Transcription Factor and Protein Regulatory Network of PmACRE1 in Pinus massoniana Response to Pine Wilt Nematode Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Cloning and Analysis of the PmACRE1 Gene Promoter in P. massoniana
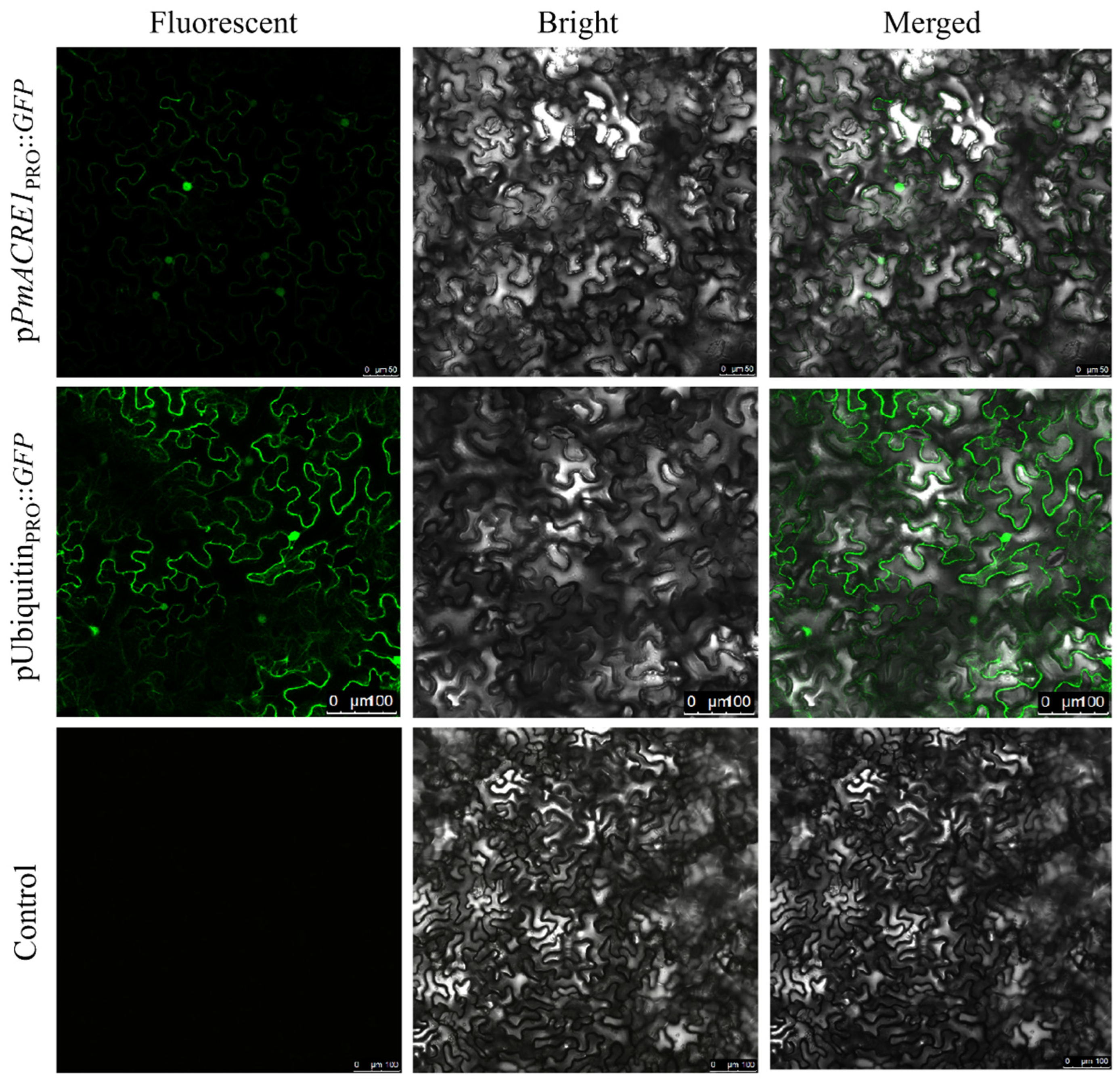
2.3. Detection of PmACRE1 Gene Promoter Activity
2.4. Isolation of Proteins Binding to the PmACRE1 Gene Promoter
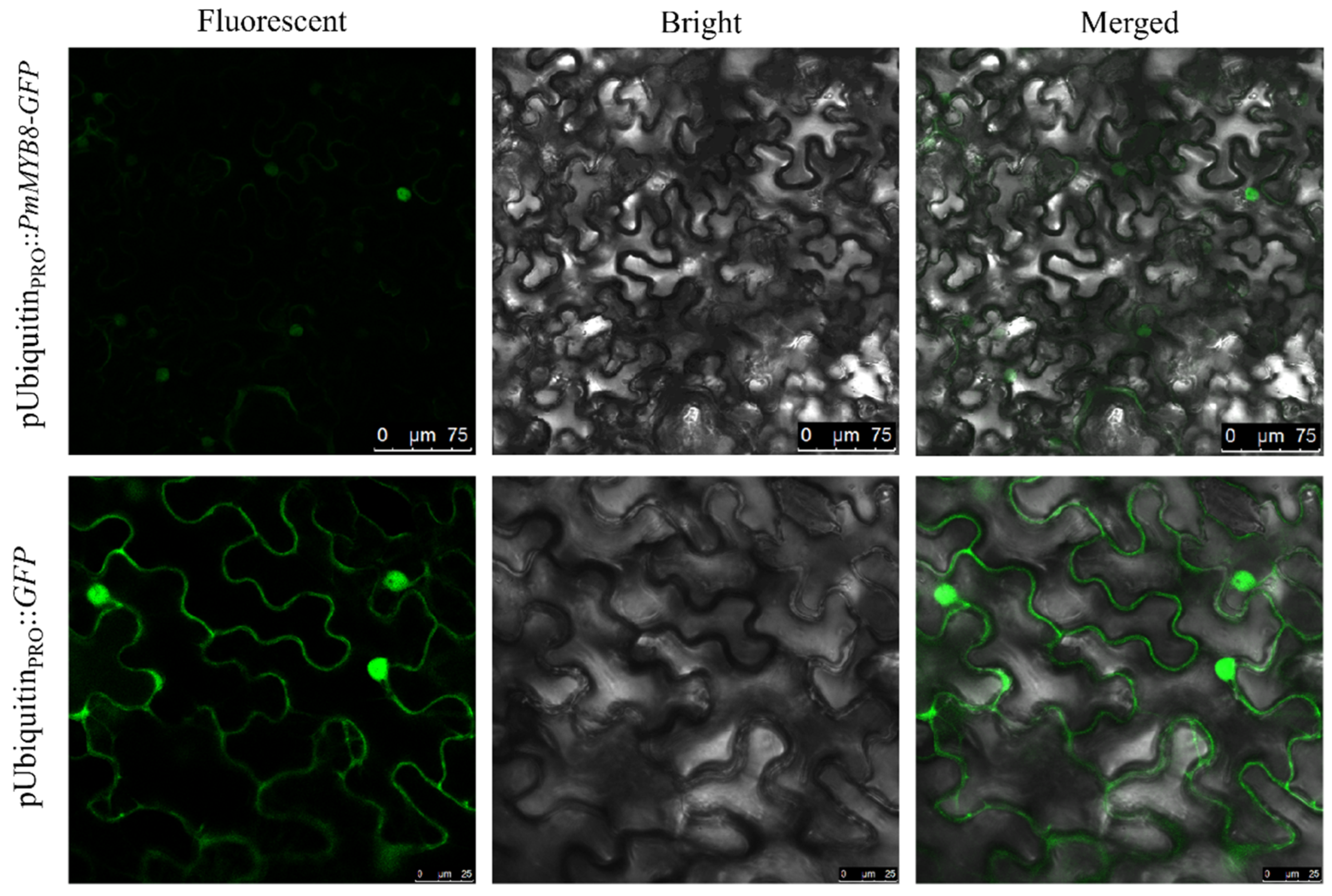
2.5. Subcellular Localization of Proteins Binding to the PmACRE1 Gene Promoter
2.6. Inoculation of Pine Wood Nematode to P. massoniana Seedlings and Proteins Extraction
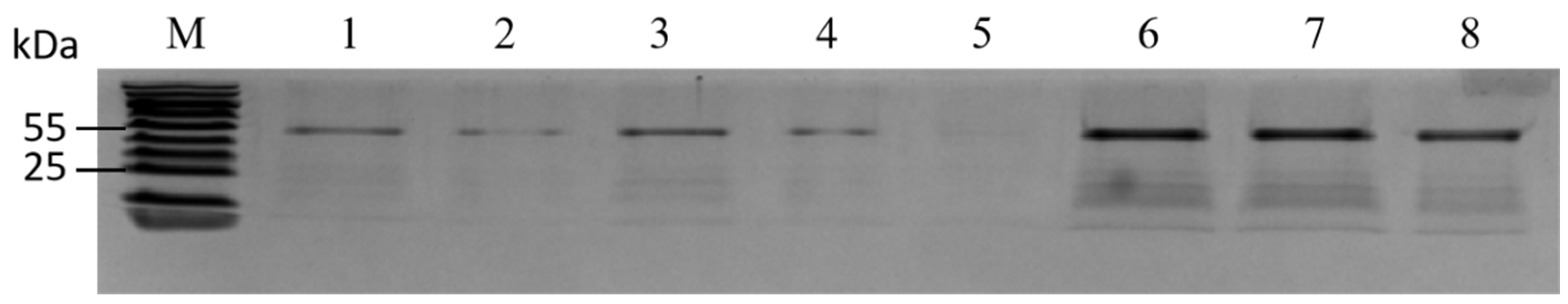
2.7. Co-IP for Isolation of PmACRE1 Interacting Proteins
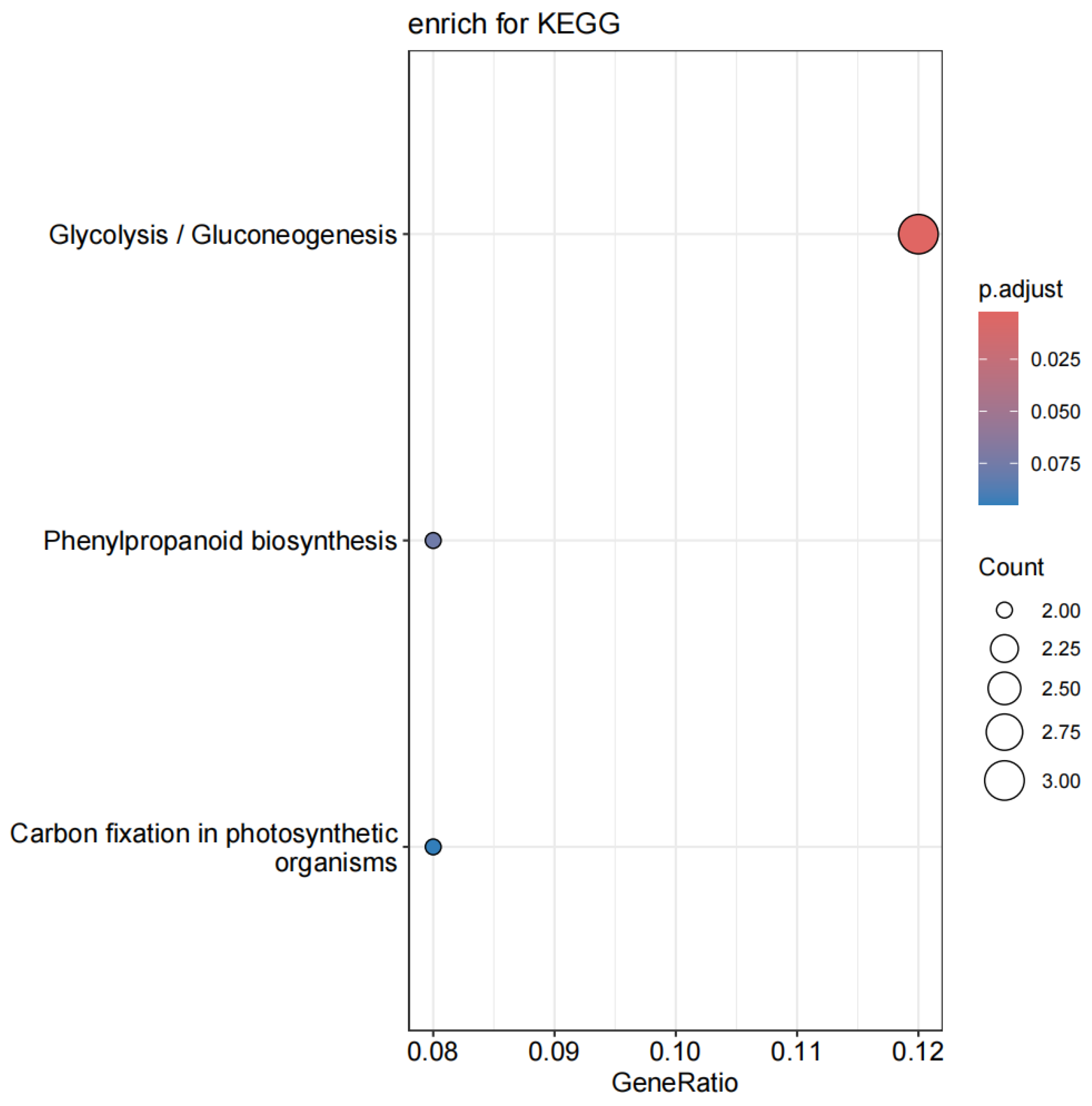
2.8. KEGG Enrichment Analysis for PmACRE1-Interacting Proteins
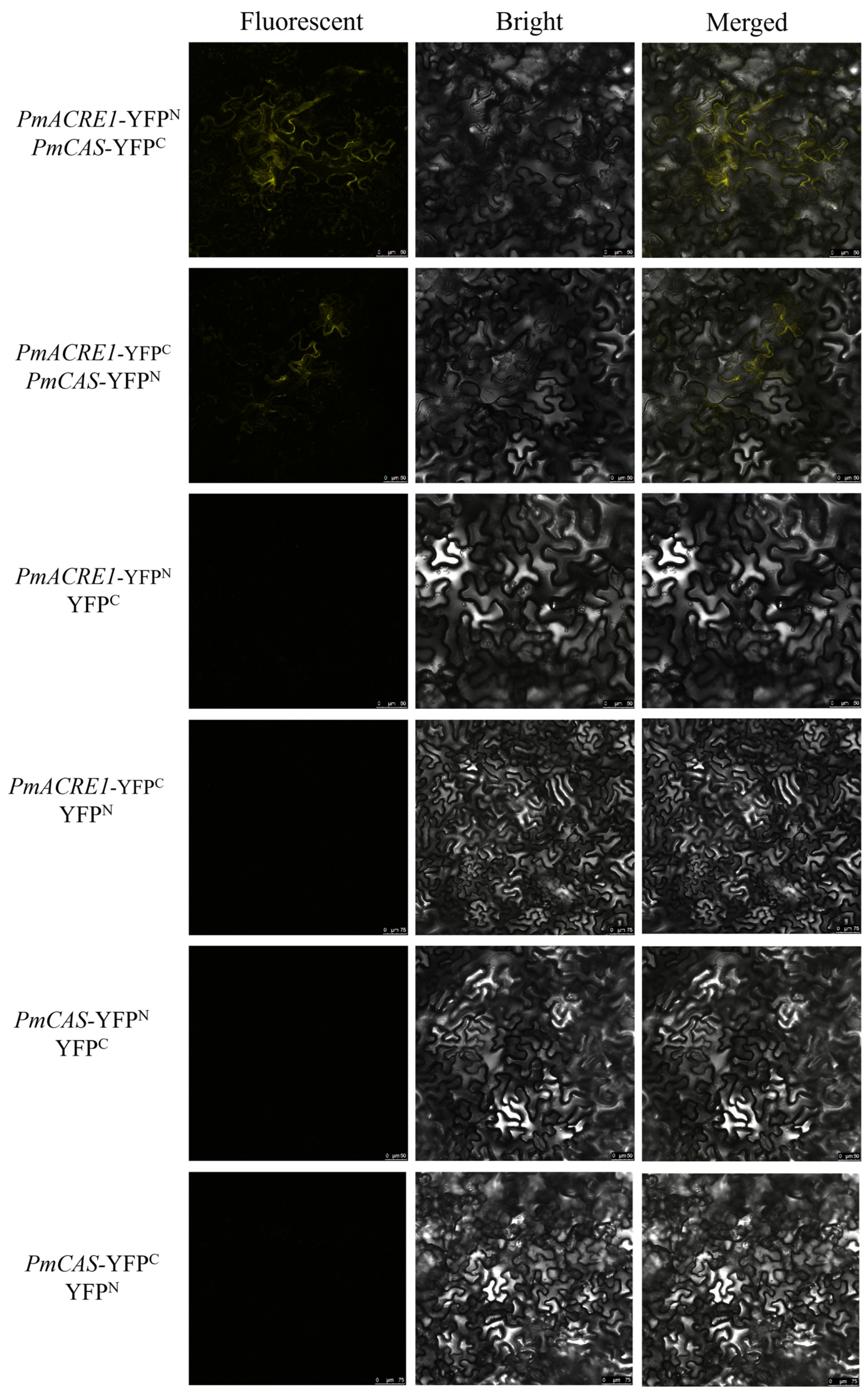
2.9. BiFC Assay for Validating Interactions among PmCCoAOMT, PmCAS, and PmACRE1
3. Results
3.1. PmACRE1 Gene Promoter and Its Cis-Elements
3.2. Activity of the PmACRE1 Gene Promoter
3.3. DNA-Binding Proteins of the PmACRE1 Gene Promoter
3.4. Subcellular Localization of the PmMYB8 Transcription Factor
3.5. Proteins Interacting with PmACRE1
3.6. KEGG Enrichment Analysis of PmACRE1 Interacting Proteins in P. massoniana
3.7. BiFC Validation of Interactions between PmACRE1 and PmCCoAOMT, PmCAS
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, X.Z.; Cao, Y.F.; Wang, L.F.; Piao, C.G.; Li, C.L. Current status of pine wilt disease and its control status. J. Environ. Entomol. 2018, 40, 256–267. [Google Scholar]
- Li, X.; Liu, X.T.; Wei, J.T.; Li, Y.; Tigabu, M.; Zhao, X.Y. Genetic improvement of Pinus koraiensis in China: Current Situation and future prospects. Forests 2020, 11, 148. [Google Scholar] [CrossRef]
- Suontama, M.; Li, Y.; Low, C.B.; Dungey, H.S. Genetic improvement of resistance to cyclaneusma needle cast in Pinus radiata. Can. J. Forest Res. 2019, 49, 128–133. [Google Scholar] [CrossRef]
- Grattapaglia, D.; Silva-Junior, O.B.; Resende, R.T.; Cappa, E.P.; Müller, B.S.F.; Tan, B.; Isik, F.; Ratcliffe, B.; El-Kassaby, Y.A. Quantitative genetics and genomics converge to accelerate forest tree breeding. Front. Plant Sci. 2018, 9, 1693. [Google Scholar] [CrossRef] [PubMed]
- Modesto, I.; Sterck, L.; Arbona, V.; Gómez-Cadenas, A.; Carrasquinho, I.; Van de Peer, Y.; Miguel, C.M. Insights into the mechanisms implicated in Pinus pinaster resistance to pinewood nematode. Front. Plant Sci. 2021, 12, 690857. [Google Scholar] [CrossRef]
- Liu, Q.; Wei, Y.; Xu, L.; Hao, Y.; Chen, X.; Zhou, Z. Transcriptomic profiling reveals differentially expressed genes associated with pine wood nematode resistance in masson pine (Pinus massoniana Lamb.). Sci. Rep. 2017, 7, 4693. [Google Scholar] [CrossRef]
- Zheng, H.Y.; Xu, M.; Xu, F.Y.; Ye, J. A comparative proteomics analysis of Pinus massoniana inoculated with Bursaphelenchus xylophilus. Pak. J. Bot. 2015, 47, 1271–1280. [Google Scholar]
