analyses. BMC Evol. Biol. 2013, 13, 84. [Google Scholar] [CrossRef]
- Park, I.; Song, J.H.; Yang, S.; Chae, S.; Moon, B.C. Plastid phylogenomic data offers novel insights into the taxonomic status of the Trichosanthes kirilowii complex (Cucurbitaceae) in south Korea. Front. Plant Sci. 2021, 12, 559511. [Google Scholar] [CrossRef]
- Li, J.; Price, M.; Su, D.M.; Zhang, Z.; Yu, Y.; Xie, D.F.; Gao, X.F. Phylogeny and comparative analysis for the plastid genomes of five Tulipa (Liliaceae). BioMed Res. Int. 2021, 2021, 6648429. [Google Scholar] [CrossRef] [PubMed]
- Almerekova, S.; Yermagambetova, M.; Ivaschenko, A.; Turuspekov, Y.; Abugalieva, S. Comparative Analysis of Plastome Sequences of Seven Tulipa L. (Liliaceae Juss.) Species from Section Kolpakowskianae Raamsd. Ex Zonn and Veldk. Int. J. Mol. Sci. 2024, 25, 7874. [Google Scholar] [CrossRef]
- Baitulin, I.O.; Sitpayeva, G.T. (Eds.) Red Book of Kazakhstan, 2nd ed.; “Art-Print XXI” Ltd.: Astana, Kazakhstan, 2014; 452p, Part 2. [Google Scholar]
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. 2024. Available online: https://powo.science.kew.org/ (accessed on 24 August 2024).
- Beshko, N.Y.; Abduraimov, O.S.; Kodirov, U.K.; Madaminov, F.M.; Mahmudov, A.V. The Current State of Cenopopulations of Some Endemic and Rare Species of the Genus Tulipa L. (Liliaceae) in the Tashkent Region (Uzbekistan). Arid Ecosyst. 2023, 13, 294–304. [Google Scholar] [CrossRef]
- Hughes, A.R.; Inouye, B.D.; Johnson, M.T.; Underwood, N.; Vellend, M. Ecological consequences of genetic diversity. Ecol. Lett. 2008, 11, 609–623. [Google Scholar] [CrossRef]
- Bidyananda, N.; Jamir, I.; Nowakowska, K.; Varte, V.; Vendrame, W.A.; Devi, R.S.; Nongdam, P. Plant Genetic Diversity Studies: Insights from DNA Marker Analyses. Int. J. Plant Biol. 2024, 15, 607–640. [Google Scholar] [CrossRef]
- Ellstrand, N.C.; Elam, D.R. Population genetic consequences of small population size: Implications for plant conservation. Annu. Rev. Ecol. Syst. 1993, 24, 217–242. [Google Scholar] [CrossRef]
- Nam, V.T.; Hang, P.L.B.; Linh, N.N.; Ly, L.H.; Hue, H.T.T.; Ha, N.H.; Hien, L.T.T. Molecular markers for analysis of plant genetic diversity. Vietnam J. Biotechnol. 2020, 18, 589–608. [Google Scholar] [CrossRef]
- Cloutier, S.; Niu, Z.; Datla, R.; Duguid, S. Development and analysis of EST-SSRs for flax (Linum usitatissimum L.). Theor. Appl. Genet. 2009, 119, 53–63. [Google Scholar] [CrossRef]
- Tang, S.; Okashah, R.A.; Cordonnier-Pratt, M.M.; Pratt, L.H.; Ed Johnson, V.; Taylor, C.A.; Knapp, S.J. EST and EST-SSR marker resources for Iris. BMC Plant Biol. 2009, 9, 72. [Google Scholar] [CrossRef]
