Alleviating Effects of Methyl Jasmonate on Pepper (Capsicum annuum L.) Seedlings under Low-Temperature Combined with Low-Light Stress
Abstract
:1. Introduction
2. Results
2.1. Effect of Exogenous MeJA on Growth Indexes of Pepper Seedlings under LL Stress
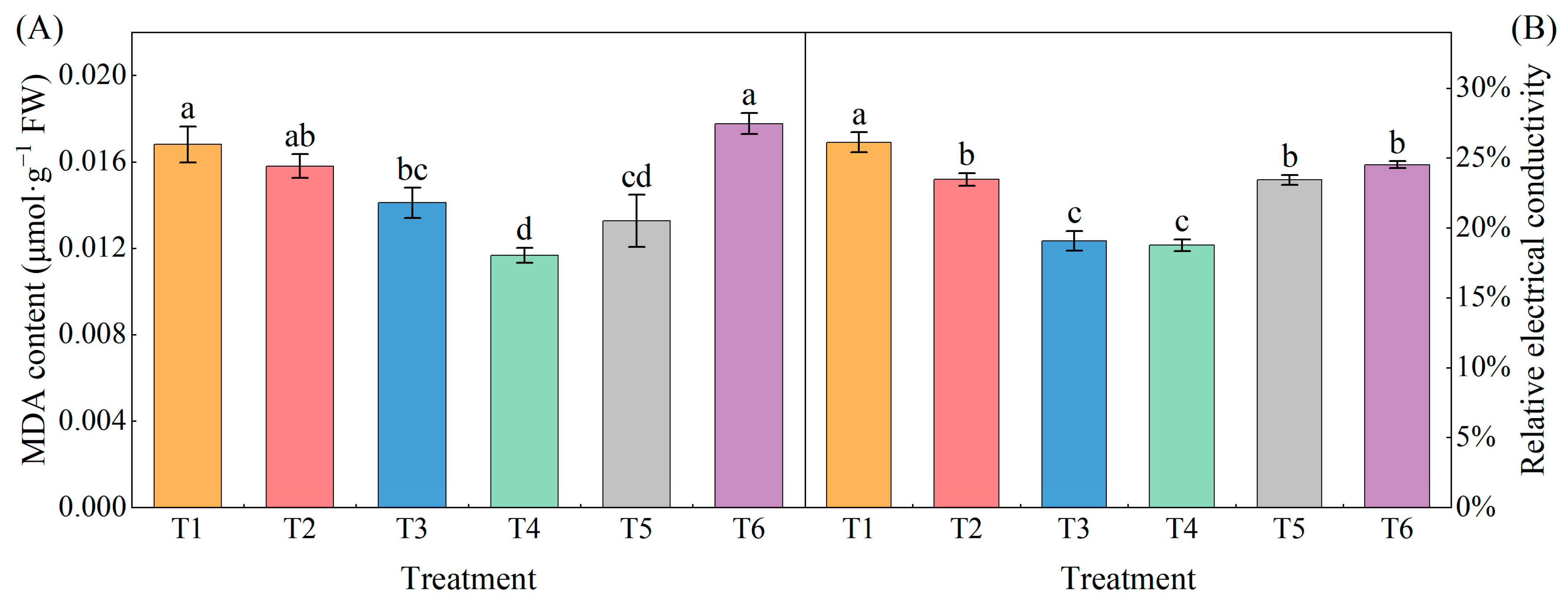
2.2. Effects of Exogenous MeJA on Malondialdehyde (MDA) and Relative Conductivity of Pepper Seedlings under LL Stress
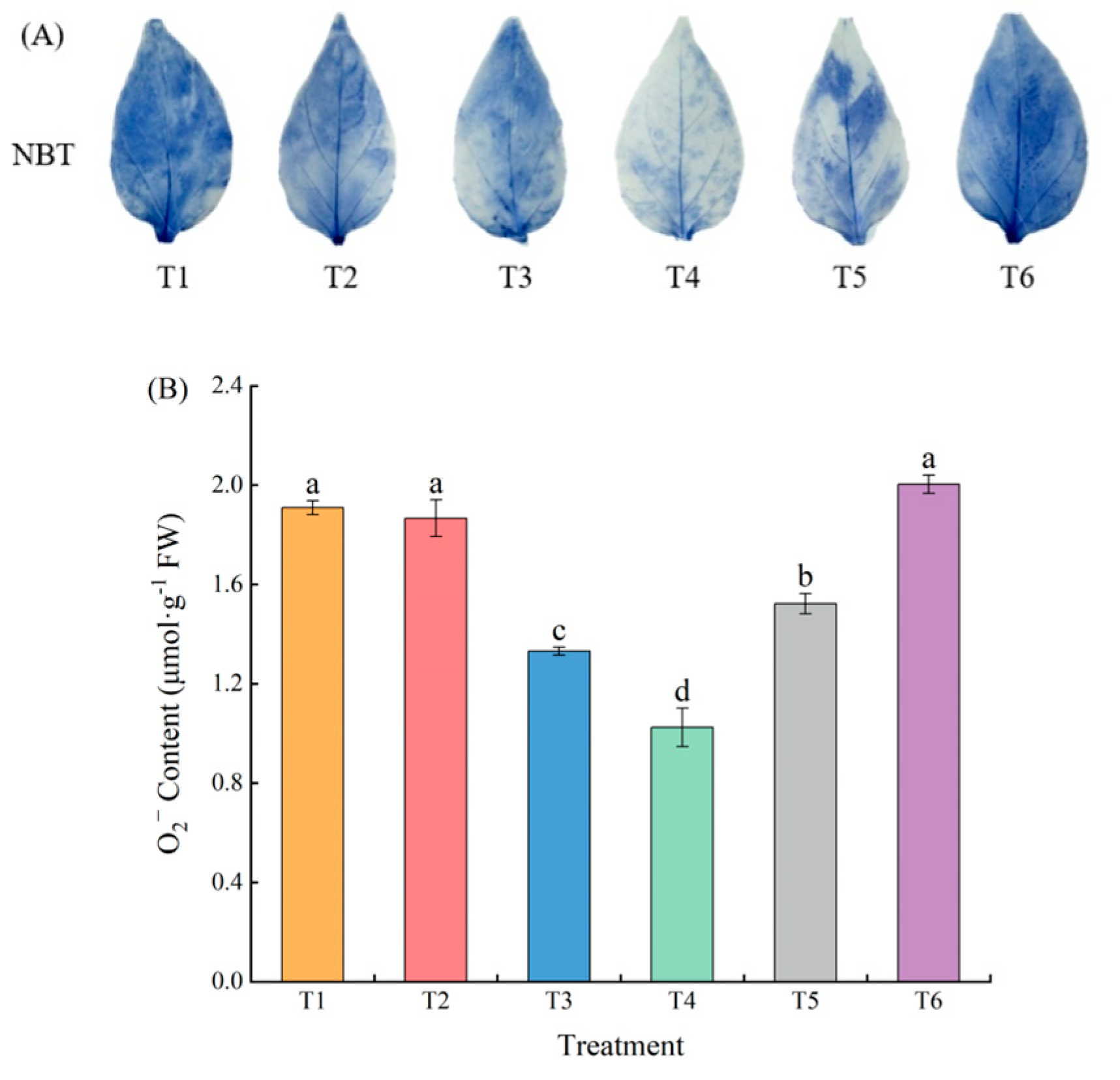
2.3. Effects of Exogenous MeJA on the Superoxide Anion Content of Pepper Seedlings under LL Stress
2.4. Effects of Exogenous MeJA on Photosynthetic Capacity of Pepper Seedlings under LL Stress
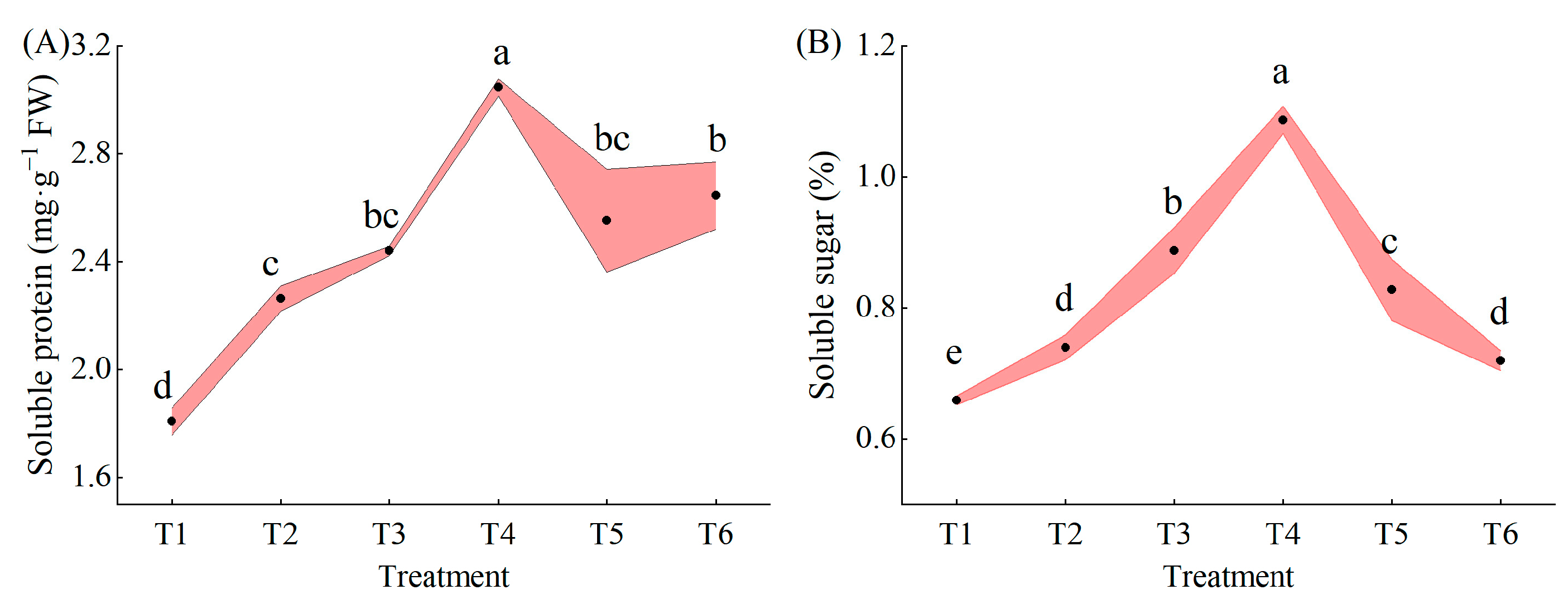
2.5. Effects of Exogenous MeJA on Soluble Protein and Soluble Sugars in Pepper Seedlings under LL Stress
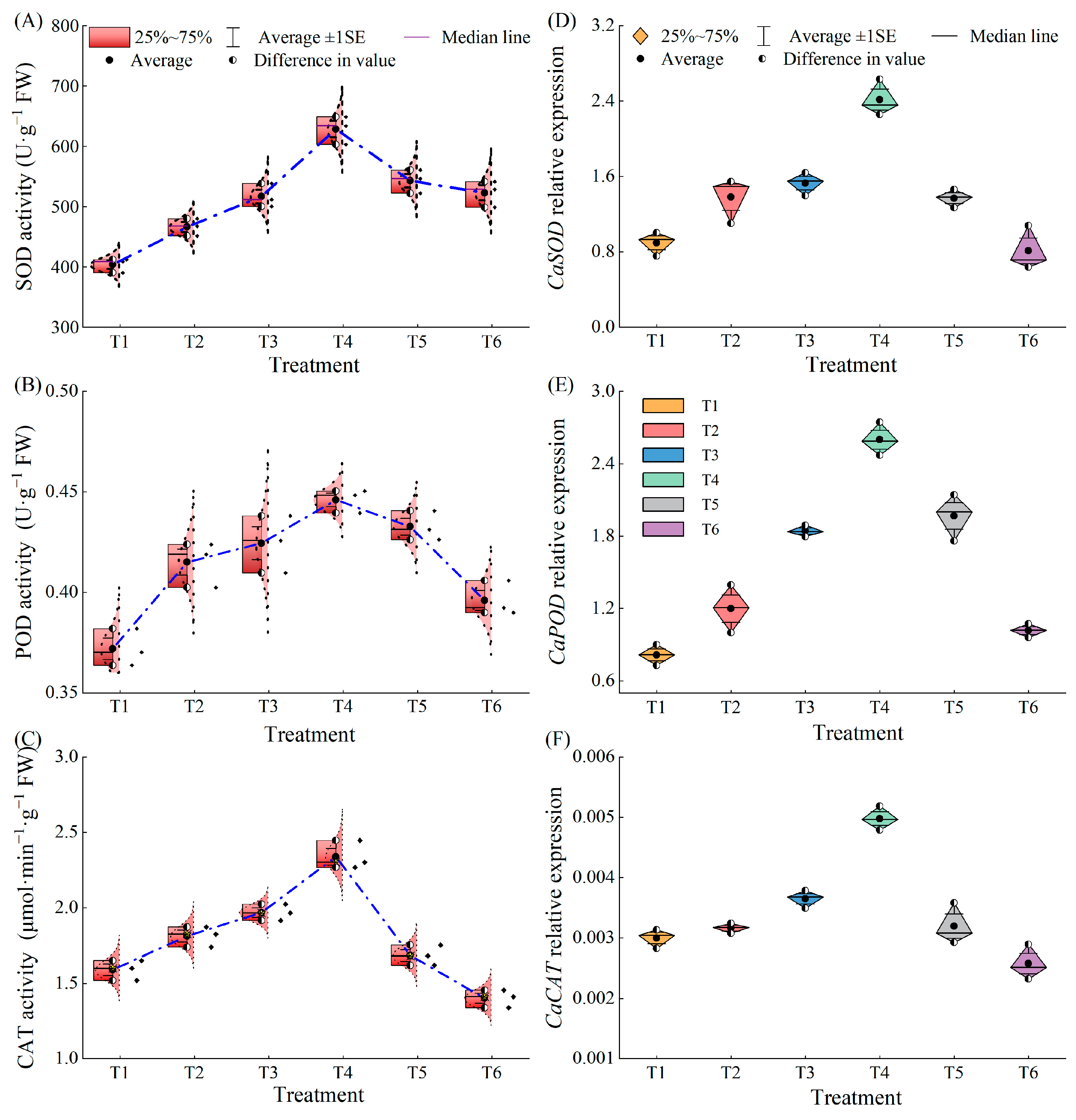
2.6. Effects of Exogenous MeJA on Antioxidant Enzyme Activities and Related Gene Expressions in Pepper Seedlings under LL Stress
3. Discussion
4. Materials and Methods
4.1. Experimental Materials and Growth Conditions
4.2. Treatments
4.2.1. Different Methyl Jasmonate (MeJA) Concentration Treatments
4.2.2. Alleviating Effects of Exogenous Methyl Jasmonate (MeJA) on Pepper Plants under LL Stress
4.3. Determinations
4.3.1. Measurements of Physiological and Biochemical Indicators
4.3.2. Determination of Relative Conductivity and MDA Content of Leaves
4.3.3. Determination of Reactive Oxygen Species Content and Histochemical Staining
4.3.4. Determination of Chlorophyll Content
4.3.5. Determination of Chlorophyll Fluorescence Parameters
4.3.6. Rapid Chlorophyll Fluorescence-Induced Kinetic Curve (OJIP) Assay
4.3.7. Determination of Soluble Protein and Soluble Sugar
4.3.8. Determination of Antioxidant Enzyme Activity
4.3.9. Measurement of Gene Expression
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bohra, A.; Gahlaut, V.; Perovic, D.; Varshney, R.K. Genetics and epigenetics: Plausible role in development of climate resilient crops. Front. Genet. 2023, 14, 1165843. [Google Scholar] [CrossRef] [PubMed]
- Boguszewska, D.; Zagdańska, B. ROS as signaling molecules and enzymes of plant response to unfavorable environmental conditions. In Oxidative Stress–Molecular Mechanisms and Biological Effects; InTech: Rijeka, Croatia, 2012; pp. 341–362. [Google Scholar]
