Biochemical and Proteomic Analyses in Drought-Tolerant Wheat Mutants Obtained by Gamma Irradiation
Abstract
:1. Introduction
2. Results
2.1. Plant Growth
2.2. Chlorophyll, Proline, and TBARS Contents
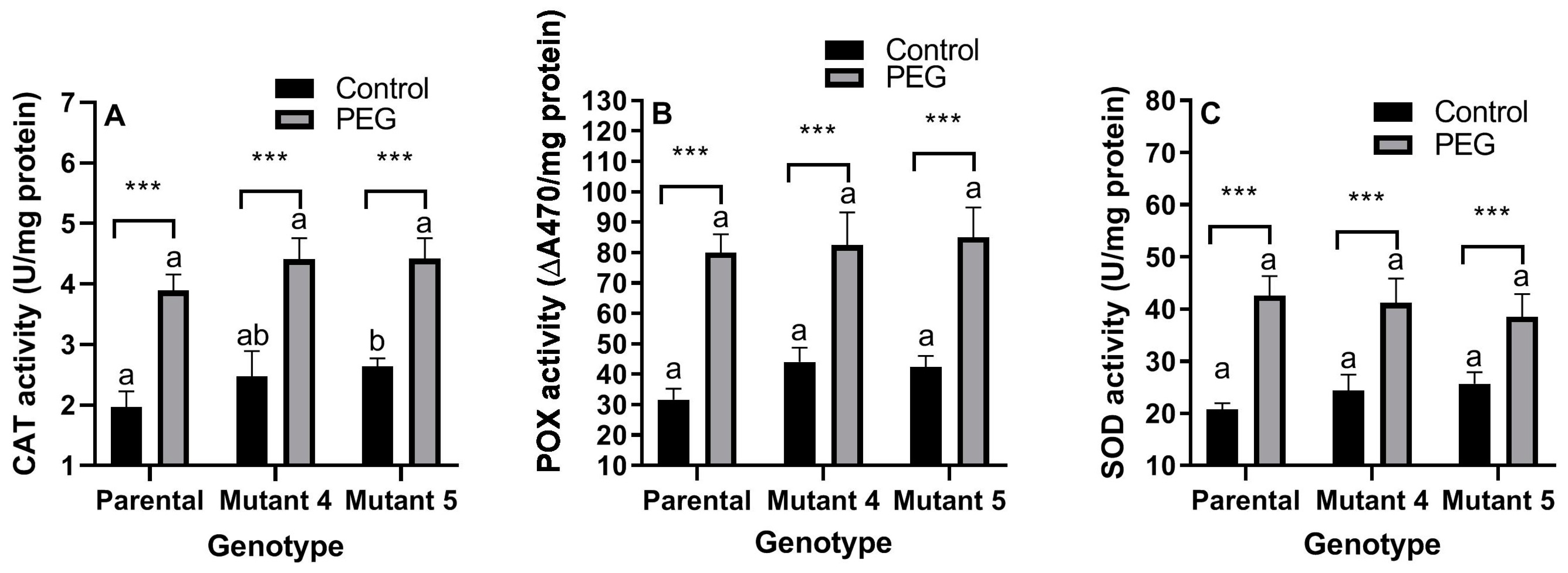
2.3. Antioxidant Enzyme Activity
2.4. Changes in mRNA Transcript Levels
2.5. Proteomic Changes
2.6. Kappa Values
2.7. STI Values
3. Discussion
3.1. Chlorophyll and TBARS Contents
3.2. Antioxidant Enzyme Activities and Proline Content
3.3. Transcriptional Regulation
3.4. Chloroplast Proteins
3.5. Mitochondrial Proteins
3.6. Other Proteins
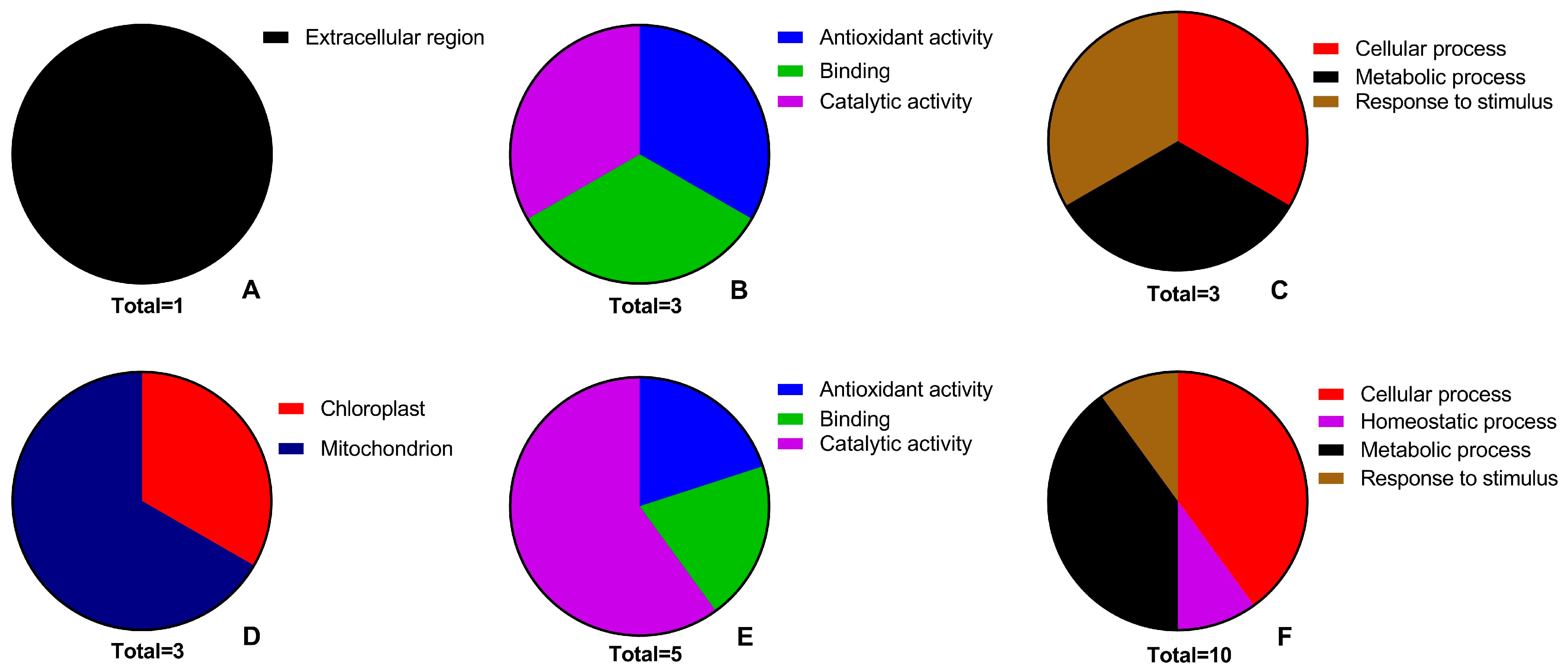
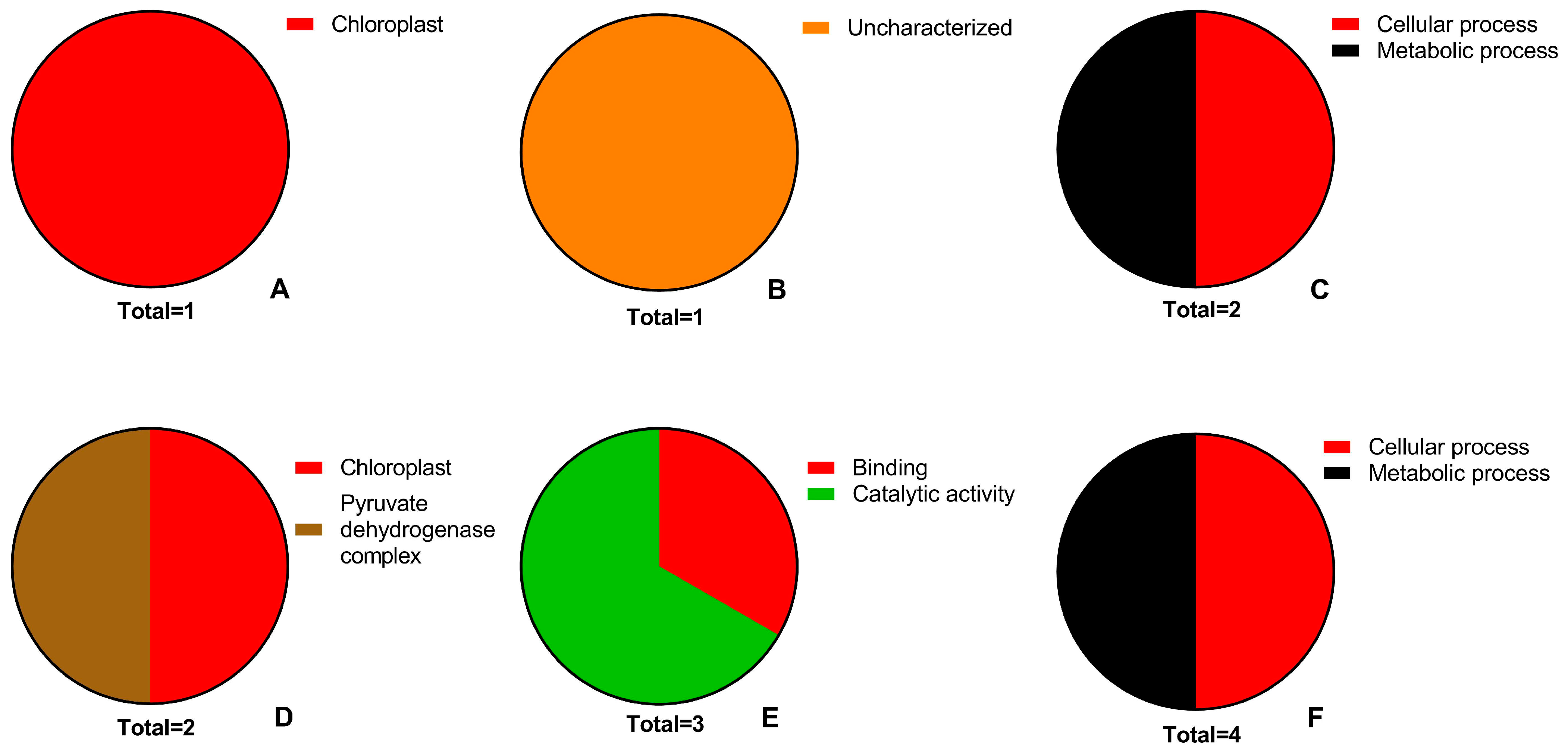
3.7. Relationship between BP Terms
3.8. STI Clustering
4. Materials and Methods
4.1. Plant Material
4.2. Measurement of Total Chlorophyll, Proline and the End Products of Lipid Peroxidation Contents
4.3. Measurement of the Enzyme Activities
4.4. qRT-PCR Analysis
4.5. Protein Extraction
4.6. 2DE Analysis
4.7. LC-MS/MS Data Analysis
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zschiesche, W.; Humbeck, K. Water. In Ecological Biochemistry: Environmental and Interspecies Interactions; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014; pp. 190–207. [Google Scholar] [CrossRef]
- Singh, P.K.; Indoliya, Y.; Agrawal, L.; Awasthi, S.; Deeba, F.; Dwivedi, S.; Chakrabarty, D.; Shirke, P.A.; Pandey, V.; Singh, N.; et al. Genomic and proteomic responses to drought stress and biotechnological interventions for enhanced drought tolerance in plants. Curr. Plant Biol. 2022, 29, 100239. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H. Mechanism of ABA signal transduction: Agricultural highlights for improving drought tolerance. J. Plant Biol. 2014, 57, 1–8. [Google Scholar] [CrossRef]
- Ali, S.; Hayat, K.; Iqbal, A.; Xie, L. Implications of abscisic acid in the drought stress tolerance of plants. Agronomy 2020, 10, 1323. [Google Scholar] [CrossRef]
- Aslam, M.M.; Waseem, M.; Jakada, B.H.; Okal, E.J.; Lei, Z.; Saqib, H.S.A.; Yuan, W.; Xu, W.; Zhang, Q. Mechanisms of abscisic Acid-Mediated drought stress responses in plants. Int. J. Mol. Sci. 2022, 23, 1084. [Google Scholar] [CrossRef]
- Romero-Perez, P.S.; Dorone, Y.; Flores, E.; Sukenik, S.; Boeynaems, S. When phased without water: Biophysics of cellular desiccation, from biomolecules to condensates. Chem. Rev. 2023, 123, 9010–9035. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Ali, Q.; Anwar, F.; Ashraf, M.; Saari, N.; Perveen, R. Ameliorating effects of exogenously applied proline on seed composition, seed oil quality and oil antioxidant activity of maize (Zea mays L.) under drought stress. Int. J. Mol. Sci. [CrossRef]
- Godoy, F.; Olivos-Hernández, K.; Stange, C.; Handford, M. Abiotic stress in crop species: Improving tolerance by applying plant metabolites. Plants 2021, 10, 186. [Google Scholar] [CrossRef]
- Rani, S.; Kumar, P.; Suneja, P. Biotechnological interventions for inducing abiotic stress tolerance in crops. Plant Gene 2021, 27, 100315. [Google Scholar] [CrossRef]