- Dillon, N.L.; Innes, D.J.; Bally, I.S.E.; Wright, C.L.; Devitt, L.C.; Dietzgen, R.G. Expressed Sequence Tag-Simple Sequence Repeat (EST-SSR) Marker Resources for Diversity Analysis of Mango (Mangifera indica L.). Diversity 2014, 6, 72–87. [Google Scholar] [CrossRef]
- Ng, K.K.; Lee, S.L.; Tsumura, Y.; Ueno, S.; Ng, C.H.; Lee, C.T. Expressed sequence tag-simple sequence repeats isolated from Shorea leprosula and their transferability to 36 species within the Dipterocarpaceae. Mol. Ecol. Resour. 2009, 9, 393–398. [Google Scholar] [CrossRef]
- Chen, C.; Bowman, K.D.; Choi, Y.A.; Dang, P.M.; Rao, M.N.; Huang, S.; Soneji, J.R.; McCollum, T.G.; Gmitter, F.G. EST-SSR genetic maps for Citrus sinensis and Poncirus trifoliata. Tree Genet. Genomes 2008, 4, 1–10. [Google Scholar] [CrossRef]
- Hwang, J.H.; Ahn, S.G.; Oh, J.Y.; Choi, Y.W.; Kang, J.S.; Park, Y.H. Functional characterization of watermelon (Citrullus lanatus L.) EST–SSR by gel electrophoresis and high-resolution melting analysis. Sci. Hortic. 2011, 130, 715–724. [Google Scholar] [CrossRef]
- Ellis, J.; Burke, J. EST-SSRs as a resource for population genetic analyses. Heredity 2007, 99, 125–132. [Google Scholar] [CrossRef]
- Ramu, P.; Billot, C.; Rami, J.F.; Senthilvel, S.; Upadhyaya, H.D.; Ananda Reddy, L.; Hash, C.T. Assessment of genetic diversity in the sorghum reference set using EST-SSR markers. Theor. Appl. Genet. 2013, 126, 2051–2064. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Yang, A.; Li, Z.; Zhang, H.; Liu, L.; Wu, Z.; Yu, F. Genetic diversity and population genetic structure of Cinnamomum camphora in South China revealed by EST-SSR markers. Forests 2019, 10, 1019. [Google Scholar] [CrossRef]
- Wu, Q.; Zang, F.; Ma, Y.; Zheng, Y.; Zang, D. Analysis of genetic diversity and population structure in endangered Populus wulianensis based on 18 newly developed EST-SSR markers. Glob. Ecol. Conserv. 2020, 24, e01329. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, Y.; Diao, X.; Yu, K.; Dai, X.; Qu, P.; Qiao, Q. Evaluation of genetic diversity and population structure of Fragaria nilgerrensis using EST-SSR markers. Gene 2021, 796, 145791. [Google Scholar] [CrossRef]
- Qi-fu, L.; Tong, O.; Yan-cheng, J.; Cai-xia, W. Tulip RAPD analysis of cultivars and wild species in Xinjiang. Acta Agric. Univ Jiangxiensis 2008, 30, 656–660. [Google Scholar]
- Kiani, M.; Memariani, F.; Zarghami, H. Molecular analysis of species of Tulipa L. from Iran based on ISSR markers. Plant Syst. Evol. 2012, 298, 1515–1522. [Google Scholar] [CrossRef]