- Hasanuzzaman, M.; Mahmud, J.A.; Anee, T.I.; Nahar, K.; Islam, M.T. Drought stress tolerance in wheat: Omics approaches in understanding and enhancing antioxidant defense. In Abiotic Stress-Mediated Sensing and Signaling in Plants: An Omics Perspective; Springer: Berlin/Heidelberg, Germany, 2018; pp. 267–307. [Google Scholar]
- Li, J.; Xie, J.; Yu, J.; Lyv, J.; Zhang, J.; Ding, D.; Li, N.; Zhang, J.; Bakpa, E.P.; Yang, Y. Melatonin enhanced low-temperature combined with low-light tolerance of pepper (Capsicum annuum L.) seedlings by regulating root growth, antioxidant defense system, and osmotic adjustment. Front. Plant Sci. 2022, 13, 998293. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Zhou, Z.; Li, Z.; Chen, Y.; Wang, Z.; Zhang, H. Rice (Oryza sativa L.) with reduced chlorophyll content exhibit higher photosynthetic rate and efficiency, improved canopy light distribution, and greater yields than normally pigmented plants. Field Crops Res. 2017, 200, 58–70. [Google Scholar] [CrossRef]
- Ding, D.; Li, J.; Xie, J.; Li, N.; Bakpa, E.P.; Han, K.; Yang, Y.; Wang, C. Exogenous zeaxanthin alleviates low temperature combined with low light induced photosynthesis inhibition and oxidative stress in pepper (Capsicum annuum L.) plants. Curr. Issues Mol. Biol. 2022, 44, 2453–2471. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Rinklebe, J.; Ahmad, P. Alleviation of arsenic toxicity in pepper plants by aminolevulinic acid and heme through modulating its sequestration and distribution within cell organelles. Environ. Pollut. 2023, 330, 121747. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Zhang, Z.; Shi, Q.; Duan, X.; Du, J.; Wu, C.; Li, X. Methyl jasmonate-and salicylic acid-induced transcription factor ZjWRKY18 regulates triterpenoid accumulation and salt stress tolerance in jujube. Int. J. Mol. Sci. 2023, 24, 3899. [Google Scholar] [CrossRef]
- Zaid, A.; Mohammad, F. Methyl jasmonate and nitrogen interact to alleviate cadmium stress in Mentha arvensis by regulating physio-biochemical damages and ROS detoxification. J. Plant Growth Regul. 2018, 37, 1331–1348. [Google Scholar] [CrossRef]
- Sharma, M.; Laxmi, A. Jasmonates: Emerging players in controlling temperature stress tolerance. Front. Plant Sci. 2016, 6, 165479. [Google Scholar] [CrossRef]
- Alhaithloul, H.A.S.; Abu-Elsaoud, A.M.; Soliman, M.H. Abiotic stress tolerance in crop plants: Role of phytohormones. In Abiotic Stress Plants; IntechOpen: London, UK, 2020; p. 233. [Google Scholar] [CrossRef]
