Impact of Abiotic Stress on Rice and the Role of DNA Methylation in Stress Response Mechanisms
Abstract
:1. Introduction
2. Impacts of Abiotic Stress Factors on Rice
2.1. Drought Stress
2.2. Temperature Stress
2.3. Salt Stress
2.4. Heavy Metal Stress
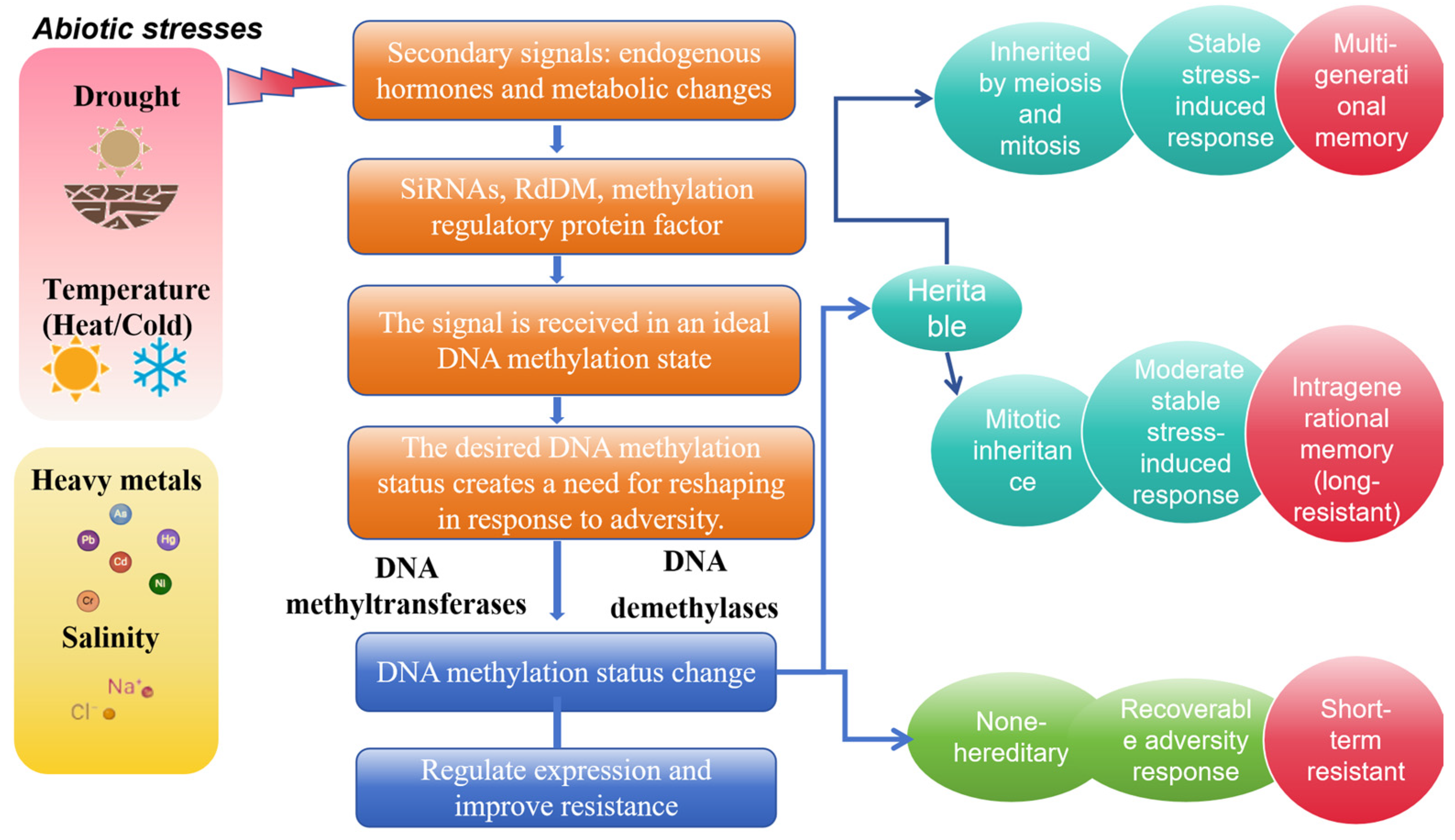
3. DNA Methylation Regulatory Mechanisms of Rice in Response to Abiotic Stress
3.1. DNA Methylation in Response to Drought Stress
3.2. DNA Methylation in Response to Temperature Stress
3.3. DNA Methylation in Response to Salt Stress
3.4. DNA Methylation in Response to Heavy Metal Stress
4. Discussion and Future Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, J.; Xing, Y.; Xu, Y.; Wan, J. Breeding by Design for Future Rice: Genes and Genome Technologies. Crop J. 2021, 9, 491–496. [Google Scholar] [CrossRef]
- de Marsily, G. Will We Soon Run Out of Water? Ann. Nutr. Metab. 2020, 76 (Suppl. S1), 10–16. [Google Scholar] [CrossRef] [PubMed]
- Landi, S.; Hausman, J.-F.; Guerriero, G.; Esposito, S. Poaceae vs. Abiotic Stress: Focus on Drought and Salt Stress, Recent Insights and Perspectives. Front. Plant Sci. 2017, 8, 1214. [Google Scholar] [CrossRef]
- Hegerl, G.C.; Brönnimann, S.; Schurer, A.; Cowan, T. The Early 20th Century Warming: Anomalies, Causes, and Consequences. WIREs Clim. Change 2018, 9, e522. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Sun, S.; Fu, G.; Hall, J.W.; Ni, Y.; He, L.; Yi, J.; Zhao, N.; Du, Y.; Pei, T.; et al. Pollution Exacerbates China’s Water Scarcity and Its Regional Inequality. Nat. Commun. 2020, 11, 650. [Google Scholar] [CrossRef]
- Ren, Y.; Huang, Z.; Jiang, H.; Wang, Z.; Wu, F.; Xiong, Y.; Yao, J. A Heat Stress Responsive NAC Transcription Factor Heterodimer Plays Key Roles in Rice Grain Filling. J. Exp. Bot. 2021, 72, 2947–2964. [Google Scholar] [CrossRef]
- Pan, Y.; Liang, H.; Gao, L.; Dai, G.; Chen, W.; Yang, X.; Qing, D.; Gao, J.; Wu, H.; Huang, J.; et al. Transcriptomic Profiling of Germinating Seeds under Cold Stress and Characterization of the Cold-Tolerant Gene LTG5 in Rice. BMC Plant Biol. 2020, 20, 371. [Google Scholar] [CrossRef]
- Bo, C.; Chen, H.; Luo, G.; Li, W.; Zhang, X.; Ma, Q.; Cheng, B.; Cai, R. Maize WRKY114 Gene Negatively Regulates Salt-Stress Tolerance in Transgenic Rice. Plant Cell Rep. 2020, 39, 135–148. [Google Scholar] [CrossRef]
- Kaur, R.; Das, S.; Bansal, S.; Singh, G.; Sardar, S.; Dhar, H.; Ram, H. Heavy Metal Stress in Rice: Uptake, Transport, Signaling, and Tolerance Mechanisms. Physiol. Plant. 2021, 173, 430–448. [Google Scholar] [CrossRef]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant Adaptation to Drought Stress. F1000Research 2016, 5, F1000 Faculty Rev-1554. [Google Scholar] [CrossRef]
- Sahebi, M.; Hanafi, M.M.; Rafii, M.Y.; Mahmud, T.M.M.; Azizi, P.; Osman, M.; Abiri, R.; Taheri, S.; Kalhori, N.; Shabanimofrad, M.; et al. Improvement of Drought Tolerance in Rice (Oryza sativa L.): Genetics, Genomic Tools, and the WRKY Gene Family. BioMed Res. Int. 2018, 2018, 3158474. [Google Scholar] [CrossRef] [PubMed]
- Ganie, S.A.; Molla, K.A.; Henry, R.J.; Bhat, K.V.; Mondal, T.K. Advances in Understanding Salt Tolerance in Rice. Theor. Appl. Genet. 2019, 132, 851–870. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-Y.; Yang, C.; Xu, J.; Lu, H.-P.; Liu, J.-X. The Hot Science in Rice Research: How Rice Plants Cope with Heat Stress. Plant Cell Environ. 2023, 46, 1087–1103. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Z.; Chong, K.; Xu, Y. Chilling Tolerance in Rice: Past and Present. J. Plant Physiol. 2022, 268, 153576. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-N.; Zhu, C.; Jiang, J.; Zhang, H.; Zhu, J.-K.; Duan, C.-G. Epigenetic Regulation in Plant Abiotic Stress Responses. J. Integr. Plant Biol. 2020, 62, 563–580. [Google Scholar] [CrossRef]
- Baulcombe, D.C.; Dean, C. Epigenetic Regulation in Plant Responses to the Environment. Cold Spring Harb. Perspect. Biol. 2014, 6, a019471. [Google Scholar] [CrossRef]
- Rajkumar, M.S.; Shankar, R.; Garg, R.; Jain, M. Bisulphite Sequencing Reveals Dynamic DNA Methylation under Desiccation and Salinity Stresses in Rice Cultivars. Genomics 2020, 112, 3537–3548. [Google Scholar] [CrossRef]
- Santos, A.P.; Serra, T.; Figueiredo, D.D.; Barros, P.; Lourenço, T.; Chander, S.; Oliveira, M.M.; Saibo, N.J.M. Transcription Regulation of Abiotic Stress Responses in Rice: A Combined Action of Transcription Factors and Epigenetic Mechanisms. OMICS 2011, 15, 839–857. [Google Scholar] [CrossRef]
- Xia, H.; Huang, W.; Xiong, J.; Yan, S.; Tao, T.; Li, J.; Wu, J.; Luo, L. Differentially Methylated Epiloci Generated from Numerous Genotypes of Contrasting Tolerances Are Associated with Osmotic-Tolerance in Rice Seedlings. Front. Plant Sci. 2017, 8, 11. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, X.; Pan, Y.; Zhu, L.; Fu, B.; Li, Z. DNA Methylation Changes Detected by Methylation-Sensitive Amplified Polymorphism in Two Contrasting Rice Genotypes under Salt Stress. J. Genet. Genom. 2011, 38, 419–424. [Google Scholar] [CrossRef]
- Folsom, J.J.; Begcy, K.; Hao, X.; Wang, D.; Walia, H. Rice Fertilization-Independent Endosperm1 Regulates Seed Size under Heat Stress by Controlling Early Endosperm Development. Plant Physiol. 2014, 165, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Cong, W.; Li, N.; Miao, Y.; Huang, Y.; Zhao, W.; Kang, Y.; Zhang, B.; Wang, J.; Zhang, J.; Lv, Y.; et al. DNA Hypomethylation-Associated Transcriptional Rewiring Enables Resistance to Heavy Metal Mercury (Hg) Stress in Rice. J. Hazard. Mater. 2024, 461, 132649. [Google Scholar] [CrossRef] [PubMed]
- Baillo, E.H.; Kimotho, R.N.; Zhang, Z.; Xu, P. Transcription Factors Associated with Abiotic and Biotic Stress Tolerance and Their Potential for Crops Improvement. Genes 2019, 10, 771. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, M.G.; Jan, A.; Ishizaki, T.; Valencia, M.; Dedicova, B.; Maruyama, K.; Ogata, T.; Todaka, D.; Yamaguchi-Shinozaki, K.; Nakashima, K.; et al. Expression of the CCCH-Tandem Zinc Finger Protein Gene OsTZF5 under a Stress-Inducible Promoter Mitigates the Effect of Drought Stress on Rice Grain Yield under Field Conditions. Plant Biotechnol. J. 2020, 18, 1711–1721. [Google Scholar] [CrossRef]
- Dash, P.K.; Rai, R.; Rai, V.; Pasupalak, S. Drought Induced Signaling in Rice: Delineating Canonical and Non-Canonical Pathways. Front. Chem. 2018, 6, 264. [Google Scholar] [CrossRef]
- Ozga, J.A.; Kaur, H.; Savada, R.P.; Reinecke, D.M. Hormonal Regulation of Reproductive Growth under Normal and Heat-Stress Conditions in Legume and Other Model Crop Species. J. Exp. Bot. 2017, 68, 1885–1894. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, H.; Huai, J.; Peng, F.; Wu, J.; Lin, R.; Fang, X. Condensation of SEUSS Promotes Hyperosmotic Stress Tolerance in Arabidopsis. Nat. Chem. Biol. 2022, 18, 1361–1369. [Google Scholar] [CrossRef]
- Wehner, F.; Olsen, H.; Tinel, H.; Kinne-Saffran, E.; Kinne, R.K.H. Cell Volume Regulation: Osmolytes, Osmolyte Transport, and Signal Transduction. In Reviews of Physiology, Biochemistry and Pharmacology; Springer: Cham, Switzerland, 2003; Volume 148, pp. 1–80. [Google Scholar] [CrossRef]
- Hu, H.; Xiong, L. Genetic Engineering and Breeding of Drought-Resistant Crops. Annu. Rev. Plant Biol. 2014, 65, 715–741. [Google Scholar] [CrossRef]