- Xu, L.; Liu, Z.Y.; Zhang, K.; Lu, Q.; Liang, J.; Zhang, X.Y. Characterization of the Pinus massoniana transcriptional response to Bursaphelenchus xylophilus infection using suppression subtractive hybridization. Int. J. Mol. Sci. 2013, 14, 11356–11375. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Q.; Zhou, Z.; Yin, H.; Xie, Y. Overexpression of geranyl diphosphate synthase (PmGPPS1) boosts monoterpene and diterpene production involved in the response to pine wood nematode invasion. Tree Physiol. 2022, 42, 411–424. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Q.; Zhou, Z.; Yin, H.; Xie, Y.; Wei, Y. Two terpene synthases in resistant Pinus massoniana contribute to defence against Bursaphelenchus xylophilus. Plant Cell Environ. 2021, 41, 257–274. [Google Scholar] [CrossRef]
- Xie, W.F.; Huang, A.Z.; Li, H.M.; Feng, L.Z.; Zhang, F.P.; Guo, W.X. Identification and comparative analysis of microRNAs in Pinus massoniana infected by Bursaphelenchus xylophilus. Plant Growth Regul. 2017, 83, 223–232. [Google Scholar] [CrossRef]
- Xie, W.F.; Liang, G.H.; Huang, A.Z.; Zhang, F.P.; Guo, W.X. Comparative study on the mRNA expression of Pinus massoniana infected by Bursaphelenchus xylophilus. J. For. Res. 2020, 31, 75–86. [Google Scholar] [CrossRef]
- Xie, W.F.; Liang, G.H. Expression correlation between miRNA and mRNA from needle leaves of Pinus massoniana with Bursaphelenchus xylophilus Infestation. Forest Res. 2018, 31, 7–14. [Google Scholar]
- Dangl, J.L.; Jones, J.D.G. Plant pathogens and integrated defence responses to infection. Nature 2001, 411, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, B.; Gao, K.; Zhao, Y.; Li, W.; Deng, L.; Zhou, Z.; Liu, Q. Comprehensive analysis and functional verification of the Pinus massoniana NBS-LRR gene family involved in the resistance to Bursaphelenchus xylophilus. Int. J. Mol. Sci. 2023, 24, 1812. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Xu, Y.P.; Zheng, Z.; Cao, J.S.; Cai, X.Z. Comparative transcript profiling by cDNA-AFLP reveals similar patterns of Avr4/Cf-4- and Avr9/Cf-9-dependent defence gene expression. Mol. Plant Pathol. 2007, 8, 515–527. [Google Scholar] [CrossRef]
- González-Lamothe, R.; Tsitsigiannis, D.I.; Ludwig, A.A.; Panicot, M.; Shirasu, K.; Jones, J.D. The U-box protein CMPG1 is required for efficient activation of defense mechanisms triggered by multiple resistance genes in tobacco and tomato. Plant Cell. 2006, 18, 1067–1083. [Google Scholar] [CrossRef]
- Rowland, O.; Ludwig, A.A.; Merrick, C.; Baillieul, F.; Tracy, F.; Durrant, W.E.; Fitz-Laylin, L.; Nekrasov, V.; Yoshioka, H.; Jonathan, J.D.G. Functional analysis of Avr9/Cf-9 rapidly elicited genes identifies a protein kinase, ACIK1, that is essential for full Cf-9-dependent disease resistance in tomato. Plant Cell. 2005, 17, 295–310. [Google Scholar] [CrossRef]
- Durrant, W.E.; Rowland, O.; Piedras, P.; Hammond-Kosack, K.E.; Jones, J.D. cDNA-AFLP reveals a striking overlap in race-specific resistance and wound response gene expression profiles. Plant Cell. 2000, 12, 963–977. [Google Scholar] [CrossRef]
- Ni, X.; Tian, Z.; Liu, J.; Song, B.; Xie, C. Cloning and molecular characterization of the potato RING finger protein gene StRFP1 and its function in potato broad-spectrum resistance against Phytophthora infestans. J. Plant Physiol. 2010, 167, 488–496. [Google Scholar] [CrossRef]