- Lata, C.; Shivhare, R. Engineering cereal crops for enhanced abiotic stress tolerance. Proc. Indian Natl. Sci. Acad. 2021, 87, 63–83. [Google Scholar] [CrossRef]
- Zafar, S.A.; Zaidi, S.S.A.; Gaba, Y.; Singla-Pareek, S.L.; Dhankher, O.P.; Li, X.; Mansoor, S.; Pareek, A. Engineering abiotic stress tolerance via CRISPR/Cas-mediated genome editing. J. Exp. Bot. 2020, 71, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Suprasanna, P.; Mirajkar, S.J.; Patade, V.Y.; Jain, S.M. 17. Induced mutagenesis for improving plant abiotic stress tolerance. In Mutagenesis: Exploring Genetic Diversity of Crops; Wageningen Academic Publishers: Wageningen, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Ahloowalia, B.S.; Maluszynski, M. Induced mutations in plant breeding. Euphytica 2001, 118, 167–173. [Google Scholar] [CrossRef]
- Ma, L.; Kong, F.; Sun, K.; Wang, T.; Guo, T. From classical radiation to modern radiation: Past, present, and future of radiation mutation breeding. Front. Public Health 2021, 9, 768071. [Google Scholar] [CrossRef] [PubMed]
- Mutant Variety Search. Available online: https://nucleus.iaea.org/sites/mvd/SitePages/Search.aspx (accessed on 2 May 2024).
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Budak, H.; Hussain, B.; Khan, Z.; Ozturk, N.Z.; Ullah, N. From genetics to functional genomics: Improvement in drought signaling and tolerance in wheat. Front. Plant Sci. 2015, 6, 1012. [Google Scholar] [CrossRef]