- Zhu, J.-K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Yu, B.; Chao, D.-Y.; Zhao, Y. How Plants Sense and Respond to Osmotic Stress. J. Integr. Plant Biol. 2024, 66, 394–423. [Google Scholar] [CrossRef]
- Tena, G.; Boudsocq, M.; Sheen, J. Protein Kinase Signaling Networks in Plant Innate Immunity. Curr. Opin. Plant Biol. 2011, 14, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Seybold, H.; Trempel, F.; Ranf, S.; Scheel, D.; Romeis, T.; Lee, J. Ca2+ Signalling in Plant Immune Response: From Pattern Recognition Receptors to Ca2+ Decoding Mechanisms. New Phytol. 2014, 204, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Oladosu, Y.; Rafii, M.Y.; Samuel, C.; Fatai, A.; Magaji, U.; Kareem, I.; Kamarudin, Z.S.; Muhammad, I.; Kolapo, K. Drought Resistance in Rice from Conventional to Molecular Breeding: A Review. Int. J. Mol. Sci. 2019, 20, 3519. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Transcriptional Regulatory Networks in Cellular Responses and Tolerance to Dehydration and Cold Stresses. Annu. Rev. Plant Biol. 2006, 57, 781–803. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding Plant Responses to Drought—From Genes to the Whole Plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Yin, X.; Cui, Y.; Wang, M.; Xia, X. Overexpression of a Novel MYB-Related Transcription Factor, OsMYBR1, Confers Improved Drought Tolerance and Decreased ABA Sensitivity in Rice. Biochem. Biophys. Res. Commun. 2017, 490, 1355–1361. [Google Scholar] [CrossRef]
- Xiong, H.; Li, J.; Liu, P.; Duan, J.; Zhao, Y.; Guo, X.; Li, Y.; Zhang, H.; Ali, J.; Li, Z. Overexpression of OsMYB48-1, a Novel MYB-Related Transcription Factor, Enhances Drought and Salinity Tolerance in Rice. PLoS ONE 2014, 9, e92913. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kamiya, N.; Morinaka, Y.; Matsuoka, M.; Sazuka, T. Auxin Biosynthesis by the YUCCA Genes in Rice. Plant Physiol. 2007, 143, 1362–1371. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Zhang, W.; Yan, S.; Wang, R.; Zhao, J.; Li, Y.; Qi, Z.; Sun, Z.; Zhu, Z. The Putative Auxin Efflux Carrier OsPIN3t Is Involved in the Drought Stress Response and Drought Tolerance. Plant J. 2012, 72, 805–816. [Google Scholar] [CrossRef]
- Du, H.; Wu, N.; Fu, J.; Wang, S.; Li, X.; Xiao, J.; Xiong, L. A GH3 Family Member, OsGH3-2, Modulates Auxin and Abscisic Acid Levels and Differentially Affects Drought and Cold Tolerance in Rice. J. Exp. Bot. 2012, 63, 6467–6480. [Google Scholar] [CrossRef]
- Fàbregas, N.; Lozano-Elena, F.; Blasco-Escámez, D.; Tohge, T.; Martínez-Andújar, C.; Albacete, A.; Osorio, S.; Bustamante, M.; Riechmann, J.L.; Nomura, T.; et al. Overexpression of the Vascular Brassinosteroid Receptor BRL3 Confers Drought Resistance without Penalizing Plant Growth. Nat. Commun. 2018, 9, 4680. [Google Scholar] [CrossRef] [PubMed]
- Pires, M.V.; Pereira Júnior, A.A.; Medeiros, D.B.; Daloso, D.M.; Pham, P.A.; Barros, K.A.; Engqvist, M.K.M.; Florian, A.; Krahnert, I.; Maurino, V.G.; et al. The Influence of Alternative Pathways of Respiration That Utilize Branched-Chain Amino Acids Following Water Shortage in Arabidopsis. Plant Cell Environ. 2016, 39, 1304–1319. [Google Scholar] [CrossRef] [PubMed]
- Krasensky, J.; Jonak, C. Drought, Salt, and Temperature Stress-Induced Metabolic Rearrangements and Regulatory Networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef] [PubMed]
- Urano, K.; Maruyama, K.; Ogata, Y.; Morishita, Y.; Takeda, M.; Sakurai, N.; Suzuki, H.; Saito, K.; Shibata, D.; Kobayashi, M.; et al. Characterization of the ABA-Regulated Global Responses to Dehydration in Arabidopsis by Metabolomics. Plant J. 2009, 57, 1065–1078. [Google Scholar] [CrossRef] [PubMed]
- Szabados, L.; Savouré, A. Proline: A Multifunctional Amino Acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.; Joung, J.-G.; Fei, Z.; Jander, G. Interdependence of Threonine, Methionine and Isoleucine Metabolism in Plants: Accumulation and Transcriptional Regulation under Abiotic Stress. Amino Acids 2010, 39, 933–947. [Google Scholar] [CrossRef]
- Obata, T.; Witt, S.; Lisec, J.; Palacios-Rojas, N.; Florez-Sarasa, I.; Yousfi, S.; Araus, J.L.; Cairns, J.E.; Fernie, A.R. Metabolite Profiles of Maize Leaves in Drought, Heat, and Combined Stress Field Trials Reveal the Relationship between Metabolism and Grain Yield. Plant Physiol. 2015, 169, 2665–2683. [Google Scholar] [CrossRef]
- Mahmood, T.; Khalid, S.; Abdullah, M.; Ahmed, Z.; Shah, M.K.N.; Ghafoor, A.; Du, X. Insights into Drought Stress Signaling in Plants and the Molecular Genetic Basis of Cotton Drought Tolerance. Cells 2019, 9, 105. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Yonekura-Sakakibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Sakakibara, H.; Shinozaki, K.; et al. Enhancement of Oxidative and Drought Tolerance in Arabidopsis by Overaccumulation of Antioxidant Flavonoids. Plant J. 2014, 77, 367–379. [Google Scholar] [CrossRef]
- Li, B.; Feng, Y.; Zong, Y.; Zhang, D.; Hao, X.; Li, P. Elevated CO2-Induced Changes in Photosynthesis, Antioxidant Enzymes and Signal Transduction Enzyme of Soybean under Drought Stress. Plant Physiol. Biochem. 2020, 154, 105–114. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, H.; Zhang, D.; Li, J.; Xiong, H.; Yu, J.; Li, J.; Rashid, M.A.R.; Li, G.; Ma, X.; et al. Genetic Analysis of Cold Tolerance at the Germination and Booting Stages in Rice by Association Mapping. PLoS ONE 2015, 10, e0120590. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, C.; Linderholm, H.W.; Fu, Y.; Cai, W.; Xu, J.; Zhuang, L.; Wu, M.; Shi, Y.; Wang, G.; et al. The Negative Impact of Increasing Temperatures on Rice Yields in Southern China. Sci. Total Environ. 2022, 820, 153262. [Google Scholar] [CrossRef] [PubMed]
- Quinn, P.J. Effects of Temperature on Cell Membranes. Symp. Soc. Exp. Biol. 1988, 42, 237–258. [Google Scholar] [PubMed]
- Zhang, Q.; Chen, Q.; Wang, S.; Hong, Y.; Wang, Z. Rice and Cold Stress: Methods for Its Evaluation and Summary of Cold Tolerance-Related Quantitative Trait Loci. Rice 2014, 7, 24. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, S. COLD1: A Cold Sensor in Rice. Sci. China Life Sci. 2015, 58, 409–410. [Google Scholar] [CrossRef]
- Cui, Y.; Lu, S.; Li, Z.; Cheng, J.; Hu, P.; Zhu, T.; Wang, X.; Jin, M.; Wang, X.; Li, L.; et al. CYCLIC NUCLEOTIDE-GATED ION CHANNELs 14 and 16 Promote Tolerance to Heat and Chilling in Rice. Plant Physiol. 2020, 183, 1794–1808. [Google Scholar] [CrossRef]
- Shi, H.; Zhong, S. Light and Temperature Perceptions Go through a Phase Separation. Curr. Opin. Plant Biol. 2023, 74, 102397. [Google Scholar] [CrossRef]
- Hayashi, S.; Wakasa, Y.; Takahashi, H.; Kawakatsu, T.; Takaiwa, F. Signal Transduction by IRE1-Mediated Splicing of bZIP50 and Other Stress Sensors in the Endoplasmic Reticulum Stress Response of Rice. Plant J. 2012, 69, 946–956. [Google Scholar] [CrossRef]
- Kim, M.-H.; Jeon, J.; Lee, S.; Lee, J.H.; Gao, L.; Lee, B.-H.; Park, J.M.; Kim, Y.J.; Kwak, J.M. Proteasome Subunit RPT2a Promotes PTGS through Repressing RNA Quality Control in Arabidopsis. Nat. Plants 2019, 5, 1273–1282. [Google Scholar] [CrossRef]
- Liu, L.; Chen, X. RNA Quality Control as a Key to Suppressing RNA Silencing of Endogenous Genes in Plants. Mol. Plant 2016, 9, 826–836. [Google Scholar] [CrossRef]