- Asif, A.; Baig, M.A.; Siddiqui, M.B. Role of jasmonates and salicylates in plant allelopathy. In Jasmonates and Salicylates Signaling in Plants; Springer: Berlin/Heidelberg, Germany, 2021; pp. 115–127. [Google Scholar]
- Phan, H.; Schläppi, M. Low temperature antioxidant activity QTL associate with genomic regions involved in physiological cold stress tolerance responses in rice (Oryza Sativa L.). Genes 2021, 12, 1700. [Google Scholar] [CrossRef]
- Li, L.; Staden, J.v.; Jäger, A. Effects of plant growth regulators on the antioxidant system in seedlings of two maize cultivars subjected to water stress. Plant Growth Regul. 1998, 25, 81–87. [Google Scholar] [CrossRef]
- Tang, C.; Xie, J.; Lv, J.; Li, J.; Zhang, J.; Wang, C.; Liang, G. Alleviating damage of photosystem and oxidative stress from chilling stress with exogenous zeaxanthin in pepper (Capsicum annuum L.) seedlings. Plant Physiol. Biochem. 2021, 162, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Saadati, S.; Baninasab, B.; Mobli, M.; Gholami, M. Enhancement of freezing tolerance of olive leaves by foliar application of methyl jasmonate and 24–epibrassinolide through changes in some metabolites and antioxidant activity. Sci. Hortic. 2021, 284, 110127. [Google Scholar] [CrossRef]
- Safavi-Rizi, V.; Uellendahl, K.; Öhrlein, B.; Safavi-Rizi, H.; Stöhr, C. Cross-stress tolerance: Mild nitrogen (N) deficiency effects on drought stress response of tomato (Solanum lycopersicum L.). Plant-Environ. Interact. 2021, 2, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, P.; Gan, Y.; Yu, J.; Xie, J. Brassinosteroid alleviates chilling-induced oxidative stress in pepper by enhancing antioxidation systems and maintenance of photosystem II. Acta Physiol. Plant. 2015, 37, 222. [Google Scholar] [CrossRef]
- Arshad, M.; Ullah, S.; Khurshid, K.; Ali, A. Estimation of leaf water content from mid-and thermal-infrared spectra by coupling genetic algorithm and partial least squares regression. J. Appl. Remote Sens. 2018, 12, 022203. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Fujita, M. Exogenous spermidine alleviates low temperature injury in mung bean (Vigna radiata L.) seedlings by modulating ascorbate-glutathione and glyoxalase pathway. Int. J. Mol. Sci. 2015, 16, 30117–30132. [Google Scholar] [CrossRef] [PubMed]