- Tang, N.; Shahin, A.; Bijman, P.; Liu, J.; van Tuyl, J.; Arens, P. Genetic diversity and structure in a collection of tulip cultivars assessed by SNP markers. Sci. Hortic. 2013, 161, 286–292. [Google Scholar] [CrossRef]
- Pourkhaloee, A.; Khosh-Khui, M.; Arens, P.; Salehi, H.; Razi, H.; Niazi, A.; van Tuyl, J. Molecular analysis of genetic diversity, population structure, and phylogeny of wild and cultivated tulips (Tulipa L.) by genic microsatellites. Hortic. Environ. Biotechnol. 2018, 59, 875–888. [Google Scholar] [CrossRef]
- Shahin, A.; van Kaauwen, M.; Esselink, D.; Bargsten, J.W.; van Tuyl, J.M.; Visser, R.G.; Arens, P. Generation and analysis of expressed sequence tags in the extreme large genomes Lilium and Tulipa. BMC Genom. 2012, 13, 640. [Google Scholar] [CrossRef]
- Ferrer, M.M.; Eguiarte, L.E.; Montana, C. Genetic structure and outcrossing rates in Flourensia cernua (Asteraceae) growing at different densities in the South-western Chihuahuan Desert. Ann. Bot. 2004, 94, 419–426. [Google Scholar] [CrossRef]
- Shen, Y.; Cheng, Y.; Li, K.; Li, H. Integrating phylogeographic analysis and geospatial methods to infer historical dispersal routes and glacial refugia of Liriodendron chinense. Forests 2019, 10, 565. [Google Scholar] [CrossRef]
- Ghasemi Ghehsareh, M.; Salehi, H.; Khosh-Khui, M.; Niazi, A. Application of ISSR markers to analyze molecular relationships in Iranian jasmine (Jasminum spp.) accessions. Mol. Biotechnol. 2015, 57, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sharma, V.K.; Dikshit, H.K.; Singh, D.; Aski, M.; Prakash, P.; Kaushik, S.C.; Singh, G.; Kumar, S.; Sarker, A. Microsatellite marker-based genetic diversity analysis of elite lentil lines differing in grain iron and zinc concentration. J. Plant Biochem. Biotechnol. 2017, 26, 199–207. [Google Scholar] [CrossRef]
- Wu, Z.H.; Shi, J.; Xi, M.L.; Jiang, F.X.; Deng, M.W.; Dayanandan, S. Inter-simple sequence repeat data reveals high genetic diversity in wild populations of the narrowly distributed endemic Lilium regale in the Minjiang River Valley of China. PLoS ONE 2015, 10, e0118831. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.S.; Hsiao, J.Y. Altitudinal genetic differentiation and diversity of Taiwan lily (Lilium longiflorum var. formosanum; Liliaceae) using RAPD markers and morphological characters. Int. J. Plant Sci. 2001, 162, 287–295. [Google Scholar] [CrossRef]
- Asgari, D.; Babaei, A.; Naghavi, M.R.; Kiani, M. Biodiversity status of Tulipa (Liliaceae) in Iran inferred from molecular characterization. Hortic. Environ. Biotechnol. 2020, 61, 559–567. [Google Scholar] [CrossRef]