- Reddy, K.R.; Seghal, A.; Jumaa, S.; Bheemanahalli, R.; Kakar, N.; Redoña, E.D.; Wijewardana, C.; Alsajri, F.A.; Chastain, D.; Gao, W.; et al. Morpho-Physiological Characterization of Diverse Rice Genotypes for Seedling Stage High- and Low-Temperature Tolerance. Agronomy 2021, 11, 112. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Li, F.; Liu, H.; Yang, W.; Chong, K.; Xu, Y. OsMAPK3 Phosphorylates OsbHLH002/OsICE1 and Inhibits Its Ubiquitination to Activate OsTPP1 and Enhances Rice Chilling Tolerance. Dev. Cell 2017, 43, 731–743.e5. [Google Scholar] [CrossRef] [PubMed]
- Sunkar, R.; Chinnusamy, V.; Zhu, J.; Zhu, J.-K. Small RNAs as Big Players in Plant Abiotic Stress Responses and Nutrient Deprivation. Trends Plant Sci. 2007, 12, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Ganie, S.A.; Wani, S.H.; Henry, R.; Hensel, G. Improving Rice Salt Tolerance by Precision Breeding in a New Era. Curr. Opin. Plant Biol. 2021, 60, 101996. [Google Scholar] [CrossRef] [PubMed]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant Salt-Tolerance Mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef]
- Reddy, I.N.B.L.; Kim, B.-K.; Yoon, I.-S.; Kim, K.-H.; Kwon, T.-R. Salt Tolerance in Rice: Focus on Mechanisms and Approaches. Rice Sci. 2017, 24, 123–144. [Google Scholar] [CrossRef]
- Tao, J.-J.; Chen, H.-W.; Ma, B.; Zhang, W.-K.; Chen, S.-Y.; Zhang, J.-S. The Role of Ethylene in Plants Under Salinity Stress. Front. Plant Sci. 2015, 6, 1059. [Google Scholar] [CrossRef]
- Wang, J.; Huang, R. Modulation of Ethylene and Ascorbic Acid on Reactive Oxygen Species Scavenging in Plant Salt Response. Front. Plant Sci. 2019, 10, 319. [Google Scholar] [CrossRef]
- Liu, C.; Mao, B.; Yuan, D.; Chu, C.; Duan, M. Salt Tolerance in Rice: Physiological Responses and Molecular Mechanisms. Crop J. 2022, 10, 13–25. [Google Scholar] [CrossRef]
- Bassil, E.; Coku, A.; Blumwald, E. Cellular Ion Homeostasis: Emerging Roles of Intracellular NHX Na+/H+ Antiporters in Plant Growth and Development. J. Exp. Bot. 2012, 63, 5727–5740. [Google Scholar] [CrossRef]
- Cheng, X.; He, Q.; Tang, S.; Wang, H.; Zhang, X.; Lv, M.; Liu, H.; Gao, Q.; Zhou, Y.; Wang, Q.; et al. The miR172/IDS1 Signaling Module Confers Salt Tolerance through Maintaining ROS Homeostasis in Cereal Crops. New Phytol. 2021, 230, 1017–1033. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Xue, N.; Han, Z. A Meta-Analysis of Heavy Metals Pollution in Farmland and Urban Soils in China over the Past 20 Years. J. Environ. Sci. 2021, 101, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Q.; Liao, X.; Li, X.; Zheng, S.; Zhao, F. Phytoexclusion of Heavy Metals Using Low Heavy Metal Accumulating Cultivars: A Green Technology. J. Hazard. Mater. 2021, 413, 125427. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Zhao, Q.; Sun, H.; Geng, L.; Yang, Z.; Liu, W. Characteristics of Heavy Metals in Soils and Grains of Wheat and Maize from Farmland Irrigated with Sewage. Environ. Sci. Pollut. Res. Int. 2019, 26, 5554–5563. [Google Scholar] [CrossRef]
- Qin, G.; Niu, Z.; Yu, J.; Li, Z.; Ma, J.; Xiang, P. Soil Heavy Metal Pollution and Food Safety in China: Effects, Sources and Removing Technology. Chemosphere 2021, 267, 129205. [Google Scholar] [CrossRef]
- He, M.; Shen, H.; Li, Z.; Wang, L.; Wang, F.; Zhao, K.; Liu, X.; Wendroth, O.; Xu, J. Ten-Year Regional Monitoring of Soil-Rice Grain Contamination by Heavy Metals with Implications for Target Remediation and Food Safety. Environ. Pollut. 2019, 244, 431–439. [Google Scholar] [CrossRef]
- Jiang, M.; Jiang, J.; Li, S.; Li, M.; Tan, Y.; Song, S.; Shu, Q.; Huang, J. Glutamate Alleviates Cadmium Toxicity in Rice via Suppressing Cadmium Uptake and Translocation. J. Hazard. Mater. 2020, 384, 121319. [Google Scholar] [CrossRef]
- van Dijk, J.R.; Kranchev, M.; Blust, R.; Cuypers, A.; Vissenberg, K. Arabidopsis Root Growth and Development under Metal Exposure Presented in an Adverse Outcome Pathway Framework. Plant Cell Environ. 2022, 45, 737–750. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, H.; Jiang, H.; Lu, W.; Pan, J.; Qian, Q.; Xue, D. Measuring the Damage of Heavy Metal Cadmium in Rice Seedlings by SRAP Analysis Combined with Physiological and Biochemical Parameters. J. Sci. Food Agric. 2015, 95, 2292–2298. [Google Scholar] [CrossRef]
- Riaz, M.; Kamran, M.; Rizwan, M.; Ali, S.; Zhou, Y.; Núñez-Delgado, A.; Wang, X. Boron Application Mitigates Cd Toxicity in Leaves of Rice by Subcellular Distribution, Cell Wall Adsorption and Antioxidant System. Ecotoxicol. Environ. Saf. 2021, 222, 112540. [Google Scholar] [CrossRef]
- Ueno, D.; Yamaji, N.; Kono, I.; Huang, C.F.; Ando, T.; Yano, M.; Ma, J.F. Gene Limiting Cadmium Accumulation in Rice. Proc. Natl. Acad. Sci. USA 2010, 107, 16500–16505. [Google Scholar] [CrossRef] [PubMed]
- Miyadate, H.; Adachi, S.; Hiraizumi, A.; Tezuka, K.; Nakazawa, N.; Kawamoto, T.; Katou, K.; Kodama, I.; Sakurai, K.; Takahashi, H.; et al. OsHMA3, a P1B-Type of ATPase Affects Root-to-Shoot Cadmium Translocation in Rice by Mediating Efflux into Vacuoles. New Phytol. 2011, 189, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wei, S.; Yang, Y.; Ding, Z.; Wang, Q.; Zhao, J.; Liu, X.; Chu, X.; Tian, J.; Wu, N.; et al. Identification of Cadmium-Binding Proteins from Rice (Oryza sativa L.). Int. J. Biol. Macromol. 2018, 119, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Xu, W.; Xie, J.; Gao, Y.; Wu, L.; Sun, L.; Feng, L.; Chen, X.; Zhang, T.; Dai, C.; et al. Variation of a Major Facilitator Superfamily Gene Contributes to Differential Cadmium Accumulation between Rice Subspecies. Nat. Commun. 2019, 10, 2562. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, H.; Reinfelder, J.R.; Liang, X.; Sun, C.; Liu, C.; Li, F.; Yi, J. A Transcriptomic (RNA-Seq) Analysis of Genes Responsive to Both Cadmium and Arsenic Stress in Rice Root. Sci. Total Environ. 2019, 666, 445–460. [Google Scholar] [CrossRef]
- Mir, A.R.; Pichtel, J.; Hayat, S. Copper: Uptake, Toxicity and Tolerance in Plants and Management of Cu-Contaminated Soil. Biometals 2021, 34, 737–759. [Google Scholar] [CrossRef]
- Cao, H.; Chen, D.; Kuang, L.; Yan, T.; Gao, F.; Wu, D. Metabolomic Analysis Reveals the Molecular Responses to Copper Toxicity in Rice (Oryza sativa). Plant Physiol. Biochem. 2023, 199, 107727. [Google Scholar] [CrossRef]
- Begcy, K.; Dresselhaus, T. Epigenetic Responses to Abiotic Stresses during Reproductive Development in Cereals. Plant Reprod. 2018, 31, 343–355. [Google Scholar] [CrossRef]
- Akhter, Z.; Bi, Z.; Ali, K.; Sun, C.; Fiaz, S.; Haider, F.U.; Bai, J. In Response to Abiotic Stress, DNA Methylation Confers EpiGenetic Changes in Plants. Plants 2021, 10, 1096. [Google Scholar] [CrossRef]
- Law, J.A.; Jacobsen, S.E. Establishing, Maintaining and Modifying DNA Methylation Patterns in Plants and Animals. Nat. Rev. Genet. 2010, 11, 204–220. [Google Scholar] [CrossRef]
- He, X.-J.; Chen, T.; Zhu, J.-K. Regulation and Function of DNA Methylation in Plants and Animals. Cell Res. 2011, 21, 442–465. [Google Scholar] [CrossRef] [PubMed]
- Colot, V.; Rossignol, J.L. Eukaryotic DNA Methylation as an Evolutionary Device. Bioessays 1999, 21, 402–411. [Google Scholar] [CrossRef]