- de Vega, D.; Holden, N.; Hedley, P.E.; Morris, J.; Luna, E.; Newton, A. Chitosan primes plant defence mechanisms against Botrytis cinerea, including expression of Avr9/Cf-9 rapidly elicited genes. Plant Cell Environ. 2021, 44, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Asiegbu, F.O. Induction of Pinus sylvestris PsACRE, a homology of Avr9/Cf-9; rapidly elicited defense-related gene following infection with root rot fungus Heterobasidion annosum. Plant Sci. 2004, 167, 535–540. [Google Scholar] [CrossRef]
- Nicaise, V.; Roux, M.; Zipfel, C. Recent advances in PAMP-triggered immunity against bacteria: Pattern recognition receptors watch over and raise the alarm. Plant Physiol. 2009, 150, 1638–1647. [Google Scholar] [CrossRef] [PubMed]
- de Vega, D.; Newton, A.C.; Sadanandom, A. Post-translational modifications in priming the plant immune system: Ripe for exploitation? FEBS Letters 2018, 592, 1929–1936. [Google Scholar] [CrossRef] [PubMed]
- Schwessinger, B.; Zipfel, C. News from the frontline: Recent insights into PAMP-triggered immunity in plants. Curr. Opin. Plant Biol. 2008, 11, 389–395. [Google Scholar] [CrossRef]
- Xie, W.F.; Xu, X.M.; Qiu, W.J.; Lai, X.L.; Liu, M.X.; Zhang, F.P. Expression of PmACRE1 in Arabidopsis thaliana enables host defence against Bursaphelenchus xylophilus infection. BMC Plant Biol. 2022, 22, 541. [Google Scholar] [CrossRef]
- Fang, C.X.; Yang, L.K.; Chen, W.X.; Li, L.L.; Zhang, P.L.; Li, Y.Z.; He, H.B.; Lin, W.X. MYB57 transcriptionally regulates MAPK11 to interact with PAL2;3 and modulate rice allelopathy. J. Exp. Bot. 2020, 71, 2127–2141. [Google Scholar] [CrossRef]
- Yi, S.; Jin, W.T.; Yuan, Y.N.; Fang, Y.H. An optimized CTAB method for genomic DNA extraction from freshly-picked Pinnae of Fern, Adiantum capillus-veneris L. Bio-Protocol 2018, 8, e2906. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Zhang, K.Y.; Lin, M.S.; Luo, H.W.; Lin, F.Y. Pathogenicity determination of Bursaphelenchus xylophilus isolates to Pine thunbergii. J.Nanjing Agric. Univ. 2002, 25, 43–46. [Google Scholar]
- Zhu, L.H.; Zhang, X.Y.; Xia, X.R.; Wan, Y.; Dai, S.J.; Ye, J.R. Pathogenicity of aseptic Bursaphelenchus xylophilus on Pinus massoniana. Sci. Silvae Sin. 2020, 56, 63–69. [Google Scholar]
- Wang, Y.; Jin, M.; Wang, Y.; Yang, Y.; Yu, A. Infestation of pine seedlings (Pinus thunbergii Parl.) with pine wood nematode, Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae) through needle leaves. J. Asia-Pac. Entomol. 2024, 27, 102252. [Google Scholar] [CrossRef]
- An, Y.; Li, Y.; Ma, L.; Li, D.; Zhang, W.; Feng, Y.; Liu, Z.; Wang, X.; Wen, X.; Zhang, X. Transcriptomic response of Pinus massoniana to infection stress from the pine wood nematode Bursaphelenchus xylophilus. Stress. Biol. 2023, 3, 50. [Google Scholar] [CrossRef]
- Shin, H.; Lee, H.; Woo, K.S.; Noh, E.W.; Koo, Y.B.; Lee, K.J. Identification of genes upregulated by pine wood nematode inoculation in Japanese red pine. Tree Physiol. 2009, 29, 411–421. [Google Scholar] [CrossRef]
- dos Santos, C.S.S.; de Vasconcelos, M.W. Identification of genes differentially expressed in Pinus pinaster and Pinus pinea after infection with the pine wood nematode. Eur. J. Plant Pathol. 2012, 132, 407–418. [Google Scholar] [CrossRef]