- Sen, A.; Ozturk, I.; Yaycili, O.; Alikamanoglu, S. Drought tolerance in irradiated wheat mutants studied by genetic and biochemical markers. J. Plant Growth Regul. 2017, 36, 669–679. [Google Scholar] [CrossRef]
- Sen, A.; Kecoglu, I.; Ahmed, M.; Parlatan, U.; Unlu, M.B. Differentiation of advanced generation mutant wheat lines: Conventional techniques versus Raman spectroscopy. Front. Plant Sci. 2023, 14, 1116876. [Google Scholar] [CrossRef]
- Haak, D.C.; Fukao, T.; Grene, R.; Hua, Z.; Ivanov, R.; Perrella, G.; Li, S. Multilevel regulation of abiotic stress responses in plants. Front. Plant Sci. 2017, 8, 1564. [Google Scholar] [CrossRef]
- Schaumlöffel, D. The omics tool box. In Ecological Biochemistry: Environmental and Interspecies Interactions; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 342–365. [Google Scholar] [CrossRef]
- Wang, X.; Cai, X.; Xu, C.; Wang, Q.; Dai, S. Drought-Responsive mechanisms in plant leaves revealed by proteomics. Int. J. Mol. Sci. 2016, 17, 1706. [Google Scholar] [CrossRef]
- Jiang, S.S.; Liang, X.N.; Li, X.; Wang, S.L.; Lv, D.W.; Ma, C.Y.; Li, X.H.; Ma, W.J.; Yan, Y.M. Wheat drought-responsive grain proteome analysis by linear and nonlinear 2-DE and MALDI-TOF mass spectrometry. Int. J. Mol. Sci. 2012, 13, 16065–16083. [Google Scholar] [CrossRef]
- UniProt Knowledgebase. Available online: https://www.uniprot.org/uniprotkb/A0A3B6LWV1/entry (accessed on 2 April 2024).