- Zhang, X.; Yazaki, J.; Sundaresan, A.; Cokus, S.; Chan, S.W.-L.; Chen, H.; Henderson, I.R.; Shinn, P.; Pellegrini, M.; Jacobsen, S.E.; et al. Genome-Wide High-Resolution Mapping and Functional Analysis of DNA Methylation in Arabidopsis. Cell 2006, 126, 1189–1201. [Google Scholar] [CrossRef] [PubMed]
- Henderson, I.R.; Jacobsen, S.E. Epigenetic Inheritance in Plants. Nature 2007, 447, 418–424. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.-K. RNA-Directed DNA Methylation. Curr. Opin. Plant Biol. 2011, 14, 142–147. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, H.-L.; Daxinger, L.; Pontes, O.; He, X.; Qian, W.; Lin, H.; Xie, M.; Lorkovic, Z.J.; Zhang, S.; et al. An RNA Polymerase II- and AGO4-Associated Protein Acts in RNA-Directed DNA Methylation. Nature 2010, 465, 106–109. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Zhu, J.-K. RNA-Directed DNA Methylation and Demethylation in Plants. Sci. China C Life Sci. 2009, 52, 331–343. [Google Scholar] [CrossRef]
- Matzke, M.A.; Mosher, R.A. RNA-Directed DNA Methylation: An Epigenetic Pathway of Increasing Complexity. Nat. Rev. Genet. 2014, 15, 394–408. [Google Scholar] [CrossRef]
- Matzke, M.A.; Kanno, T.; Matzke, A.J.M. RNA-Directed DNA Methylation: The Evolution of a Complex Epigenetic Pathway in Flowering Plants. Annu. Rev. Plant Biol. 2015, 66, 243–267. [Google Scholar] [CrossRef]
- Wendte, J.M.; Pikaard, C.S. The RNAs of RNA-Directed DNA Methylation. Biochim. Biophys. Acta (BBA) Gene Regul. Mech. 2017, 1860, 140–148. [Google Scholar] [CrossRef]
- Henderson, I.R.; Zhang, X.; Lu, C.; Johnson, L.; Meyers, B.C.; Green, P.J.; Jacobsen, S.E. Dissecting Arabidopsis thaliana DICER Function in Small RNA Processing, Gene Silencing and DNA Methylation Patterning. Nat. Genet. 2006, 38, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Bologna, N.G.; Voinnet, O. The Diversity, Biogenesis, and Activities of Endogenous Silencing Small RNAs in Arabidopsis. Annu. Rev. Plant Biol. 2014, 65, 473–503. [Google Scholar] [CrossRef] [PubMed]
- Stroud, H.; Greenberg, M.V.C.; Feng, S.; Bernatavichute, Y.V.; Jacobsen, S.E. Comprehensive Analysis of Silencing Mutants Reveals Complex Regulation of the Arabidopsis Methylome. Cell 2013, 152, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Chen, X. Regulation of Small RNA Stability: Methylation and Beyond. Cell Res. 2012, 22, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Haag, J.R.; Pikaard, C.S. Multisubunit RNA Polymerases IV and V: Purveyors of Non-Coding RNA for Plant Gene Silencing. Nat. Rev. Mol. Cell Biol. 2011, 12, 483–492. [Google Scholar] [CrossRef]
- Fang, X.; Qi, Y. RNAi in Plants: An Argonaute-Centered View. Plant Cell 2016, 28, 272–285. [Google Scholar] [CrossRef]
- McCue, A.D.; Panda, K.; Nuthikattu, S.; Choudury, S.G.; Thomas, E.N.; Slotkin, R.K. ARGONAUTE 6 Bridges Transposable Element mRNA-Derived siRNAs to the Establishment of DNA Methylation. EMBO J. 2015, 34, 20–35. [Google Scholar] [CrossRef]
- Eun, C.; Lorkovic, Z.J.; Naumann, U.; Long, Q.; Havecker, E.R.; Simon, S.A.; Meyers, B.C.; Matzke, A.J.M.; Matzke, M. AGO6 Functions in RNA-Mediated Transcriptional Gene Silencing in Shoot and Root Meristems in Arabidopsis thaliana. PLoS ONE 2011, 6, e25730. [Google Scholar] [CrossRef]
- Cao, X.; Aufsatz, W.; Zilberman, D.; Mette, M.F.; Huang, M.S.; Matzke, M.; Jacobsen, S.E. Role of the DRM and CMT3 Methyltransferases in RNA-Directed DNA Methylation. Curr. Biol. 2003, 13, 2212–2217. [Google Scholar] [CrossRef]
- Cuerda-Gil, D.; Slotkin, R.K. Non-Canonical RNA-Directed DNA Methylation. Nat. Plants 2016, 2, 16163. [Google Scholar] [CrossRef]
- Panda, K.; Ji, L.; Neumann, D.A.; Daron, J.; Schmitz, R.J.; Slotkin, R.K. Full-Length Autonomous Transposable Elements Are Preferentially Targeted by Expression-Dependent Forms of RNA-Directed DNA Methylation. Genome Biol. 2016, 17, 170. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Mao, L.; Qi, Y. Roles of Dicer-like and Argonaute Proteins in TAS-Derived Small Interfering RNA-Triggered DNA Methylation. Plant Physiol. 2012, 160, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Nuthikattu, S.; McCue, A.D.; Panda, K.; Fultz, D.; DeFraia, C.; Thomas, E.N.; Slotkin, R.K. The Initiation of Epigenetic Silencing of Active Transposable Elements Is Triggered by RDR6 and 21-22 Nucleotide Small Interfering RNAs. Plant Physiol. 2013, 162, 116–131. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Hale, C.J.; Law, J.A.; Johnson, L.M.; Feng, S.; Tu, A.; Jacobsen, S.E. DDR Complex Facilitates Global Association of RNA Polymerase V to Promoters and Evolutionarily Young Transposons. Nat. Struct. Mol. Biol. 2012, 19, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.M.; Du, J.; Hale, C.J.; Bischof, S.; Feng, S.; Chodavarapu, R.K.; Zhong, X.; Marson, G.; Pellegrini, M.; Segal, D.J.; et al. SRA- and SET-Domain-Containing Proteins Link RNA Polymerase V Occupancy to DNA Methylation. Nature 2014, 507, 124–128. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, K.; Qian, W.; Duan, C.-G.; Wang, B.; Zhang, H.; Wang, P.; Zhu, X.; Lang, Z.; Yang, Y.; et al. An Rrp6-like Protein Positively Regulates Noncoding RNA Levels and DNA Methylation in Arabidopsis. Mol. Cell 2014, 54, 418–430. [Google Scholar] [CrossRef]
- Ausin, I.; Mockler, T.C.; Chory, J.; Jacobsen, S.E. IDN1 and IDN2 Are Required for de Novo DNA Methylation in Arabidopsis thaliana. Nat. Struct. Mol. Biol. 2009, 16, 1325–1327. [Google Scholar] [CrossRef]
- Finke, A.; Kuhlmann, M.; Mette, M.F. IDN2 Has a Role Downstream of siRNA Formation in RNA-Directed DNA Methylation. Epigenetics 2012, 7, 950–960. [Google Scholar] [CrossRef]
- Ronemus, M.J.; Galbiati, M.; Ticknor, C.; Chen, J.; Dellaporta, S.L. Demethylation-Induced Developmental Pleiotropy in Arabidopsis. Science 1996, 273, 654–657. [Google Scholar] [CrossRef]
- Yamauchi, T.; Moritoh, S.; Johzuka-Hisatomi, Y.; Ono, A.; Terada, R.; Nakamura, I.; Iida, S. Alternative Splicing of the Rice OsMET1 Genes Encoding Maintenance DNA Methyltransferase. J. Plant Physiol. 2008, 165, 1774–1782. [Google Scholar] [CrossRef]
- Lindroth, A.M.; Saarikoski, P.; Flygh, G.; Clapham, D.; Grönroos, R.; Thelander, M.; Ronne, H.; von Arnold, S. Two S-Adenosylmethionine Synthetase-Encoding Genes Differentially Expressed during Adventitious Root Development in Pinus contorta. Plant Mol. Biol. 2001, 46, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Stroud, H.; Do, T.; Du, J.; Zhong, X.; Feng, S.; Johnson, L.; Patel, D.J.; Jacobsen, S.E. Non-CG Methylation Patterns Shape the Epigenetic Landscape in Arabidopsis. Nat. Struct. Mol. Biol. 2014, 21, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Seymour, D.K.; Becker, C. The Causes and Consequences of DNA Methylome Variation in Plants. Curr. Opin. Plant Biol. 2017, 36, 56–63. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhao, C.; Zhang, Q.; Zinta, G.; Wang, D.; Lozano-Durán, R.; Zhu, J.-K. Pathway Conversion Enables a Double-Lock Mechanism to Maintain DNA Methylation and Genome Stability. Proc. Natl. Acad. Sci. USA 2021, 118, e2107320118. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhong, X.; Bernatavichute, Y.V.; Stroud, H.; Feng, S.; Caro, E.; Vashisht, A.A.; Terragni, J.; Chin, H.G.; Tu, A.; et al. Dual Binding of Chromomethylase Domains to H3K9me2-Containing Nucleosomes Directs DNA Methylation in Plants. Cell 2012, 151, 167–180. [Google Scholar] [CrossRef]