- Hirao, T.; Matsunaga, K.; Hirakawa, H.; Shirasawa, K.; Isoda, K.; Mishima, K.; Tamura, M.; Watanabe, A. Construction of genetic linkage map and identification of a novel major locus for resistance to pine wood nematode in Japanese black pine (Pinus thunbergii). BMC Plant Biol. 2019, 19, 424. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wu, X.Q.; Wen, T.Y.; Feng, Y.Q.; Zhang, Y. Transcriptomic analysis reveals differentially expressed genes associated with pine wood nematode resistance in resistant Pinus thunbergii. Tree Physiol. 2023, 7, 995–1008. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, W.; Chen, Z.X.; Han, B.; Haque, M.E.; Liu, A.Z. Gene structure, expression pattern and interaction of Nuclear Factor-Y family in castor bean (Ricinus communis). Planta 2018, 247, 559–572. [Google Scholar] [CrossRef]
- Kuang, J.F.; Wu, C.J.; Guo, Y.F.; Walther, D.; Shan, W.; Chen, J.Y.; Chen, L.; Lu, W.J. Deciphering transcriptional regulators of banana fruit ripening by regulatory network analysis. Plant Biotech. J. 2020, 19, 477–489. [Google Scholar] [CrossRef]
- Zhu, J.; Wei, X.; Yin, C.; Zhou, H.; Yan, J.; He, W.; Yan, J.; Li, H. ZmEREB57 regulates OPDA synthesis and enhances salt stress tolerance through two distinct signalling pathways in Zea mays. Plant Cell Environ. 2023, 46, 2867–2883. [Google Scholar] [CrossRef]
- Xie, L.; Liu, S.; Zhang, Y.; Tian, W.; Xu, D.; Li, J.; Luo, X.; Li, L.; Bian, Y.; Li, F.; et al. Efficient proteome-wide identification of transcription factors targeting Glu-1: A case study for functional validation of TaB3-2A1 in wheat. Plant Biotech. J. 2023, 21, 1952–1965. [Google Scholar] [CrossRef]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef]
- Craven-Bartle, B.; Pascual, M.B.; Cánovas, F.M.; Avila, C.A. Myb transcription factor regulates genes of the phenylalanine pathway in maritime pine. Plant J. 2013, 74, 755–766. [Google Scholar] [CrossRef]
- Pandey, A.; Misra, P.; Chandrashekar, K.; Trivedi, P.K. Development of AtMYB12-expressing transgenic tobacco callus culture for production of rutin with biopesticidal potential. Plant Cell Rep. 2012, 31, 1867–1876. [Google Scholar] [CrossRef]
- Yu, Y.; Guo, D.D.; Min, D.H.; Cao, T.; Ning, L.; Jiang, Q.Y.; Sun, X.J.; Zhang, H.; Tang, W.S.; Gao, S.Q.; et al. Foxtail millet MYB-like transcription factor SiMYB16 confers salt tolerance in transgenic rice by regulating phenylpropane pathway. Plant Physiol. Biochem. 2023, 195, 310–321. [Google Scholar] [CrossRef]
- Liu, D.L.; Xue, Y.S.; Wang, R.Z.; Song, B.B.; Xue, C.; Shan, Y.F.; Xue, Z.L.; Wu, J. PbrMYB4, a R2R3-MYB protein, regulates pear stone cell lignification through activation of lignin biosynthesis genes. Hortic. Plant J. 2024; in press. [Google Scholar] [CrossRef]
- Fan, T.; Fan, Y.; Yang, Y.; Qian, D.; Niu, Y.; An, L.; Xiang, Y. SEC1A and SEC6 synergistically regulate pollen tube polar growth. J. Integr. Plant Biol. 2023, 65, 1717–1733. [Google Scholar] [CrossRef]
- Koh, H.; Joo, H.; Lim, C.W.; Lee, S.C. Roles of the pepper JAZ protein CaJAZ1-03 and its interacting partner RING-type E3 ligase CaASRF1 in regulating ABA signaling and drought responses. Plant Cell Environ. 2023, 46, 3242–3257. [Google Scholar] [CrossRef]
- Wang, H.M.; Yu, Y.C.; Fu, C.X.; Zhou, G.K.; Gao, H.H. Progress of a key enzyme-caffeoyl-CoA 3-O-methyltransferase in lignin biosynthesis. Genom. Appl. Biol. 2014, 33, 458–466. [Google Scholar]