- Talaat, N.B. Drought stress alleviator melatonin reconfigures water-stressed barley (Hordeum vulgare L.) plants’ photosynthetic efficiency, antioxidant capacity, and endogenous phytohormone profile. Int. J. Mol. Sci. 2023, 24, 16228. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Sharma, P. Effect of low light stress on photosynthetic pigments and antioxidative enzymes in field grown Indian mustard (Brassica juncea L.) genotypes. J. Agric. Sci. Technol. 2021, 11, 61–72. [Google Scholar]
- Yu, X.; He, J.; Li, H. Effects of exogenous melatonin on photosynthetic characteristics of eggplant seedlings under low temperature and weak light stress. In Proceedings of the 2015 6th International Conference on Manufacturing Science and Engineering, Guangzhou, China, 28–29 November 2015; pp. 724–728. [Google Scholar]
- Han, C.; Liu, Y.; Shi, W.; Qiao, Y.; Wang, L.; Tian, Y.; Fan, M.; Deng, Z.; Lau, O.S.; De Jaeger, G. KIN10 promotes stomatal development through stabilization of the SPEECHLESS transcription factor. Nat. Commun. 2020, 11, 4214. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.-l.; Mao, S.-l.; Wang, L.-h.; Zhang, B.-x.; Zhang, Z.-x. Effect of low light on the characteristics of photosynthesis and chlorophyll a fluorescence during leaf development of sweet pepper. J. Integr. Agric. 2012, 11, 1633–1643. [Google Scholar] [CrossRef]
- Yu, J.-Q.; Zhou, Y.-H.; Huang, L.-F.; Allen, D.J. Chill-induced inhibition of photosynthesis: Genotypic variation within Cucumis sativus. Plant Cell Physiol. 2002, 43, 1182–1188. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Han, H.; Feng, H.L.; Zhao, S.J.; Meng, Q.W. Overexpression and suppression of violaxanthin de-epoxidase affects the sensitivity of photosystem ii photoinhibition to high light and chilling stress in transgenic tobacco. J. Integr. Plant Biol. 2010, 52, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Rácz, A.; Czégény, G.; Kutyáncsánin, D.; Nagy, N.; Hideg, É.; Csepregi, K. Fight against cold: Photosynthetic and antioxidant responses of different bell pepper cultivars (Capsicum annuum L.) to cold stress. Biol. Futur. 2023, 74, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, M.; Sperdouli, I.; Adamakis, I.-D.S.; Şaş, B.; İşgören, S.; Moustaka, J.; Morales, F. Mechanistic Approach on Melatonin-Induced Hormesis of Photosystem II Function in the Medicinal Plant Mentha spicata. Plants 2023, 12, 4025. [Google Scholar] [CrossRef]
- Zhang, H.; Fan, J.; Peng, Q.; Song, W.; Zhou, Y.; Xia, C.; Ma, J. Effects of chlormequat chloride and paclobutrazol on the growth and chlorophyll fluorescence kinetics of Daphne genkwa. Russ. J. Plant Physiol. 2023, 70, 110. [Google Scholar] [CrossRef]