- Pourkhaloee, A.; Khosh-Khui, M.; Arens, P.; Salehi, H.; Razi, H.; Niazi, A.; Afsharifar, A.; Van Tuyl, J. Genetic Diversity and Population Structure of Iranian Tulips Revealed by EST-SSR and NBS-LRR Markers. Int. J. Hortic. Sci. Technol. 2017, 4, 167–182. [Google Scholar] [CrossRef]
- Trapnell, D.W.; Hamrick, J.L.; Giannasi, D.E. Genetic variation and species boundaries in Calopogon (Orchidaceae). Syst. Bot. 2004, 29, 308–315. [Google Scholar] [CrossRef]
- Drummond, C.S.; Hamilton, M.B. Hierarchical components of genetic variation at a species boundary: Population structure in two sympatric varieties of Lupinus microcarpus (Leguminosae). Mol. Ecol. 2007, 16, 753–769. [Google Scholar] [CrossRef]
- Muñiz-Castro, M.Á.; Castro-Félix, P.; Carranza-Aranda, A.S.; Vázquez-García, J.A.; Santerre, A. Population genetics, species boundaries, and conservation in the Magnolia pacifica species complex along a continentality and moisture gradient in western Mexico. Bot. Sci. 2020, 98, 500–516. [Google Scholar] [CrossRef]
- Yu, Z.; Fredua-Agyeman, R.; Hwang, S.-F.; Strelkov, S.E. Molecular genetic diversity and population structure analyses of rutabaga accessions from Nordic countries as revealed by single nucleotide polymorphism markers. BMC Genom. 2021, 22, 442. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yun, L.; Gao, Z.; Wang, T.; Ren, X.; Zhao, Y. EST-SSR primer development and genetic structure analysis of Psathyrostachys juncea Nevski. Front. Plant Sci. 2022, 13, 837787. [Google Scholar] [CrossRef] [PubMed]
- Eren, B.; Keskin, B.; Demirel, F.; Demirel, S.; Türkoğlu, A.; Yilmaz, A.; Haliloğlu, K. Assessment of genetic diversity and population structure in local alfalfa genotypes using iPBS molecular markers. Genet. Resour. Crop Evol. 2023, 70, 617–628. [Google Scholar] [CrossRef]
- Kark, S. Within-Population Diversity in the Distribution Range: Partridges as a Research Model; Department of Evolution, Systematics and Ecology, The Hebrew University of Jerusalem, and The Desertification and Restoration Ecology Research Center: Jerusalem, Israel, 1999. [Google Scholar]
- Volis, S.; Mendlinger, S.; Orlovsky, N. Variability in phenotypic traits in core and peripheral populations of wild barley Hordeum spontaneum Koch. Hereditas 2001, 133, 235–247. [Google Scholar] [CrossRef]
- Yermagambetova, M.; Almerekova, S.; Turginov, O.; Sultangaziev, O.; Abugalieva, S.; Turuspekov, Y. Genetic Diversity and Population Structure of Juniperus seravschanica Kom. Collected in Central Asia. Plants 2023, 12, 2961. [Google Scholar] [CrossRef]