- Du, J.; Johnson, L.M.; Groth, M.; Feng, S.; Hale, C.J.; Li, S.; Vashisht, A.A.; Wohlschlegel, J.A.; Patel, D.J.; Jacobsen, S.E. Mechanism of DNA Methylation-Directed Histone Methylation by KRYPTONITE. Mol. Cell 2014, 55, 495–504. [Google Scholar] [CrossRef]
- Ebbs, M.L.; Bartee, L.; Bender, J. H3 Lysine 9 Methylation Is Maintained on a Transcribed Inverted Repeat by Combined Action of SUVH6 and SUVH4 Methyltransferases. Mol. Cell. Biol. 2005, 25, 10507–10515. [Google Scholar] [CrossRef]
- Liu, Z.-W.; Shao, C.-R.; Zhang, C.-J.; Zhou, J.-X.; Zhang, S.-W.; Li, L.; Chen, S.; Huang, H.-W.; Cai, T.; He, X.-J. The SET Domain Proteins SUVH2 and SUVH9 Are Required for Pol V Occupancy at RNA-Directed DNA Methylation Loci. PLoS Genet. 2014, 10, e1003948. [Google Scholar] [CrossRef]
- Cheng, C.; Tarutani, Y.; Miyao, A.; Ito, T.; Yamazaki, M.; Sakai, H.; Fukai, E.; Hirochika, H. Loss of Function Mutations in the Rice Chromomethylase OsCMT3a Cause a Burst of Transposition. Plant J. 2015, 83, 1069–1081. [Google Scholar] [CrossRef]
- Hu, D.; Yu, Y.; Wang, C.; Long, Y.; Liu, Y.; Feng, L.; Lu, D.; Liu, B.; Jia, J.; Xia, R.; et al. Multiplex CRISPR-Cas9 Editing of DNA Methyltransferases in Rice Uncovers a Class of Non-CG Methylation Specific for GC-Rich Regions. Plant Cell 2021, 33, 2950–2964. [Google Scholar] [CrossRef]
- Wang, L.; Yuan, J.; Ma, Y.; Jiao, W.; Ye, W.; Yang, D.-L.; Yi, C.; Chen, Z.J. Rice Interploidy Crosses Disrupt Epigenetic Regulation, Gene Expression, and Seed Development. Mol. Plant 2018, 11, 300–314. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Cao, X. Transposon-Mediated Epigenetic Regulation Contributes to Phenotypic Diversity and Environmental Adaptation in Rice. Curr. Opin. Plant Biol. 2017, 36, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Akinmusola, R.Y.; Wilkins, C.-A.; Doughty, J. DDM1-Mediated TE Silencing in Plants. Plants 2023, 12, 437. [Google Scholar] [CrossRef]
- Higo, H.; Tahir, M.; Takashima, K.; Miura, A.; Watanabe, K.; Tagiri, A.; Ugaki, M.; Ishikawa, R.; Eiguchi, M.; Kurata, N.; et al. DDM1 (Decrease in DNA Methylation) Genes in Rice (Oryza sativa). Mol. Genet. Genom. 2012, 287, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Zhou, C.; Zhou, Q.; Zhou, S.; Yang, W.; Zhao, Y.; Li, G.; Zhou, D.-X. Analysis of Chromatin Regulators Reveals Specific Features of Rice DNA Methylation Pathways. Plant Physiol. 2016, 171, 2041–2054. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Morales-Ruiz, T.; Ariza, R.R.; Roldán-Arjona, T.; David, L.; Zhu, J.K. ROS1, a Repressor of Transcriptional Gene Silencing in Arabidopsis, Encodes a DNA Glycosylase/Lyase. Cell 2002, 111, 803–814. [Google Scholar] [CrossRef]
- Ortega-Galisteo, A.P.; Morales-Ruiz, T.; Ariza, R.R.; Roldán-Arjona, T. Arabidopsis DEMETER-LIKE Proteins DML2 and DML3 Are Required for Appropriate Distribution of DNA Methylation Marks. Plant Mol. Biol. 2008, 67, 671–681. [Google Scholar] [CrossRef]
- Kim, M.Y.; Ono, A.; Scholten, S.; Kinoshita, T.; Zilberman, D.; Okamoto, T.; Fischer, R.L. DNA Demethylation by ROS1a in Rice Vegetative Cells Promotes Methylation in Sperm. Proc. Natl. Acad. Sci. USA 2019, 116, 9652–9657. [Google Scholar] [CrossRef]
- La, H.; Ding, B.; Mishra, G.P.; Zhou, B.; Yang, H.; Bellizzi, M.d.R.; Chen, S.; Meyers, B.C.; Peng, Z.; Zhu, J.-K.; et al. A 5-Methylcytosine DNA Glycosylase/Lyase Demethylates the Retrotransposon Tos17 and Promotes Its Transposition in Rice. Proc. Natl. Acad. Sci. USA 2011, 108, 15498–15503. [Google Scholar] [CrossRef]
- Zhou, S.; Li, X.; Liu, Q.; Zhao, Y.; Jiang, W.; Wu, A.; Zhou, D.-X. DNA Demethylases Remodel DNA Methylation in Rice Gametes and Zygote and Are Required for Reproduction. Mol. Plant 2021, 14, 1569–1583. [Google Scholar] [CrossRef]
- Wang, W.-S.; Pan, Y.-J.; Zhao, X.-Q.; Dwivedi, D.; Zhu, L.-H.; Ali, J.; Fu, B.-Y.; Li, Z.-K. Drought-Induced Site-Specific DNA Methylation and Its Association with Drought Tolerance in Rice (Oryza sativa L.). J. Exp. Bot. 2011, 62, 1951–1960. [Google Scholar] [CrossRef] [PubMed]
- Auler, P.A.; Nogueira do Amaral, M.; Bolacel Braga, E.J.; Maserti, B. Drought Stress Memory in Rice Guard Cells: Proteome Changes and Genomic Stability of DNA. Plant Physiol. Biochem. 2021, 169, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Chen, L.; Xia, H.; Wei, H.; Lou, Q.; Li, M.; Li, T.; Luo, L. Transgenerational Epimutations Induced by Multi-Generation Drought Imposition Mediate Rice Plant’s Adaptation to Drought Condition. Sci. Rep. 2017, 7, 39843. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Chen, L.; Li, M.; Lou, Q.; Xia, H.; Wang, P.; Li, T.; Liu, H.; Luo, L. Transgenerational Variations in DNA Methylation Induced by Drought Stress in Two Rice Varieties with Distinguished Difference to Drought Resistance. PLoS ONE 2013, 8, e80253. [Google Scholar] [CrossRef]
- Garg, R.; Narayana Chevala, V.; Shankar, R.; Jain, M. Divergent DNA Methylation Patterns Associated with Gene Expression in Rice Cultivars with Contrasting Drought and Salinity Stress Response. Sci. Rep. 2015, 5, 14922. [Google Scholar] [CrossRef]
- Li, P.; Yang, H.; Wang, L.; Liu, H.; Huo, H.; Zhang, C.; Liu, A.; Zhu, A.; Hu, J.; Lin, Y.; et al. Physiological and Transcriptome Analyses Reveal Short-Term Responses and Formation of Memory Under Drought Stress in Rice. Front. Genet. 2019, 10, 55. [Google Scholar] [CrossRef]
- Li, W.-Q.; Zhang, M.-J.; Gan, P.-F.; Qiao, L.; Yang, S.-Q.; Miao, H.; Wang, G.-F.; Zhang, M.-M.; Liu, W.-T.; Li, H.-F.; et al. CLD1/SRL1 Modulates Leaf Rolling by Affecting Cell Wall Formation, Epidermis Integrity and Water Homeostasis in Rice. Plant J. 2017, 92, 904–923. [Google Scholar] [CrossRef]
- Cao, L.; Lu, X.; Wang, G.; Zhang, P.; Fu, J.; Wang, Z.; Wei, L.; Wang, T. Transcriptional Regulatory Networks in Response to Drought Stress and Rewatering in Maize (Zea mays L.). Mol. Genet. Genom. 2021, 296, 1203–1219. [Google Scholar] [CrossRef]
- Mao, H.; Wang, H.; Liu, S.; Li, Z.; Yang, X.; Yan, J.; Li, J.; Tran, L.-S.P.; Qin, F. A Transposable Element in a NAC Gene Is Associated with Drought Tolerance in Maize Seedlings. Nat. Commun. 2015, 6, 8326. [Google Scholar] [CrossRef]
- Duan, H.; Li, J.; Zhu, Y.; Jia, W.; Wang, H.; Jiang, L.; Zhou, Y. Responsive Changes of DNA Methylation in Wheat (Triticum aestivum) under Water Deficit. Sci. Rep. 2020, 10, 7938. [Google Scholar] [CrossRef]
- Fei, Y.; Xue, Y.; Du, P.; Yang, S.; Deng, X. Expression Analysis and Promoter Methylation under Osmotic and Salinity Stress of TaGAPC1 in Wheat (Triticum aestivum L). Protoplasma 2017, 254, 987–996. [Google Scholar] [CrossRef] [PubMed]
- González, R.M.; Ricardi, M.M.; Iusem, N.D. Atypical Epigenetic Mark in an Atypical Location: Cytosine Methylation at Asymmetric (CNN) Sites within the Body of a Non-Repetitive Tomato Gene. BMC Plant Biol. 2011, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Van Dooren, T.J.M.; Silveira, A.B.; Gilbault, E.; Jiménez-Gómez, J.M.; Martin, A.; Bach, L.; Tisné, S.; Quadrana, L.; Loudet, O.; Colot, V. Mild Drought in the Vegetative Stage Induces Phenotypic, Gene Expression, and DNA Methylation Plasticity in Arabidopsis but No Transgenerational Effects. J. Exp. Bot. 2020, 71, 3588–3602. [Google Scholar] [CrossRef] [PubMed]