- Oukarroum, A.; Lebrihi, A.; El Gharous, M.; Goltsev, V.; Strasser, R.J. Desiccation-induced changes of photosynthetic transport in Parmelina tiliacea (Hoffm.) Ach. analysed by simultaneous measurements of the kinetics of prompt fluorescence, delayed fluorescence and modulated 820 nm reflection. J. Lumin. 2018, 198, 302–308. [Google Scholar] [CrossRef]
- Goussi, R.; Manaa, A.; Derbali, W.; Cantamessa, S.; Abdelly, C.; Barbato, R. Comparative analysis of salt stress, duration and intensity, on the chloroplast ultrastructure and photosynthetic apparatus in Thellungiella salsuginea. J. Photochem. Photobiol. B Biol. 2018, 183, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Çiçek, N.; Oukarroum, A.; Strasser, R.J.; Schansker, G. Salt stress effects on the photosynthetic electron transport chain in two chickpea lines differing in their salt stress tolerance. Photosynth. Res. 2018, 136, 291–301. [Google Scholar] [CrossRef]
- Tietz, S.; Hall, C.C.; Cruz, J.A.; Kramer, D.M. NPQ (T): A Chlorophyll Fluorescence Parameter for Rapid Estimation and Imaging of Non-Photochemical Quenching of Excitons in Photosystem-II-Associated Antenna Complexes; 0140-7791; Wiley Online Library: Hoboken, NJ, USA, 2017. [Google Scholar]
- Lotfi, R.; Kalaji, H.; Valizadeh, G.; Khalilvand Behrozyar, E.; Hemati, A.; Gharavi-Kochebagh, P.; Ghassemi, A. Effects of humic acid on photosynthetic efficiency of rapeseed plants growing under different watering conditions. Photosynthetica 2018, 56, 962–970. [Google Scholar] [CrossRef]
- Kiran, B.R.; Mohan, S.V. Photosynthetic transients in Chlorella sorokiniana during phycoremediation of dairy wastewater under distinct light intensities. Bioresour. Technol. 2021, 340, 125593. [Google Scholar]
- Liang, Y.; Chen, H.; Tang, M.J.; Yang, P.F.; Shen, S.H. Responses of Jatropha curcas seedlings to cold stress: Photosynthesis-related proteins and chlorophyll fluorescence characteristics. Physiol. Plant. 2007, 131, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive oxygen species in plant signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef]
- Choudhary, A.; Kumar, A.; Kaur, N. ROS and oxidative burst: Roots in plant development. Plant Divers. 2020, 42, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef]