- Doyle, J. DNA protocols for plants-CTAB total DNA isolation. In Molecular Techniques in Taxonomy; Hewitt, G., Johnston, A., Eds.; Springer: Berlin/Heidelberg, Germany, 1991; pp. 283–293. [Google Scholar]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Amiryousefi, A.; Hyvönen, J.; Poczai, P. iMEC: Online marker efficiency calculator9. Appl. Plant Sci. 2018, 6, e01159. Available online: https://irscope.shinyapps.io/iMEC/ (accessed on 22 May 2024). [CrossRef]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 4–9. [Google Scholar]
- Earl, D.A.; vonHoldt, B.M. Structure harvester: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software structure: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- Jakobsson, M.; Rosenberg, N.A. CLUMPP: A cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics 2007, 23, 1801–1806. [Google Scholar] [CrossRef] [PubMed]
| Species | EST-SSRs | N | Na | Ne | I | h | PIC |
|---|---|---|---|---|---|---|---|
| Tulipa alberti | Ca-2572 | 207 | 3 | 2.4 | 0.959 | 0.581 | 0.578 |
| Ca-3952 | 207 | 1 | 1.0 | 0.000 | 0.000 | 0.000 | |
| Ca-5526 | 207 | 2 | 1.8 | 0.643 | 0.453 | 0.451 | |
| Ca-5553 | 207 | 4 | 1.7 | 0.692 | 0.418 | 0.416 | |
| Ca-6950 | 207 | 2 | 1.9 | 0.673 | 0.483 | 0.480 | |
| Ca-7862 | 207 | 1 | 1.0 | 0.000 | 0.000 | 0.000 | |
| Ca-8508 | 207 | 4 | 3.4 | 1.286 | 0.707 | 0.703 | |
| Ca-13333 | 207 | 3 | 2.3 | 0.946 | 0.565 | 0.562 | |
| Ca-15730 | 207 | 3 | 1.9 | 0.760 | 0.473 | 0.471 | |
| Kn-834 | 207 | 4 | 2.9 | 1.118 | 0.661 | 0.658 | |
| Kn-2291 | 207 | 2 | 1.8 | 0.637 | 0.447 | 0.444 | |
| Kn-7480 | 207 | 1 | 1.0 | 0.000 | 0.000 | 0.000 | |
| Kn-30956 | 207 | 1 | 1.0 | 0.000 | 0.000 | 0.000 | |
| Mean | 207 | 2.4 | 1.9 | 0.593 | 0.368 | 0.366 | |
| SE | 0.331 | 0.209 | 0.126 | 0.075 | 0.267 | ||
| Tulipa greigii | Ca-2572 | 216 | 5 | 2.3 | 1.099 | 0.572 | 0.569 |
| Ca-3952 | 216 | 4 | 2.5 | 1.118 | 0.599 | 0.597 | |
| Ca-5526 | 216 | 5 | 3.8 | 1.371 | 0.737 | 0.734 | |
| Ca-5553 | 216 | 4 | 2.0 | 0.917 | 0.501 | 0.499 | |
| Ca-6950 | 216 | 3 | 1.8 | 0.747 | 0.432 | 0.430 | |
| Ca-7862 | 216 | 3 | 1.7 | 0.703 | 0.397 | 0.395 | |
| Ca-8508 | 216 | 6 | 4.5 | 1.603 | 0.782 | 0.779 | |
| Ca-13333 | 216 | 2 | 2.0 | 0.690 | 0.499 | 0.497 | |
| Ca-15730 | 216 | 5 | 4.0 | 1.471 | 0.753 | 0.750 | |
| Kn-834 | 216 | 3 | 2.0 | 0.872 | 0.506 | 0.504 | |
| Kn-2291 | 216 | 3 | 2.5 | 0.997 | 0.610 | 0.607 | |
| Kn-7480 | 216 | 4 | 3.3 | 1.289 | 0.704 | 0.700 | |
| Kn-30956 | 216 | 3 | 2.3 | 0.920 | 0.567 | 0.564 | |
| Mean | 216 | 3.8 | 2.7 | 1.061 | 0.589 | 0.586 | |
| SE | 0.317 | 0.257 | 0.082 | 0.034 | 0.124 | ||
| Total | Ca-2572 | 423 | 5 | 2.0 | 0.685 | 0.431 | 0.567 |
| Ca-3952 | 423 | 4 | 1.5 | 0.368 | 0.240 | 0.616 | |
| Ca-5526 | 423 | 5 | 1.9 | 0.648 | 0.424 | 0.706 | |