- UniProt Knowledgebase. Available online: https://www.uniprot.org/uniprotkb/Q9S714/entry (accessed on 2 April 2024).
- Kyte, J.; Doolittle, R.F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982, 157, 105–132. [Google Scholar] [CrossRef] [PubMed]
- ProtParam. Available online: https://web.expasy.org/protparam/ (accessed on 22 July 2024).
- UniProt Knowledgebase. Available online: https://www.uniprot.org/uniprotkb/P11383/entry (accessed on 2 April 2024).
- UniProt Knowledgebase. Available online: https://www.uniprot.org/uniprotkb/A0A3B6DE81/entry (accessed on 2 April 2024).
- UniProt Knowledgebase. Available online: https://www.uniprot.org/uniprotkb/A0A3B6CFK1/entry (accessed on 2 April 2024).
- UniProt Knowledgebase. Available online: https://www.uniprot.org/uniprotkb/A0A0C4BJ55/entry (accessed on 2 April 2024).
- UniProt Knowledgebase. Available online: https://www.uniprot.org/uniprotkb/A0A3B6TLZ2/entry (accessed on 2 April 2024).
- UniProt Knowledgebase. Available online: https://www.uniprot.org/uniprotkb/A0A3B6LWF0/entry (accessed on 2 April 2024).
- Kapoor, D.; Singh, S.; Kumar, V.; Romero, R.; Prasad, R.; Singh, J. Antioxidant enzymes regulation in plants in reference to reactive oxygen species (ROS) and reactive nitrogen species (RNS). Plant Gene 2019, 19, 100182. [Google Scholar] [CrossRef]
- Agathokleous, E.; Feng, Z.; Peñuelas, J. Chlorophyll hormesis: Are chlorophylls major components of stress biology in higher plants? Sci. Total Environ. 2020, 726, 138637. [Google Scholar] [CrossRef] [PubMed]
- Dalal, V.K. Modulation of photosynthesis and other proteins during water–stress. Mol. Biol. Rep. 2021, 48, 3681–3693. [Google Scholar] [CrossRef] [PubMed]
- Takamiya, K.I.; Tsuchiya, T.; Ohta, H. Degradation pathway(s) of chlorophyll: What has gene cloning revealed? Trends Plant Sci. 2000, 5, 426–431. [Google Scholar] [CrossRef]
- Sheoran, S.; Thakur, V.; Narwal, S.; Turan, R.; Mamrutha, H.M.; Singh, V.; Tiwari, V.; Sharma, I. Differential activity and expression profile of antioxidant enzymes and physiological changes in wheat (Triticum aestivum L.) under drought. Appl. Biochem. Biotechnol. 2015, 177, 1282–1298. [Google Scholar] [CrossRef]
- Anjum, N.A.; Sofo, A.; Scopa, A.; Roychoudhury, A.; Gill, S.S.; Iqbal, M.; Lukatkin, A.S.; Pereira, E.; Duarte, A.C.; Ahmad, I. Lipids and proteins—Major targets of oxidative modifications in abiotic stressed plants. Environ. Sci. Pollut. Res. 2014, 22, 4099–4121. [Google Scholar] [CrossRef]
- Hosseinifard, M.; Stefaniak, S.; Javid, M.G.; Soltani, E.; Wojtyla, L.; Garnczarska, M. Contribution of exogenous proline to abiotic stresses tolerance in plants: A review. Int. J. Mol. Sci. 2022, 23, 5186. [Google Scholar] [CrossRef]
- Franco, A.A.; Odom, R.S.; Rando, T.A. Regulation of antioxidant enzyme gene expression in response to oxidative stress and during differentiation of mouse skeletal muscle. Free Radic. Biol. Med. 1999, 27, 1122–1132. [Google Scholar] [CrossRef]
- Nasirzadeh, L.; Sorkhilaleloo, B.; Hervan, E.M.; Fatehi, F. Changes in antioxidant enzyme activities and gene expression profiles under drought stress in tolerant, intermediate, and susceptible wheat genotypes. Cereal Res. Commun. 2020, 49, 83–89. [Google Scholar] [CrossRef]