- Gul, N.; Masoodi, K.Z.; Ramazan, S.; Mir, J.I.; Aslam, S. Study on the impact of exogenously applied methyl jasmonate concentrations on Solanum lycopersicum under low temperature stress. BMC Plant Biol. 2023, 23, 437. [Google Scholar] [CrossRef]
- Mullarky, E.; Cantley, L.C. Diverting glycolysis to combat oxidative stress. In Innovative Medicine: Basic Research and Development; Springer: Berlin/Heidelberg, Germany, 2015; pp. 3–23. [Google Scholar] [CrossRef]
- Iwaniuk, P.; Kaczyński, P.; Pietkun, M.; Łozowicka, B. Evaluation of titanium and silicon role in mitigation of fungicides toxicity in wheat expressed at the level of biochemical and antioxidant profile. Chemosphere 2022, 308, 136284. [Google Scholar] [CrossRef] [PubMed]
- Iwaniuk, P.; Łuniewski, S.; Kaczyński, P.; Łozowicka, B. The Influence of Humic Acids and Nitrophenols on Metabolic Compounds and Pesticide Behavior in Wheat under Biotic Stress. Agronomy 2023, 13, 1378. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, S.; Chen, Z.; Wang, X.; Jiang, Q.; Zhao, J.; Duan, B.; Xi, Z. Transcriptomic analysis provides insights into the abscisic acid mediates brassinosteroid-induced cold resistance of grapevine (Vitis vinifera L.). Plant Growth Regul. 2023, 101, 845–860. [Google Scholar] [CrossRef]
- Farooq, M.; Ali, S.; Hameed, A.; Bharwana, S.; Rizwan, M.; Ishaque, W.; Farid, M.; Mahmood, K.; Iqbal, Z. Cadmium stress in cotton seedlings: Physiological, photosynthesis and oxidative damages alleviated by glycinebetaine. S. Afr. J. Bot. 2016, 104, 61–68. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef] [PubMed]
- Aldesuquy, H.; Ghanem, H. Exogenous salicylic acid and trehalose ameliorate short term drought stress in wheat cultivars by up-regulating membrane characteristics and antioxidant defense system. J. Hortic 2015, 2, 1–10. [Google Scholar] [CrossRef]
- Xie, X.; He, Z.; Chen, N.; Tang, Z.; Wang, Q.; Cai, Y. The roles of environmental factors in regulation of oxidative stress in plant. BioMed Res. Int. 2019, 2019, 9732325. [Google Scholar] [CrossRef] [PubMed]
- Couée, I.; Sulmon, C.; Gouesbet, G.; El Amrani, A. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J. Exp. Bot. 2006, 57, 449–459. [Google Scholar] [CrossRef]
- Zhou, B.; Deng, Y.-S.; Kong, F.-Y.; Li, B.; Meng, Q.-W. Overexpression of a tomato carotenoid ɛ-hydroxylase gene alleviates sensitivity to chilling stress in transgenic tobacco. Plant Physiol. Biochem. 2013, 70, 235–245. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Jiang, W.; Liu, D. Cadmium accumulation, activities of antioxidant enzymes, and malondialdehyde (MDA) content in Pistia stratiotes L. Environ. Sci. Pollut. Res. 2013, 20, 1117–1123. [Google Scholar] [CrossRef]