| Ca-5553 | 423 | 4 | 1.6 | 0.513 | 0.309 | 0.676 | |
| Ca-6950 | 423 | 3 | 1.7 | 0.578 | 0.398 | 0.467 | |
| Ca-7862 | 423 | 3 | 1.3 | 0.203 | 0.135 | 0.195 | |
| Ca-8508 | 423 | 6 | 3.0 | 1.182 | 0.688 | 0.748 | |
| Ca-13333 | 423 | 3 | 1.7 | 0.555 | 0.379 | 0.584 | |
| Ca-15730 | 423 | 5 | 2.5 | 0.941 | 0.572 | 0.714 | |
| Kn-834 | 423 | 4 | 2.1 | 0.751 | 0.496 | 0.598 | |
| Kn-2291 | 423 | 3 | 1.8 | 0.647 | 0.439 | 0.558 | |
| Kn-7480 | 423 | 4 | 1.8 | 0.465 | 0.275 | 0.453 | |
| Kn-30956 | 423 | 3 | 1.5 | 0.369 | 0.245 | 0.398 | |
| Mean | 423 | 4 | 1.9 | 0.608 | 0.387 | 0.560 | |
| SE | 0.065 | 0.047 | 0.026 | 0.016 | 0.152 |
| Species | Population ID | Na | Ne | I | h | PPL |
|---|---|---|---|---|---|---|
| Tulipa alberti | T.A. Pop 1 | 1.8 | 1.5 | 0.414 | 0.277 | 69.23% |
| T.A. Pop 2 | 2.0 | 1.7 | 0.465 | 0.299 | 61.54% | |
| T.A. Pop 3 | 1.8 | 1.6 | 0.439 | 0.300 | 61.54% | |
| T.A. Pop 4 | 1.6 | 1.4 | 0.315 | 0.219 | 46.15% | |
| T.A. Pop 5 | 1.9 | 1.5 | 0.413 | 0.274 | 69.23% | |
| T.A. Pop 6 | 2.1 | 1.7 | 0.488 | 0.312 | 61.54% | |
| T.A. Pop 7 | 2.0 | 1.8 | 0.529 | 0.353 | 69.23% | |
| T.A. Pop 8 | 1.6 | 1.4 | 0.303 | 0.205 | 46.15% | |
| T.A. Pop 9 | 2.0 | 1.6 | 0.441 | 0.274 | 61.54% | |
| T.A. Pop 10 | 1.8 | 1.5 | 0.380 | 0.258 | 53.85% | |
| T.A. Pop 11 | 1.8 | 1.6 | 0.429 | 0.280 | 53.85% | |
| Mean | 1.9 | 1.6 | 0.420 | 0.277 | 59.4% | |
| SE | 0.154 | 0.121 | 0.068 | 0.041 | 0.085 | |
| Tulipa greigii | T.Gr. Pop 1 | 3.2 | 2.7 | 0.993 | 0.609 | 100.00% |
| T.Gr. Pop 2 | 2.6 | 1.9 | 0.638 | 0.392 | 69.23% | |
| T.Gr. Pop 3 | 2.7 | 2.2 | 0.731 | 0.439 | 69.23% | |
| T.Gr. Pop 4 | 3.4 | 2.4 | 0.947 | 0.558 | 100.00% | |
| T.Gr. Pop 5 | 3.3 | 2.4 | 0.929 | 0.558 | 100.00% | |
| T.Gr. Pop 6 | 2.9 | 2.3 | 0.822 | 0.495 | 84.62% | |
| T.Gr. Pop 7 | 2.6 | 2.0 | 0.747 | 0.481 | 100.00% | |
| T.Gr. Pop 8 | 1.8 | 1.5 | 0.406 | 0.318 | 69.23% | |
| T.Gr. Pop 9 | 2.3 | 1.8 | 0.615 | 0.410 | 84.62% | |
| T.Gr. Pop 10 | 2.9 | 2.2 | 0.830 | 0.513 | 92.31% | |
| T.Gr. Pop 11 | 2.8 | 2.2 | 0.804 | 0.529 | 100.00% | |
| T.Gr. Pop 12 | 3.2 | 2.4 | 0.904 | 0.548 | 92.31% | |
| Mean | 2.8 | 2.2 | 0.781 | 0.487 | 88.5% | |
| SE | 0.466 | 0.323 | 0.167 | 0.084 | 0.129 | |
| Total | Mean | 2.4 | 1.9 | 0.608 | 0.387 | 74.6% |
| SE | 0.065 | 0.047 | 0.026 | 0.016 | 3.82% |
| Species | Source | df | SS | MS | Est.Var. | % | PhiPT | Nm | p Values |
|---|---|---|---|---|---|---|---|---|---|
| Tulioa alberti | Among Pops | 10 | 197.049 | 19.705 | 0.904 | 25% | |||
| Within Pops | 196 | 536.796 | 2.739 | 2.739 | 75% | ||||
| Total | 206 | 733.845 | 3.642 | 100% | 0.248 | 0.746 | <0.001 | ||