- Arora, H.; Singh, R.K.; Sharma, S.; Sharma, N.; Panchal, A.; Das, T.; Prasad, A.; Prasad, M. DNA Methylation Dynamics in Response to Abiotic and Pathogen Stress in Plants. Plant Cell Rep. 2022, 41, 1931–1944. [Google Scholar] [CrossRef]
- Waseem, M.; Huang, F.; Wang, Q.; Aslam, M.M.; Abbas, F.; Ahmad, F.; Ashraf, U.; Hassan, W.; Fiaz, S.; Ye, X.; et al. Identification, Methylation Profiling, and Expression Analysis of Stress-Responsive Cytochrome P450 Genes in Rice under Abiotic and Phytohormones Stresses. GM Crops Food 2021, 12, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lu, Z.; Wang, L.; Jin, B. Plant Responses to Heat Stress: Physiology, Transcription, Noncoding RNAs, and Epigenetics. Int. J. Mol. Sci. 2020, 22, 117. [Google Scholar] [CrossRef]
- Santos, A.P.; Ferreira, L.; Maroco, J.; Oliveira, M.M. Abiotic Stress and Induced DNA Hypomethylation Cause Interphase Chromatin Structural Changes in Rice rDNA Loci. Cytogenet. Genome Res. 2011, 132, 297–303. [Google Scholar] [CrossRef]
- Yang, L.; Lang, C.; Wu, Y.; Meng, D.; Yang, T.; Li, D.; Jin, T.; Zhou, X. ROS1-Mediated Decrease in DNA Methylation and Increase in Expression of Defense Genes and Stress Response Genes in Arabidopsis thaliana Due to Abiotic Stresses. BMC Plant Biol. 2022, 22, 104. [Google Scholar] [CrossRef]
- Tong, W.; Li, R.; Huang, J.; Zhao, H.; Ge, R.; Wu, Q.; Mallano, A.I.; Wang, Y.; Li, F.; Deng, W.; et al. Divergent DNA Methylation Contributes to Duplicated Gene Evolution and Chilling Response in Tea Plants. Plant J. 2021, 106, 1312–1327. [Google Scholar] [CrossRef]
- Guo, H.; Wu, T.; Li, S.; He, Q.; Yang, Z.; Zhang, W.; Gan, Y.; Sun, P.; Xiang, G.; Zhang, H.; et al. The Methylation Patterns and Transcriptional Responses to Chilling Stress at the Seedling Stage in Rice. Int. J. Mol. Sci. 2019, 20, 5089. [Google Scholar] [CrossRef]
- Liu, T.; Li, Y.; Duan, W.; Huang, F.; Hou, X. Cold Acclimation Alters DNA Methylation Patterns and Confers Tolerance to Heat and Increases Growth Rate in Brassica rapa. J. Exp. Bot. 2017, 68, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Min, L.; Wang, M.; Wang, C.; Zhao, Y.; Li, Y.; Fang, Q.; Wu, Y.; Xie, S.; Ding, Y.; et al. Disrupted Genome Methylation in Response to High Temperature Has Distinct Affects on Microspore Abortion and Anther Indehiscence. Plant Cell 2018, 30, 1387–1403. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.M.; Horie, T. Genomics, Physiology, and Molecular Breeding Approaches for Improving Salt Tolerance. Annu. Rev. Plant Biol. 2017, 68, 405–434. [Google Scholar] [CrossRef] [PubMed]
- Yaish, M.W.; Al-Lawati, A.; Al-Harrasi, I.; Patankar, H.V. Genome-Wide DNA Methylation Analysis in Response to Salinity in the Model Plant Caliph Medic (Medicago truncatula). BMC Genom. 2018, 19, 78. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Huang, F.; Qin, Q.; Zhao, X.; Li, Z.; Fu, B. Comparative Analysis of DNA Methylation Changes in Two Rice Genotypes under Salt Stress and Subsequent Recovery. Biochem. Biophys. Res. Commun. 2015, 465, 790–796. [Google Scholar] [CrossRef]
- Feng, Q.; Yang, C.; Lin, X.; Wang, J.; Ou, X.; Zhang, C.; Chen, Y.; Liu, B. Salt and Alkaline Stress Induced Transgenerational Alteration in DNA Methylation of Rice (‘Oryza sativa’). Aust. J. Crop Sci. 2012, 6, 877–883. [Google Scholar]
- Ferreira, L.J.; Azevedo, V.; Maroco, J.; Oliveira, M.M.; Santos, A.P. Salt Tolerant and Sensitive Rice Varieties Display Differential Methylome Flexibility under Salt Stress. PLoS ONE 2015, 10, e0124060. [Google Scholar] [CrossRef]
- Paul, A.; Dasgupta, P.; Roy, D.; Chaudhuri, S. Comparative Analysis of Histone Modifications and DNA Methylation at OsBZ8 Locus under Salinity Stress in IR64 and Nonabokra Rice Varieties. Plant Mol. Biol. 2017, 95, 63–88. [Google Scholar] [CrossRef]
- Wang, M.; Qin, L.; Xie, C.; Li, W.; Yuan, J.; Kong, L.; Yu, W.; Xia, G.; Liu, S. Induced and Constitutive DNA Methylation in a Salinity-Tolerant Wheat Introgression Line. Plant Cell Physiol. 2014, 55, 1354–1365. [Google Scholar] [CrossRef]
- Huang, C.-F.; Miki, D.; Tang, K.; Zhou, H.-R.; Zheng, Z.; Chen, W.; Ma, Z.-Y.; Yang, L.; Zhang, H.; Liu, R.; et al. A Pre-mRNA-Splicing Factor Is Required for RNA-Directed DNA Methylation in Arabidopsis. PLoS Genet. 2013, 9, e1003779. [Google Scholar] [CrossRef]
- Karan, R.; DeLeon, T.; Biradar, H.; Subudhi, P.K. Salt Stress Induced Variation in DNA Methylation Pattern and Its Influence on Gene Expression in Contrasting Rice Genotypes. PLoS ONE 2012, 7, e40203. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Fang, B.; Huang, F.; Dai, L.; Tang, Z.; Tian, J.; Cao, G.; Meng, X.; Liu, Y.; Lei, B.; et al. Mass Spectrometry-Based Metabolomics Investigation on Two Different Indica Rice Grains (Oryza sativa L.) under Cadmium Stress. Food Chem. 2021, 343, 128472. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.J.; Liu, X.S.; Tao, H.; Tan, S.K.; Chu, S.S.; Oono, Y.; Zhang, X.D.; Chen, J.; Yang, Z.M. Variation of DNA Methylation Patterns Associated with Gene Expression in Rice (Oryza sativa) Exposed to Cadmium. Plant Cell Environ. 2016, 39, 2629–2649. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Cong, W.; Mu, J.; Fu, T.; Zhuang, T.; Yan, Y.; Kang, Y.; Yu, L.; Zhao, W.; Li, H.; et al. Various Potentially Toxic Element Tolerances in Different Rice Genotypes Correlate with Distinct Physiological Responses and Alterations in DNA Methylation. Chemosphere 2022, 292, 133462. [Google Scholar] [CrossRef] [PubMed]
- Cong, W.; Miao, Y.; Xu, L.; Zhang, Y.; Yuan, C.; Wang, J.; Zhuang, T.; Lin, X.; Jiang, L.; Wang, N.; et al. Transgenerational Memory of Gene Expression Changes Induced by Heavy Metal Stress in Rice (Oryza sativa L.). BMC Plant Biol. 2019, 19, 282. [Google Scholar] [CrossRef]
- Ou, X.; Zhang, Y.; Xu, C.; Lin, X.; Zang, Q.; Zhuang, T.; Jiang, L.; von Wettstein, D.; Liu, B. Transgenerational Inheritance of Modified DNA Methylation Patterns and Enhanced Tolerance Induced by Heavy Metal Stress in Rice (Oryza sativa L.). PLoS ONE 2012, 7, e41143. [Google Scholar] [CrossRef]
- Liu, X.S.; Feng, S.J.; Zhang, B.Q.; Wang, M.Q.; Cao, H.W.; Rono, J.K.; Chen, X.; Yang, Z.M. OsZIP1 Functions as a Metal Efflux Transporter Limiting Excess Zinc, Copper and Cadmium Accumulation in Rice. BMC Plant Biol. 2019, 19, 283. [Google Scholar] [CrossRef]
- Feng, S.J.; Liu, X.S.; Cao, H.W.; Yang, Z.M. Identification of a Rice Metallochaperone for Cadmium Tolerance by an Epigenetic Mechanism and Potential Use for Clean up in Wetland. Environ. Pollut. 2021, 288, 117837. [Google Scholar] [CrossRef]
- Ding, H.; Gao, J.; Qin, C.; Ma, H.; Huang, H.; Song, P.; Luo, X.; Lin, H.; Shen, Y.; Pan, G.; et al. The Dynamics of DNA Methylation in Maize Roots under Pb Stress. Int. J. Mol. Sci. 2014, 15, 23537–23554. [Google Scholar] [CrossRef]
- Mager, S.; Schönberger, B.; Ludewig, U. The Transcriptome of Zinc Deficient Maize Roots and Its Relationship to DNA Methylation Loss. BMC Plant Biol. 2018, 18, 372. [Google Scholar] [CrossRef]