- Tak, H.; Negi, S.; Ganapathi, T.R. Overexpression of MusaMYB31, a R2R3 type MYB transcription factor gene indicate its role as a negative regulator of lignin biosynthesis in banana. PLoS ONE 2017, 12, e172695. [Google Scholar] [CrossRef]
- Wagner, A.; Tobimatsu, Y.; Phillips, L.; Flint, H.; Torr, K.; Donaldson, L.; Pears, L.; Ralph, J. CCoAOMT suppression modifies lignin composition in Pinus radiata. Plant J. 2011, 67, 119–129. [Google Scholar] [CrossRef]
- Zhang, Y.; Lv, H.P.; Ma, C.Y.; Guo, L.; Tan, J.F.; Peng, Q.H.; Lin, Z. Cloning of a caffeoyl-coenzyme A O-methyltransferase from Camellia sinensis and analysis of its catalytic activity. J. Zhejiang Univ. Sci. B 2015, 16, 103–112. [Google Scholar] [CrossRef]
- Do, C.T.; Pollet, B.; Thévenin, J.; Sibout, R.; Denoue, D.; Barrière, Y.; Lapierre, C.; Jouanin, L. Both caffeoyl Coenzyme A 3-O-methyltransferase 1 and caffeic acid O-methyltransferase 1 are involved in redundant functions for lignin, flavonoids and sinapoyl malate biosynthesis in Arabidopsis. Planta 2007, 226, 1117–1129. [Google Scholar] [CrossRef]
- Day, A.; Neutelings, G.; Nolin, F.; Grec, S.; Habrant, A.; Crônier, D.; Maher, B.; Rolando, C.; David, H.; Chabbert, B.; et al. Caffeoyl coenzyme A O-methyltransferase down-regulation is associated with modifications in lignin and cell-wall architecture in flax secondary xylem. Plant Physiol. Biochem. 2009, 47, 9–19. [Google Scholar] [CrossRef]
- Jia, L.J.; Tang, H.Y.; Wang, W.Q.; Yuan, T.L.; Wei, W.Q.; Pang, B.; Gong, X.M.; Wang, S.F.; Li, Y.J.; Zhang, D.; et al. A linear nonribosomal octapeptide from Fusarium graminearum facilitates cell-to-cell invasion of wheat. Nat. Commun. 2019, 10, 1–20. [Google Scholar] [CrossRef]
- Nomura, H.; Komori, T.; Uemura, S.; Kanda, Y.; Shimotani, K.; Nakai, K.; Furuichi, T.; Takebayashi, K.; Sugimoto, T.; Sano, S.; et al. Chloroplast-mediated activation of plant immune signalling in Arabidopsis. Nat. Commun. 2012, 3, 926. [Google Scholar] [CrossRef]
- Nomura, H.; Komori, T.; Kobori, M.; Nakahira, Y.; Shiina, T. Evidence for chloroplast control of external Ca2+-induced cytosolic Ca2+ transients and stomatal closure. Plant J. 2008, 53, 988–998. [Google Scholar] [CrossRef]
- Madhu; Sharma, A.; Kaur, A.; Upadhyay, S.K. Glutathione peroxidases in plants: Innumerable role in abiotic stress tolerance and plant development. J. Plant Growth Regul. 2023, 42, 598–613. [Google Scholar] [CrossRef]
- Bela, K.; Bangash, S.A.K.; Riyazuddin; Csiszár, J. Plant Glutathione Peroxidases: Antioxidant Enzymes in Plant Stress Responses and Tolerance. In Glutathione in Plant Growth, Development, and Stress Tolerance; Hossain, M., Mostofa, M., Diaz-Vivancos, P., Burritt, D., Fujita, M., Tran, L.S., Eds.; Springer: Cham, Switzerland, 2017; pp. 113–126. [Google Scholar]
- Fukuda, H. Signals that control plant vascular cell differentiation. Nat. Rev. Mol. Cell Biol. 2004, 5, 379–391. [Google Scholar] [CrossRef]
- Aloni, R. Role of hormones in controlling vascular differentiation and the mechanism of lateral root initiation. Planta 2013, 238, 819–830. [Google Scholar] [CrossRef]
- Li, Y.H.; Han, S.Q.; Qi, Y.H. Advances in structure and function of auxin response factor in plants. J. Integr. Plant Biol. 2022, 65, 617–632. [Google Scholar] [CrossRef]