| Tulipa greigii | Among Pops | 11 | 512.880 | 46.625 | 2.204 | 23% | |||
| Within Pops | 204 | 1468.755 | 7.200 | 7.200 | 77% | ||||
| Total | 215 | 1981.634 | 9.403 | 100% | 0.234 | 1.283 | <0.001 | ||
| Total | Among Pops | 1 | 234.507 | 234.507 | 1.094 | 26% | |||
| Within Pops | 421 | 1316.394 | 3.127 | 3.127 | 74% | ||||
| Total | 422 | 1550.901 | 4.221 | 100% | 0.259 | 1.428 | <0.001 |
| Species | Population ID | Sample Size | Altitude | Collection Sites |
|---|---|---|---|---|
| Tulipa alberti | T.A. Pop 1 | 15 | 1100 | Zhetyzhol ridge, western part of the Trans-Ili Alatau, Zhambyl region |
| T.A. Pop 2 | 21 | 600 | Qonayev district, Trans-Ili Alatau, Almaty region | |
| T.A. Pop 3 | 21 | 611 | Chulak Mountains, Trans-Ili Alatau, Almaty region | |
| T.A. Pop 4 | 17 | 510 | The right bank of the Ili River, in the area of the Kapchagai reservoir, gravelly slopes, Trans-Ili Alatau, Almaty region | |
| T.A. Pop 5 | 21 | 575 | Malaysary pass, Trans-Ili Alatau, Almaty region | |
| T.A. Pop 6 | 21 | 1064 | Zhetyzhol ridge, eastern slope, Kenen village, western part of the Trans-Ili Alatau, Zhambyl region | |
| T.A. Pop 7 | 21 | 1072 | Zhetyzhol ridge, southwestern slope, Kenen village, western part of the Trans-Ili Alatau, Zhambyl region | |
| T.A. Pop 8 | 13 | 700 | Right bank of the Tarylgan river, Chu-Ili mountains, Zhambyl region | |
| T.A. Pop 9 | 19 | 620 | Kurtinsky district, near the Kurtinsky reservoir, Chu-Ili mountains, Almaty region | |
| T.A. Pop 10 | 17 | 870 | Tamgaly tas, Chu-Ili mountains, Almaty region | |
| T.A. Pop 11 | 21 | 880 | Anrakai Mountains, Chu-Ili mountains, Zhambyl region | |
| Tulipa greigii | T.Gr. Pop 1 | 21 | 1054 | Kazanbulak tract, Karatau ridge, Karatau Nature Reserve, Turkestan region |
| T.Gr. Pop 2 | 20 | 1033 | Arpaozen, Kekliktas tract, Karatau ridge, Karatau Nature Reserve, Turkestan region | |
| T.Gr. Pop 3 | 20 | 932 | Shubaykyzyl hilly area, Tyulkubas district, Turkestan region | |
| T.Gr. Pop 4 | 21 | 1817 | Kaskasu gorge, Mailoshak ridge, Sairam-Ugam state national nature park (SNNP), Turkestan region | |
| T.Gr. Pop 5 | 21 | 1613 | Iirsu village, Aksu Valley, Aksu-Zhabagly state nature reserve SNR, Turkestan region | |
| T.Gr. Pop 6 | 20 | 970 | Kordai Pass, eastern slope, Trans-Ili Alatau, Zhambyl region | |
| T.Gr. Pop 7 | 21 | 1270 | Kordai Pass, southeast slope, right side of the road, Trans-Ili Alatau, Zhambyl region | |
| T.Gr. Pop 8 | 6 | 1120 | Kordai Pass, southeast slope, left side of the road, Trans-Ili Alatau, Zhambyl region | |
| T.Gr. Pop 9 | 15 | 1190 | On the right side of the road towards Taraz, western tip of the Trans-Ili Alatau, Zhambyl region | |