- Shafiq, S.; Zeb, Q.; Ali, A.; Sajjad, Y.; Nazir, R.; Widemann, E.; Liu, L. Lead, Cadmium and Zinc Phytotoxicity Alter DNA Methylation Levels to Confer Heavy Metal Tolerance in Wheat. Int. J. Mol. Sci. 2019, 20, 4676. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.K.; Ye, J.Y.; Zhang, L.L.; Chen, H.S.; Zhang, H.H.; Zhu, Y.X.; Liu, X.X.; Jin, C.W. Inhibition of DNA Demethylation Enhances Plant Tolerance to Cadmium Toxicity by Improving Iron Nutrition. Plant Cell Environ. 2020, 43, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Galati, S.; Gullì, M.; Giannelli, G.; Furini, A.; DalCorso, G.; Fragni, R.; Buschini, A.; Visioli, G. Heavy Metals Modulate DNA Compaction and Methylation at CpG Sites in the Metal Hyperaccumulator Arabidopsis Halleri. Environ. Mol. Mutagen. 2021, 62, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lang, Z.; Zhu, J.-K. Dynamics and Function of DNA Methylation in Plants. Nat. Rev. Mol. Cell Biol. 2018, 19, 489–506. [Google Scholar] [CrossRef] [PubMed]
- Bewick, A.J.; Schmitz, R.J. Gene Body DNA Methylation in Plants. Curr. Opin. Plant Biol. 2017, 36, 103–110. [Google Scholar] [CrossRef]
- Feng, Z.; Zhan, X.; Pang, J.; Liu, X.; Zhang, H.; Lang, Z.; Zhu, J.-K. Genetic Analysis Implicates a Molecular Chaperone Complex in Regulating Epigenetic Silencing of Methylated Genomic Regions. J. Integr. Plant Biol. 2021, 63, 1451–1461. [Google Scholar] [CrossRef]
- Qian, W.; Miki, D.; Lei, M.; Zhu, X.; Zhang, H.; Liu, Y.; Li, Y.; Lang, Z.; Wang, J.; Tang, K.; et al. Regulation of Active DNA Demethylation by an α-Crystallin Domain Protein in Arabidopsis. Mol. Cell 2014, 55, 361–371. [Google Scholar] [CrossRef]
- Lang, Z.; Wang, Y.; Tang, K.; Tang, D.; Datsenka, T.; Cheng, J.; Zhang, Y.; Handa, A.K.; Zhu, J.-K. Critical Roles of DNA Demethylation in the Activation of Ripening-Induced Genes and Inhibition of Ripening-Repressed Genes in Tomato Fruit. Proc. Natl. Acad. Sci. USA 2017, 114, E4511–E4519. [Google Scholar] [CrossRef]
- Ahmad, F.; Farman, K.; Waseem, M.; Rana, R.M.; Nawaz, M.A.; Rehman, H.M.; Abbas, T.; Baloch, F.S.; Akrem, A.; Huang, J.; et al. Genome-Wide Identification, Classification, Expression Profiling and DNA Methylation (5mC) Analysis of Stress-Responsive ZFP Transcription Factors in Rice (Oryza sativa L.). Gene 2019, 718, 144018. [Google Scholar] [CrossRef]
- Gotarkar, D.; Longkumer, T.; Yamamoto, N.; Nanda, A.K.; Iglesias, T.; Li, L.-F.; Miro, B.; Blanco Gonzalez, E.; Montes Bayon, M.; Olsen, K.M.; et al. A Drought-Responsive Rice Amidohydrolase Is the Elusive Plant Guanine Deaminase with the Potential to Modulate the Epigenome. Physiol. Plant 2021, 172, 1853–1866. [Google Scholar] [CrossRef]
- Shaik, R.; Ramakrishna, W. Bioinformatic Analysis of Epigenetic and microRNA Mediated Regulation of Drought Responsive Genes in Rice. PLoS ONE 2012, 7, e49331. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhu, N.; Wang, X.; Yi, Q.; Zhu, D.; Lai, Y.; Zhao, Y. Analysis of Rice Snf2 Family Proteins and Their Potential Roles in Epigenetic Regulation. Plant Physiol. Biochem. 2013, 70, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Mohan Singh, R.K.; Malik, G.; Deveshwar, P.; Tyagi, A.K.; Kapoor, S.; Kapoor, M. Rice Cytosine DNA Methyltransferases—Gene Expression Profiling during Reproductive Development and Abiotic Stress. FEBS J. 2009, 276, 6301–6311. [Google Scholar] [CrossRef] [PubMed]
- Ganie, S.A.; Dey, N.; Mondal, T.K. Promoter Methylation Regulates the Abundance of Osa-miR393a in Contrasting Rice Genotypes under Salinity Stress. Funct. Integr. Genom. 2016, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wang, Y.; Tang, L.; Tong, X.; Wang, L.; Liu, L.; Huang, S.; Zhang, J. SAPK10-Mediated Phosphorylation on WRKY72 Releases Its Suppression on Jasmonic Acid Biosynthesis and Bacterial Blight Resistance. iScience 2019, 16, 499–510. [Google Scholar] [CrossRef]
- He, C.; Zhang, H.-Y.; Zhang, Y.-X.; Fu, P.; You, L.-L.; Xiao, W.-B.; Wang, Z.-H.; Song, H.-Y.; Huang, Y.-J.; Liao, J.-L. Cytosine Methylations in the Promoter Regions of Genes Involved in the Cellular Oxidation Equilibrium Pathways Affect Rice Heat Tolerance. BMC Genom. 2020, 21, 560. [Google Scholar] [CrossRef]


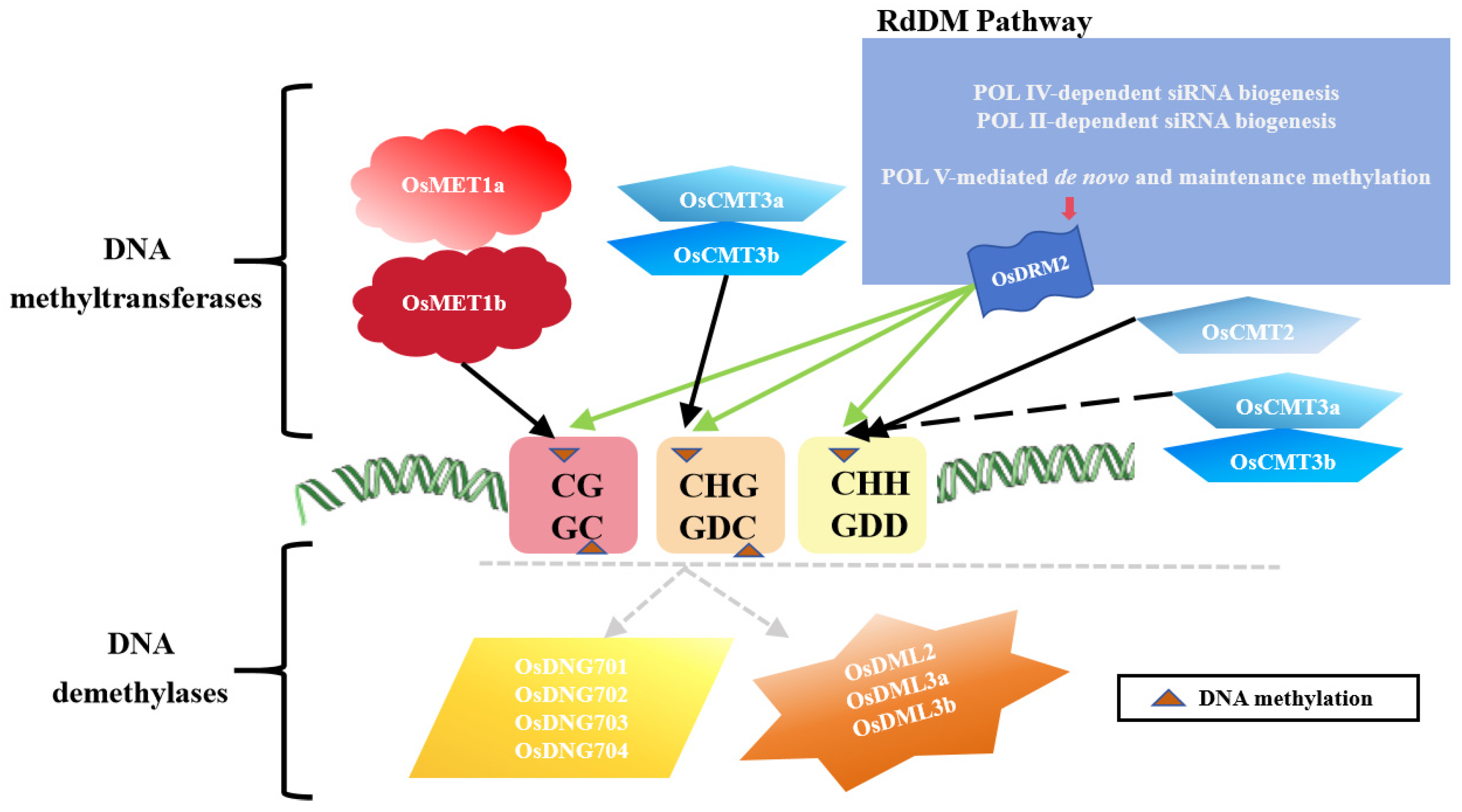
| Gene Name | Annotation | Locus ID | Reference | Function | |
|---|---|---|---|---|---|
| OsMYBR1 | MYB Transcription factor | LOC_Os04g49450 | [36] | Drought Stress | OsMYBR1-RNAi with higher than WT survival after restored watering, lower relative conductivity and malondialdehyde content, and higher proline content, which negatively regulated drought tolerance in rice |
| OsMYB48; OsMYB48-1 | MYB Transcription factor | LOC_Os01g74410 | [37] | Drought Stress | OE-OsMYB48-1 showed reduced water loss rate, low malondialdehyde, and high proline, hypersensitivity to ABA during germination and after germination, and accumulated more endogenous ABA under drought stress. |
| OsPIN3t; OsPIN10a; OsPIN3a | PIN-FORMED | LOC_Os01g45550 | [39] | Drought Stress | OsPIN3t Is an auxin export carrier localized to the plasma membrane, involved in the polar auxin transport, but also involved in the drought stress response in rice |
| OsGH3-2; OsGH3.2 | indole-3-acetic acid-amido synthetase | LOC_Os01g55940 | [40] | Drought Stress | OsGH 3-2 encodes an enzyme that catalyzes IAA-binding amino acids, which is induced by drought but inhibited by cooling. It is involved in the regulation of auxin and abscisic acid content in rice and plays positive and negative roles in regulating cold tolerance and drought resistance, respectively |
| SRL1; CLD1 | SEMI-ROLLED LEAF1 | LOC_Os07g01240 | [129] | Drought Stress | SEMI-ROLLED LEAF1 encodes a glycosylphosphatidylinositol-anchored protein localized to the plasma membrane. SRL 1 negatively regulates vesicular cell formation by negatively regulating the expression of the vacuolar H + -ATPase subunit and H + -pyrophosphatase genes |
| OsNAC10; ONAC122(ZmNAC111) | NAC Transcription factor | LOC_Os11g03300 | [131] | Drought Stress | OsNAC10 The specific expression in rice roots increases the root system, enhances the drought resistance ability, and then improves the rice yield under drought conditions |
| OsSAPK10 | Stress-Activated Protein Kinase | LOC_Os03g41460 | [139] | Drought Stress | miR2105 and the kinase OsSAPK10 co-regulate OsbZIP86 to mediate drought-induced ABA biosynthesis in rice. |
| OsNRAMP5 | natural resistance-associated macrophage protein | LOC_Os07g15370 | [73] | Heavy Metal | Nramp5 is a resistance-related macrophage protein, the main transporter of rice root cells involved in the uptake of external metal, and is also responsible for the transport of these ions from the roots to the shoots. |
| OsIRT1 | iron-regulated transporter | LOC_Os03g46470 | [73] | Heavy Metal | OE-OsIRT1 showing increased resistance to iron deficiency at the seedling stage, sensitivity to excess zinc and cadmium, and increased zinc–iron content in shoots, roots, and mature seeds. |
| OsIRT2 | iron-regulated transporter | LOC_Os03g46454 | [73] | Heavy Metal | Rice plants can absorb cadmium ions from the soil through OsIRT1 and OsIRT2 and transport them to the ground, and OE-OsIRT2 enhances cadmium stress resistance. |
| COLD1 | Chilling Tolerance | LOC_Os04g51180 | [53] | Temperature Stress | COLD 1 encodes a regulator of G protein signaling, and overexpression of COLD1jap significantly increases rice cold tolerance, while rice lines lacking or low expression of COLD1jap are sensitive to cold. |