- Cancé, C.; Martin-Arevalillo, R.; Boubekeur, K.; Dumas, R. Auxin response factors are keys to the many auxin doors. New Phytol. 2022, 235, 402–419. [Google Scholar] [CrossRef]
- Chen, H.; Tan, J.; Liang, X.; Tang, S.; Jia, J.; Yang, Z. Molecular mechanism of lateral bud differentiation of Pinus massoniana based on high-throughput sequencing. Sci. Rep. 2021, 11, 9033. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.; Tran, L.S. Understanding plant responses to phosphorus starvation for improvement of plant tolerance to phosphorus deficiency by biotechnological approaches. Crit. Rev. Biotechnol. 2014, 34, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Sekhwal, M.K.; Swami, A.K.; Sharma, V.; Sarin, R. Identification of drought-induced transcription factors in Sorghum bicolor using GO term semantic similarity. Cell Mol. Biol. Lett. 2015, 20, 1–23. [Google Scholar] [CrossRef]
- Zhao, F.Y.; Hu, F.; Zhang, S.Y.; Wang, K.; Zhang, C.R.; Liu, T. MAPKs regulate root growth by influencing auxin signaling and cell cycle-related gene expression in cadmium-stressed rice. Environ. Sci. Pollut. Res. Int. 2013, 20, 5449–5460. [Google Scholar] [CrossRef]

| Cis-Regulatory Element | Core Sequence | Totals | Function |
|---|---|---|---|
| AAGAA-motif | gGTAAAGAAA/GAAAGAA | 3 | Unknown |
| ABRE | CACGTG/CACGTG | 3 | cis-acting element involved in the abscisic acid responsiveness |
| ABRE3a | TACGTG | 1 | cis-acting element involved in abiotic stress and signaling pathway |
| ABRE4 | CACGTA | 1 | cis-acting element involved in abiotic stress and signaling pathway |
| AE-box | AGAAACTT | 1 | part of a module for light response |
| ARE | AAACCA | 5 | cis-acting regulatory element essential for the anaerobic induction |
| AT~TATA-box | TATATAAA | 3 | involved in the formation of a transcription initiation complex |
| CAAT-box | CAAAT/CAAT | 39 | common cis-acting element in promoter and enhancer regions |
| CCAAT-box | CAACGG | 1 | MYBHv1 binding site |
| ERE | ATTTTAAA | 1 | cis-acting element involved in the response to ethylene |
| G-Box | CACGTG | 2 | cis-acting regulatory element involved in light responsiveness |
| G-box | TAACACGTAG/GCCACGTGGA/TACGTG/CACGTG | 4 | cis-acting regulatory element involved in light responsiveness |
| GT1-motif | GGTTAA | 1 | light responsive element |
| LTR | CCGAAA | 1 | cis-acting element involved in low-temperature responsiveness |
| MBS | CAACTG | 2 | MYB binding site involved in drought-inducibility |
| MRE | AACCTAA | 1 | MYB binding site involved in light responsiveness |
| MYB | CAACAG/CAACCA/TAACCA | 6 | MYB binding site |
| MYB recognition site | CCGTTG | 1 | MYB binding site |
| MYB-like sequence | TAACCA | 3 | MYB binding site |
| MYC | CAATTG/CATTTG | 2 | basic helix-loop-helix (bHLH) binding motifs |
| Myb | CAACTG | 2 | MYB binding site |
| Myb-binding site | CAACAG | 2 | MYB binding site |
| O2-site | GATGATGTGG | 1 | cis-acting regulatory element involved in zein metabolism regulation |
| P-box | CCTTTTG | 1 | gibberellin-responsive element |
| STRE | AGGGG | 2 | involved in peroxisome biogenesis, function, and regulation |
| TATA | TATAAAAT | 2 | involved in the formation of a transcription initiation complex |
| TATA-box | TATATAA | 45 | core promoter element around −30 of transcription start |