| T.Gr. Pop 10 | 21 | 1010 | Merken district, Merke gorge, northern slope, Kyrgyz Alatau, Zhambyl region | |
| T.Gr. Pop 11 | 11 | 1260 | Merken district, Merke gorge, Kyrgyz Alatau, Zhambyl region | |
| T.Gr. Pop 12 | 21 | 1140 | Almalysay gorge, western tip of the Kyrgyz Alatau, Zhambyl region |
| № | Locus | Repeat Motif | Primer Sequence (5′–3′) | Product Size (bp) |
|---|---|---|---|---|
| 1 | Ca-2572 | (GAGAAG)4 | F-TGCACAGAGCCAAAGAAGTA R-TCTCCTTTCCATGTTTCCTC | 213 |
| 2 | Ca-3952 | (CAG)4 | F-ACTCAATTCACTTGCAGCAG R-GTCGTTGCAGTTGTTGTGAT | 189 |
| 3 | Ca-5526 | (GAG)6 | F-TTTACGGGAATTACTTCGAG R-ACATGGATTCCAAACAAGAG | 242 |
| 4 | Ca-5553 | (TTG)9 | F-CCGATAATTGAGGTCAGGTT R-CCGAACTCCTCGCATATAAC | 168 |
| 5 | Ca-6950 | (GAT)4 | F-ATGCAATCTTGGGAACTGAT R-CACTGTCGTCATCTTCTCCA | 198 |
| 6 | Ca-7862 | (CGC)4 | F-AATCAACGCATCATGTCAAC R-TACTGGAGGTACGCCTCCTT | 131 |
| 7 | Ca-8508 | (GTT)10 | F-AGAATTTGTCTTGCGACAGT R-TAGGGGTACCAATTTGTGTT | 325 |
| 8 | Ca-13333 | (GAT)4 | F-ATGGTTGGAAGAGGAGACTG R-AGTCATTCGATCCTCGAGTC | 242 |
| 9 | Ca-15730 | (CGC)8 | F-CATCAAAACCGACAACACC R-CGGTCAACATCATTCAAGAG | 213 |
| 10 | Kn-834 | (AT)8 | F-TCAGAAGGCTCTTCTTTCAG R-CTTTACATGGAGATAATGTTAACAA | 221 |
| 11 | Kn-1412 | (GGA)10 | F-GTCCTTTGTACGGTGATGTT R-TAGCTTCCGGAGTTCAATAG | 242 |
| 12 | Kn-2291 | (GAG AAG)4 | F-GAAGACGAAGATGATTCGAG R-TGGGTTTCACTTAAACAGCT | 275 |
| 13 | Kn-7108 | (TTTC)4 | F-TTGCTGCTTCGACTACTTTG R-GGTCATGCAACATAAACTGC | 231 |
| 14 | Kn-7480 | (GAC)9 | F-GCAACTTAGGTCAACAGAGG R-CTCCTACCAACAAAGCATTC | 268 |
| 15 | Kn-30956 | (CTC)6 | F-TGAAGCTCCTCCACTCTACC R-ACAAGGGCACTCATTCTGTT | 237 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yermagambetova, M.; Almerekova, S.; Ivashchenko, A.; Turuspekov, Y.; Abugalieva, S. Genetic Diversity of Tulipa alberti and T. greigii Populations from Kazakhstan Based on Application of Expressed Sequence Tag Simple Sequence Repeat Markers. Plants 2024, 13, 2667. https://doi.org/10.3390/plants13182667
Yermagambetova M, Almerekova S, Ivashchenko A, Turuspekov Y, Abugalieva S. Genetic Diversity of Tulipa alberti and T. greigii Populations from Kazakhstan Based on Application of Expressed Sequence Tag Simple Sequence Repeat Markers. Plants. 2024; 13(18):2667. https://doi.org/10.3390/plants13182667
Chicago/Turabian StyleYermagambetova, Moldir, Shyryn Almerekova, Anna Ivashchenko, Yerlan Turuspekov, and Saule Abugalieva. 2024. "Genetic Diversity of Tulipa alberti and T. greigii Populations from Kazakhstan Based on Application of Expressed Sequence Tag Simple Sequence Repeat Markers" Plants 13, no. 18: 2667. https://doi.org/10.3390/plants13182667