| D1; RGA1; D89 | heterotrimeric G protein α subunit | LOC_Os05g26890 | [53] | Temperature Stress | When experiments at the same light intensity, d1 showed a stronger ability to eliminate excess irradiance with increased non-photochemical quenching. Increased light avoidance and photoprotection in d1 reduced sustained photoinhibitory damage, as revealed by higher Fv/Fm. |
| OsEIL1 | ethylene-insensitive | LOC_Os03g20790 | [63] | Salt Stress | MHZ 6 encodes OsEIL1, which is homologous to EIN 3, a major transcriptional regulator of ethylene signaling in Arabidopsis, with transcriptional activation activity. MHZ 6/OsEIL1 and OsEIL2 negatively regulate salt tolerance in rice, which may be achieved through direct regulation of OsHKT2; 1 expression and Na + uptake in roots. |
| OsEIL2 | ethylene-insensitive | LOC_Os07g48630 | [63] | Salt Stress | OsEIL2 Loss of function will improve salt tolerance, with less Na + accumulation in root and shoots under salt stress; the seedlings of overexpression lines are sensitive to salt, with more Na + accumulation and lower grain size and 1000-grain weight. |
| OSBZ8; OsbZIP05; OsbZIP5 | Bzip Transcription factor | LOC_Os01g46970 | [151] | Salt Stress | OSBZ 8 plays an important role in the transcriptional regulation of vegetative tissues in rice. OSBZ 8 is present in the ABRE-DNA: protein complex, and when treating seedlings with NaCl increases complex formation. OSBZ 8 is regulated at both the transcriptional and post-transcriptional levels. There is a positive correlation between OSBZ8 expression and salt resistance. |
| Gene Name | Annotation | Locus ID | Reference | Function | |
|---|---|---|---|---|---|
| ZFP31 | LSD0 subclass family protein | LOC_Os07g17400 | [190] | Drought Stress | zinc finger, RING-type, The methylation content of gene ZFP31 decreased, and the expression level increased under salt stress |
| ZP160 | LSD1 subclass family protein | LOC_Os08g12680 | [190] | Drought Stress | zinc finger domain, LSD1 subclass family protein The methylation content of gene ZP160 decreased, and the expression level increased under salt stress |
| ZP35 | ZOS11-03-C2H2 zinc finger protein | LOC_Os11g30484 | [190] | Drought Stress | zinc finger domain, LSD2 subclass family protein The ZFP35 gene was downregulated, and methylation was reduced under salt stress and drought stress |
| OsTRAB1;OsbZIP66 | bZIP Transcription factors | LOC_Os08g36790 | [146] | Drought Stress | Enhances demethylation and increases expression level under drought stress. Hypomethylation was mainly observed in the flanking region (88.88%) |
| OsRHP1 | RING-H2 finger protein | LOC_Os08g38460 | [146] | Drought Stress | |
| OsAP2.4 | AP2 domain-containing protein expressed | LOC_Os04g57340 | [146] | Drought Stress | |
| OsSGL;An-4 | SOG1-like | LOC_Os02g38130 | [146] | Drought Stress | |
| OsDOG1L-1 | SOG1-like | LOC_Os01g06560 | [146] | Drought Stress | |
| OsGDA1 | guanine deaminase 1 | LOC_Os03g61810 | [191] | Drought Stress | OsGDA1 Knockdown Impacts Xanthine Metabolism and SAH Content. Lower SAH can enhance genomic methylation, altering gene silencing or expression. |
| DUF3353 | DUF family protein | LOC_Os03g15033 | [192] | Drought Stress | DUF3353 is downregulated in drought stress and is targeted by 20 miRNAs that are part of miRBase |
| EL268 | Snf2 | LOC_Os03g51020 | [193] | Drought Stress | Differential methylation and regulation of Snf 2 family protein genes may lead to epitopes differentially methylated genome-wide when rice is subjected to osmotic (drought) stress |
| OsZIP1 | zinc-regulated transporters and iron-regulated transporter-like protein | LOC_Os01g74110 | [142] | Heavy Metal | OsZIP1 Is a metal detoxification transporter that prevents excessive accumulation of zinc, copper, and cadmium in rice. The DNA methylation of OsZIP1 histone H3K9me2 was further characterized by finding that its transcribed regional sites were demethylated |
| OsHMP | Heavy Metal Responsive Protein | LOC_Os02g37280 | [143] | Heavy Metal | heavy metal transport/detoxification protein in rice |
| Oshox22 | Homeobox-leucine zipper protein HOX1 | LOC_Os04g45810 | [146] | Salt Stress | In Pokkali, the CHH background showed hypermethylation and higher gene expression under salinity stress |
| Oschit1.1 | glycosyl hydrolase, putative, expressed | LOC_Os01g64100 | [146] | Salt Stress | In Pokkali, the CHH background showed hypermethylation and higher gene expression under salinity stress |
| OSBZ8;OsbZIP05 | bZIP Transcription factors | LOC_Os01g46970 | [169] | Salt Stress | The DNA hypomethylation status at the OsBZ 8 locus may promote transcript expression of salt tolerance genes. |
| OsBAG4 | BAG protein | LOC_Os01g61500 | [169] | Salt Stress | Enhances resistance to salt stress by protein complex OsSUVH7-OsBAG4-OsMYB106, and thus binding OsHKT1, regulates NA + to achieve. |
| OsSUVH7;SDG709 | DNA methylation reader | LOC_Os01g59620 | [169] | Salt Stress | |
| OsMYB106 | MYB Transcription factors | LOC_Os08g33660 | [169] | Salt Stress | |
| OsHKT1;5 | Na+-selective transporter | LOC_Os01g20160 | [169] | Salt Stress | |
| OsMET1-2 | DNA methyltransferase | LOC_Os07g08500 | [194] | Salt Stress | Suppresses expression under salt and drought stress |
| OsCMT2 | chromomethylase | LOC_Os05g13780 | [194] | Salt Stress | Enhances resistance to cold and salt stress |
| OsCMT3 | chromomethylase | LOC_Os10g01570/LOC_Os03g12570 | [194] | Salt Stress | Suppresses expression under salt and drought stress as DNA methylase |
| TIR1 | auxin receptor | LOC_Os05g05800 | [195] | Salt Stress | Rice produced salinity adaptation by the methylation differences in the promoter region of the osa-miR393a-TIR 1 module. |
| OST1 | Stress-Activated Protein Kinase | LOC_Os03g41460 | [196] | Temperature Stress | Interacts with and phosphorylates ICE1 to avoid ubiquitination degradation of the ICE1 protein, thereby enhancing the plant’s ability to tolerate chilling |
| PPR | PPR repeat domain-containing protein, putative, expressed | LOC_Os07g28900 | [197] | Temperature Stress | Cytosine methylations in the promoter regions of genes involved in the cellular oxidation equilibrium pathways affect rice heat tolerance |
| OsFIE1 | fertilization-independent endosperm gene | LOC_Os08g04290 | [21] | Temperature Stress | Rice fertilization-independentendosperm1 regulates seed size under heat stress by controlling early endosperm development. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, M.; Wang, S.; Wang, Y.; Wei, R.; Liang, Y.; Zuo, L.; Huo, M.; Huang, Z.; Lang, J.; Zhao, X.; et al. Impact of Abiotic Stress on Rice and the Role of DNA Methylation in Stress Response Mechanisms. Plants 2024, 13, 2700. https://doi.org/10.3390/plants13192700
Yin M, Wang S, Wang Y, Wei R, Liang Y, Zuo L, Huo M, Huang Z, Lang J, Zhao X, et al. Impact of Abiotic Stress on Rice and the Role of DNA Methylation in Stress Response Mechanisms. Plants. 2024; 13(19):2700. https://doi.org/10.3390/plants13192700
Chicago/Turabian StyleYin, Ming, Shanwen Wang, Yanfang Wang, Ronghua Wei, Yawei Liang, Liying Zuo, Mingyue Huo, Zekai Huang, Jie Lang, Xiuqin Zhao, and et al. 2024. "Impact of Abiotic Stress on Rice and the Role of DNA Methylation in Stress Response Mechanisms" Plants 13, no. 19: 2700. https://doi.org/10.3390/plants13192700