| TCA-element | CCATCTTTTT | 2 | cis-acting element involved in salicylic acid responsiveness |
| TCCC-motif | TCTCCCT | 1 | part of a light responsive element |
| Unnamed_1 | CGTGG | 2 | Unknown |
| Unnamed_2 | AACCTAACCT | 1 | Unknown |
| Unnamed_4 | CTCC | 6 | Unknown |
| W box | TTGACC | 1 | a core sequence acts as a binding site for WRKY TFs |
| WUN-motif | AAATTACT/TTATTACAT | 2 | wound-responsive element |
| chs-CMA1a | TTACTTAA | 1 | part of a light responsive element |
| circadian | CAAAGATATC | 1 | cis-acting regulatory element involved in circadian control |
| Accession | Description | Sum PEP Score | Peptides | Unique Peptides |
|---|---|---|---|---|
| AIZ74346.1 | phosphoglycerate kinase 1 [Pinus massoniana] | 109.786 | 26 | 26 |
| AHL24663.1 | ribulose-1,5-bisphosphate carboxylase/oxygenase activase large isoform [Pinus massoniana] | 116.557 | 23 | 23 |
| ULQ63856.1 | ATP synthase CF1 beta subunit (chloroplast) [Cuscuta japonica] | 47.272 | 12 | 2 |
| AIZ74323.1 | actin related protein 1 [Pinus massoniana] | 28.62 | 11 | 11 |
| QEP51812.1 | elongation factor [Pinus massoniana] | 35.434 | 10 | 1 |
| AIZ74328.1 | translation elongation factor 1-alpha [Pinus massoniana] | 33.002 | 10 | 1 |
| AFA51418.1 | extracellular calcium sensing receptor [Pinus massoniana] | 28.98 | 10 | 10 |
| AGC13142.1 | DHAR class glutathione S-transferase [Pinus tabuliformis] | 23.517 | 9 | 9 |
| ADV40957.1 | caffeoyl-CoAO-methyltransferase [Pinus radiata] | 17.378 | 7 | 7 |
| AIZ74331.1 | alpha-tubulin [Pinus massoniana] | 14.751 | 7 | 6 |
| AGT98543.1 | glutathione peroxidase 2 [Pinus tabuliformis] | 13.11 | 6 | 4 |
| AIZ74330.1 | cyclophilin [Pinus massoniana] | 14.913 | 5 | 5 |
| QSD59059.1 | heat shock 90 kDa protein [Pinus sylvestris] | 10.745 | 5 | 5 |
| CAA41404.1 | Type 1 chlorophyll a /b-binding protein [Pinus sylvestris] | 7.835 | 2 | 2 |
| ACJ70336.1 | putative ribosomal protein S10, partial [Pinus sylvestris] | 4.348 | 2 | 1 |
| AGC13149.1 | phi class glutathione S-transferase [Pinus tabuliformis] | 3.945 | 2 | 2 |
| AHA90706.1 | aquaporin [Pinus massoniana] | 3.003 | 2 | 2 |
| CBM40481.1 | MYB8 transcription factor [Pinus pinaster] | 0.752 | 1 | 1 |
| ACL14200.1 | putative ribosomal protein L34, partial [Pinus sylvestris] | 0.749 | 1 | 1 |
| AXQ01589.1 | photosystem II protein K (plastid) [Pinus pinea] | 0.714 | 1 | 1 |
| YP_008082259.1 | ribosomal protein S12 (chloroplast) [Pinus massoniana] | 0.674 | 1 | 1 |
| Accession | Description | Sum PEP Score | Peptides | Unique Peptides |
|---|---|---|---|---|
| AHL24663.1 | ribulose-1,5-bisphosphate carboxylase/oxygenase activase large isoform [Pinus massoniana] | 68.19 | 23 | 23 |
| WCL24039.1 | ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit (chloroplast) [Pinus massoniana] | 30.174 | 13 | 13 |
| QEP51812.1 | elongation factor [Pinus massoniana] | 18.672 | 8 | 2 |
| ASU09148.1 | disease resistance protein [Pinus massoniana] | 21.065 | 7 | 7 |
| AIZ74328.1 | translation elongation factor 1-alpha [Pinus massoniana] | 16.688 | 7 | 1 |
| WCL23812.1 | cytochrome f (chloroplast) [Pinus massoniana] | 14.926 | 7 | 7 |
| AFA51418.1 | extracellular calcium sensing receptor [Pinus massoniana] | 13.155 | 17 | 6 |
| AIZ74323.1 | actin related protein 1 [Pinus massoniana] | 12.518 | 6 | 6 |