- Li, N.; Pu, K.; Ding, D.; Yang, Y.; Niu, T.; Li, J.; Xie, J. Foliar Spraying of Glycine Betaine Alleviated Growth inhibition, photoinhibition, and oxidative stress in pepper (Capsicum annuum L.) seedlings under low temperatures combined with low light. Plants 2023, 12, 2563. [Google Scholar] [CrossRef]
- Li, J.; Ding, D.; Li, N.; Xie, J.; Yu, J.; Lyv, J.; Bakpa, E.P.; Zhang, J.; Wang, C.; Zhang, J. Melatonin enhances the low-temperature combined low-light tolerance of pepper (Capsicum annuum L.) seedlings by regulating photosynthesis, carotenoid, and hormone metabolism. Environ. Exp. Bot. 2022, 199, 104868. [Google Scholar] [CrossRef]
- Stirbet, A. On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and photosystem II: Basics and applications of the OJIP fluorescence transient. J. Photochem. Photobiol. B Biol. 2011, 104, 236–257. [Google Scholar] [CrossRef] [PubMed]
- Kučerová, K.; Henselová, M.; Slováková, Ľ.; Hensel, K. Effects of plasma activated water on wheat: Germination, growth parameters, photosynthetic pigments, soluble protein content, and antioxidant enzymes activity. Plasma Process. Polym. 2019, 16, 1800131. [Google Scholar] [CrossRef]


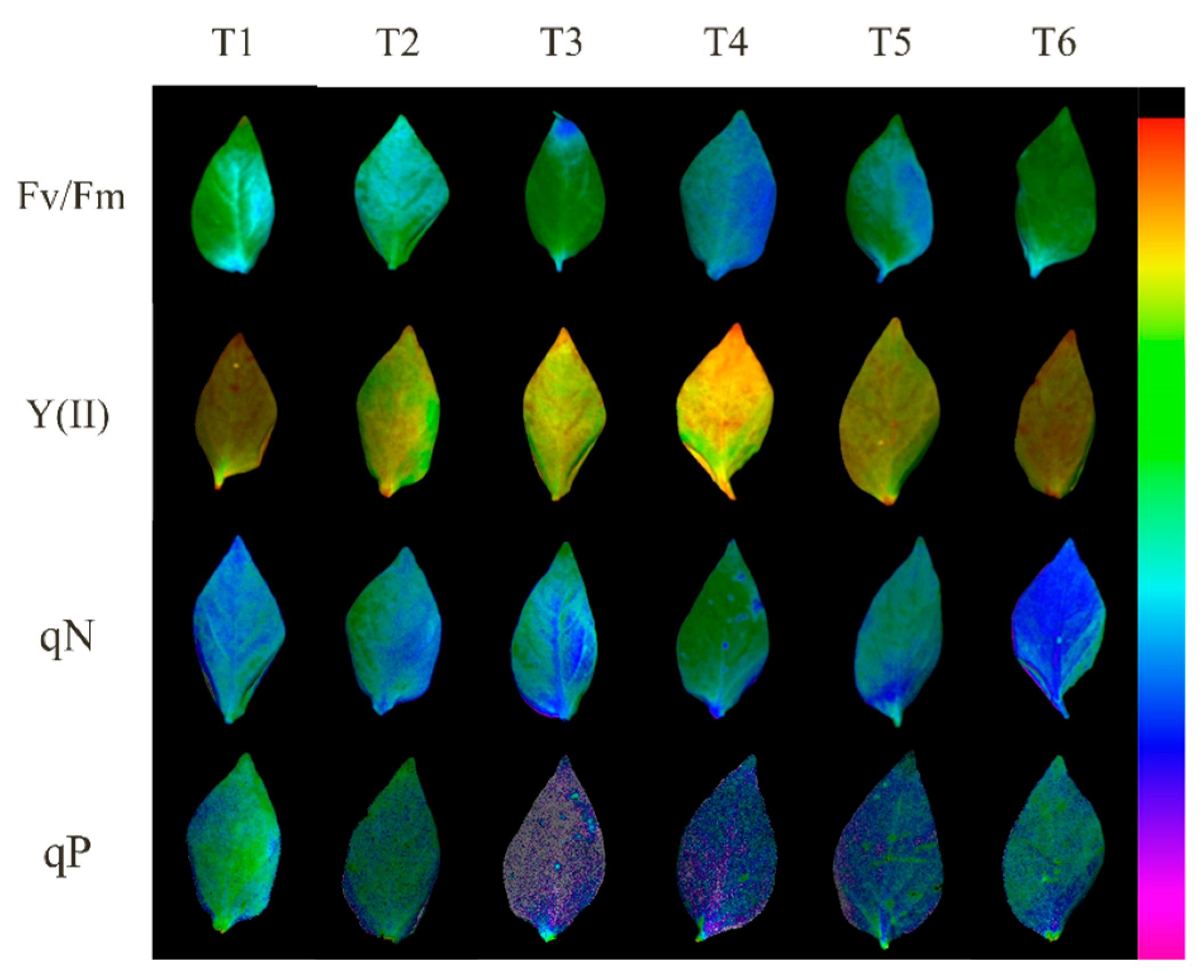
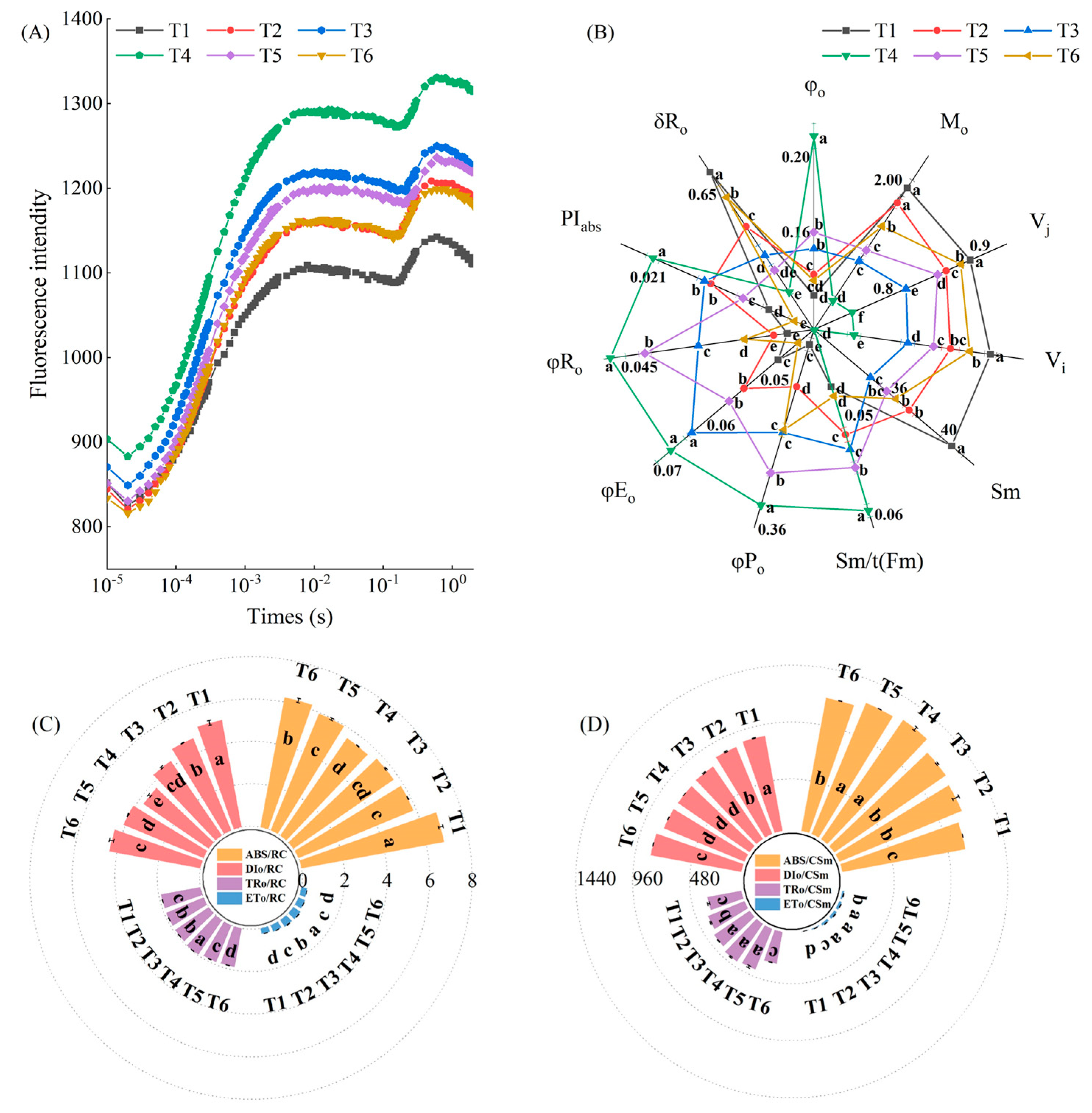
| Treatment | Fv/Fm | Y(II) | NPQ | qN | qP | 1-qP | (1-qP)/NPQ |
|---|---|---|---|---|---|---|---|
| T1 | 0.374 ± 0.007 de | 0.173 ± 0.006 c | 0.259 ± 0.008 a | 0.679 ± 0.010 b | 0.493 ± 0.011 d | 0.507 ± 0.011 a | 1.964 ± 0.100 a |
| T2 | 0.412 ± 0.009 cd | 0.191 ± 0.006 b | 0.251 ± 0.005 a | 0.570 ± 0.003 c | 0.521 ± 0.022 d | 0.479 ± 0.022 a | 1.909 ± 0.077 a |
| T3 | 0.454 ± 0.007 bc | 0.207 ± 0.002 a | 0.185 ± 0.008 b | 0.586 ± 0.016 c | 0.673 ± 0.003 b | 0.327 ± 0.003 c | 1.774 ± 0.099 a |
| T4 | 0.586 ± 0.008 a | 0.218 ± 0.003 a | 0.070 ± 0.003 d | 0.458 ± 0.007 e | 0.937 ± 0.020 a | 0.063 ± 0.020 d | 0.928 ± 0.311 b |
| T5 | 0.472 ± 0.037 b | 0.190 ± 0.005 b | 0.147 ± 0.007 c | 0.517 ± 0.004 d | 0.706 ± 0.027 b | 0.294 ± 0.027 c | 2.017 ± 0.234 a |
| T6 | 0.341 ± 0.019 e | 0.171 ± 0.003 c | 0.186 ± 0.006 b | 0.719 ± 0.007 a | 0.575 ± 0.007 c | 0.425 ± 0.007 b | 2.287 ± 0.060 a |
| Parameters | Significance |
|---|---|
| Fv/Fm | Maximum photochemical efficiency of PSII |