| WCL24807.1 | photosystem II 44 kDa protein (chloroplast) [Pinus massoniana] | 8.537 | 6 | 6 |
| WCL24370.1 | photosytem I subunit VII (chloroplast) | 8.045 | 5 | 5 |
| WCL24671.1 | photosystem II protein D1 (chloroplast) [Pinus massoniana] | 6.064 | 4 | 4 |
| WCL25227.1 | photosystem II 47 kDa protein (chloroplast) [Pinus massoniana] | 5.584 | 4 | 4 |
| AIZ74332.1 | beta-tubulin [Pinus massoniana] | 4.894 | 4 | 4 |
| AIZ74331.1 | alpha-tubulin [Pinus massoniana] | 6.451 | 3 | 3 |
| WCL23846.1 | photosystem II protein D2 (chloroplast) [Pinus massoniana] | 4.799 | 3 | 3 |
| ACY66805.1 | chlorophyll a/b-binding protein [Pinus massoniana] | 4.253 | 3 | 3 |
| AHJ86267.1 | glutathione peroxidase [Pinus massoniana] | 5.426 | 14 | 2 |
| AIZ74335.1 | polyubiquitin 3, partial [Pinus massoniana] | 2.894 | 2 | 2 |
| WCL24189.1 | ATP synthase CF1 epsilon subunit (chloroplast) [Pinus massoniana] | 2.562 | 2 | 2 |
| WCL23911.1 | ribosomal protein L2 (chloroplast) [Pinus massoniana] | 4.188 | 1 | 1 |
| AIZ74341.1 | isocitrate dehydrogenase [Pinus massoniana] | 3.581 | 1 | 1 |
| WCL38145.1 | photosystem I P700 chlorophyll a apoprotein A1 (chloroplast) [Pinus massoniana] | 2.016 | 1 | 1 |
| ACV88654.1 | cyclophilin [Pinus massoniana] | 1.319 | 1 | 1 |
| WCL24138.1 | photosystem I P700 chlorophyll a apoprotein A2 (chloroplast) [Pinus massoniana] | 1.117 | 1 | 1 |
| WCL25009.1 | cytochrome b6 (chloroplast) [Pinus massoniana] | 1.06 | 1 | 1 |
| WCL25872.1 | ATP-dependent Clp protease proteolytic subunit (chloroplast) [Pinus massoniana] | 0.863 | 1 | 1 |
| AMR43653.1 | purple acid phosphatase 1 [Pinus massoniana] | 0.861 | 1 | 1 |
| WCL25530.1 | ribosomal protein S11 (chloroplast) [Pinus massoniana] | 0.861 | 1 | 1 |
| AIF75959.1 | putative phosphofructokinase, partial [Pinus massoniana] | 0.842 | 1 | 1 |
| AVP71779.1 | auxin response factor 16 [Pinus massoniana] | 0.763 | 3 | 1 |
| UFA45708.1 | bHLH10 [Pinus massoniana] | 0.684 | 1 | 1 |
| WCL23782.1 | Ycf2 (chloroplast) [Pinus massoniana] | 0.677 | 1 | 1 |
| AHL67654.1 | caffeoyl-CoA 3-O-methyltransferase [Pinus massoniana] | 0.635 | 5 | 1 |
| AIF75747.1 | dehydrin 1 protein, partial [Pinus massoniana] | 0.621 | 5 | 1 |
| WCL25253.1 | hypothetical chloroplast RF68 (chloroplast) [Pinus massoniana] | 0.595 | 1 | 1 |
| UIB01906.1 | 2-C-methyl-D-erythritol 4-phosphate cytidylyltransferase [Pinus massoniana] | 0.584 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, W.; Lai, X.; Wu, Y.; Li, Z.; Zhu, J.; Huang, Y.; Zhang, F. Transcription Factor and Protein Regulatory Network of PmACRE1 in Pinus massoniana Response to Pine Wilt Nematode Infection. Plants 2024, 13, 2672. https://doi.org/10.3390/plants13192672
Xie W, Lai X, Wu Y, Li Z, Zhu J, Huang Y, Zhang F. Transcription Factor and Protein Regulatory Network of PmACRE1 in Pinus massoniana Response to Pine Wilt Nematode Infection. Plants. 2024; 13(19):2672. https://doi.org/10.3390/plants13192672
Chicago/Turabian StyleXie, Wanfeng, Xiaolin Lai, Yuxiao Wu, Zheyu Li, Jingwen Zhu, Yu Huang, and Feiping Zhang. 2024. "Transcription Factor and Protein Regulatory Network of PmACRE1 in Pinus massoniana Response to Pine Wilt Nematode Infection" Plants 13, no. 19: 2672. https://doi.org/10.3390/plants13192672