| PⅠ (abs) | Performance index based on absorbed light energy |
| Vj | J-point relative variable fluorescence |
| Vi | I-point relative variable fluorescence |
| Sm | Normalized total residual region above the O-J-I-P transient |
| φPo | Maximum quantum yield of primary PSII photochemistry |
| φEo | Quantum yield of electron transport flux from QA to QB |
| φRo | Quantum yield of PSII final electron acceptor reduction per photon absorbed |
| PI (abs) | Performance index based on absorbed light energy |
| ABS/RC | Average absorbed light quantum flux per PSII reaction center |
| TRo/RC | Maximum captured photonic flux per active RC |
| DIo/RC | Light quantum flux dissipated per active RC |
| ETo/RC | Light quantum flux per active RC electron transfer |
| ABS/CSm | Energy flux absorbed in the excitation cross section (CSm) |
| TRo/CSm | Energy flux captured by absorption in the excitation cross section (CSm) |
| DIo/CSm | Energy flux dissipated in the excitation cross section (CSm) |
| ETo/CSm | Energy flux for electron transfer in the excitation cross section (CSm) |
| Gene Name | Sequence (5′-3′) | GenBank Accession Number | Amplicons Size (bp) |
|---|---|---|---|
| CaSOD | F: GTGAGCCTCCAAAGGGTTCTCTTG | AF036936.2:35-721 | 127 |
| R: AAACCAAGCCACACCCAACCAG | |||
| CaPOD | F: GCCAGGACAGCAAGCCAAGG | FJ596178.1:1:68-1042 | 131 |
| R: TGAGCACCTGATAAGGCAACCATG | |||
| CaCAT | F: TTAACGCTCCCAAGTGTGCTCATC | NM_001324674.1:72-1550 | 116 |
| R: GGCAGGACGACAAGGATCAAACC | |||
| Actin | F: GTCCTTCCATCGTCCACAGG | XM_016722297.1 | 133 |
| R: GAAGGGCAAAGGTTCACAACA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pu, K.; Li, N.; Gao, Y.; Zhang, M.; Wang, T.; Xie, J.; Li, J. Alleviating Effects of Methyl Jasmonate on Pepper (Capsicum annuum L.) Seedlings under Low-Temperature Combined with Low-Light Stress. Plants 2024, 13, 2694. https://doi.org/10.3390/plants13192694
Pu K, Li N, Gao Y, Zhang M, Wang T, Xie J, Li J. Alleviating Effects of Methyl Jasmonate on Pepper (Capsicum annuum L.) Seedlings under Low-Temperature Combined with Low-Light Stress. Plants. 2024; 13(19):2694. https://doi.org/10.3390/plants13192694
Chicago/Turabian StylePu, Kaiguo, Nenghui Li, Yanqiang Gao, Miao Zhang, Tiantian Wang, Jianming Xie, and Jing Li. 2024. "Alleviating Effects of Methyl Jasmonate on Pepper (Capsicum annuum L.) Seedlings under Low-Temperature Combined with Low-Light Stress" Plants 13, no. 19: 2694. https://doi.org/10.3390/plants13192694