- Sečenji, M.; Lendvai, Á.; Miskolczi, P.; Kocsy, G.; Gallé, Á.; Szűcs, A.; Hoffmann, B.; Sárvári, É.; Schweizer, P.; Stein, N.; et al. Differences in root functions during long-term drought adaptation: Comparison of active gene sets of two wheat genotypes. Plant Biol. 2010, 12, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Yan, J.; Li, W. A member of wheat class III peroxidase gene family, TaPRX-2A, enhanced the tolerance of salt stress. BMC Plant Biol. 2020, 20, 392. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.; Jiang, Y. Reactive oxygen species, antioxidant enzyme activities and gene expression patterns in leaves and roots of Kentucky bluegrass in response to drought stress and recovery. Sci. Hortic. 2009, 120, 264–270. [Google Scholar] [CrossRef]
- Hall, J.G.; Kiefer, J. Arthrogryposis as a Syndrome: Gene Ontology Analysis. Mol. Syndromol. 2016, 7, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.E.; Bassi, R.; Boekema, E.J.; Dekker, J.P.; Jansson, S.; Leister, D.; Robinson, C.; Scheller, H.V. Structure, function and regulation of plant photosystem I. Biochim. Biophys. Acta (BBA)—Bioenerg. 2007, 1767, 335–352. [Google Scholar] [CrossRef]
- Fromme, P.; Jordan, P.; Krauss, N. Structure of photosystem I. Biochim. Biophys. Acta (BBA)—Bioenerg. 2001, 1507, 5–31. [Google Scholar] [CrossRef]
- Li, H.; Sherman, L.A.A. Redox-responsive regulator of photosynthesis gene expression in the Cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 2000, 182, 4268–4277. [Google Scholar] [CrossRef]
- Borisova-Mubarakshina, M.M.; Vetoshkina, D.V.; Ivanov, B.N. Antioxidant and signaling functions of the plastoquinone pool in higher plants. Physiol. Plant. 2019, 166, 181–198. [Google Scholar] [CrossRef]
- Zhu, D.; Luo, F.; Zou, R.; Liu, J.; Yan, Y. Integrated physiological and chloroplast proteome analysis of wheat seedling leaves under salt and osmotic stresses. J. Proteom. 2021, 234, 104097. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, H.Y.; Fan, Y.Z.; Wang, W.; Zhao, Y.Y. Comparative chloroplast proteome analysis of exogenously supplied trehalose to wheat seedlings under heat stress. Photosynthetica 2018, 56, 1123–1133. [Google Scholar] [CrossRef]
- Cleland, W.W.; Andrews, T.J.; Gutteridge, S.; Hartman, F.C.; Lorimer, G.H. Mechanism of Rubisco: The Carbamate as general base. Chem. Rev. 1998, 98, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Karami, S.; Shiran, B.; Ravash, R.; Fallahi, H. A comprehensive analysis of transcriptomic data for comparison of plants with different photosynthetic pathways in response to drought stress. PLoS ONE 2023, 18, e0287761. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Wang, Y.; Sun, W.; Lou, Q.; Mei, H.; Shen, S.; Chen, H. Drought-responsive mechanisms in rice genotypes with contrasting drought tolerance during reproductive stage. J. Plant Physiol. 2012, 169, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Cavaliere, C.; Foglia, P.; Gubbiotti, R.; Samperi, R.; Laganà, A. Analysis of drought responsive proteins in wheat (Triticum durum) by 2D-PAGE and MALDI-TOF mass spectrometry. Plant Sci. 2009, 177, 570–576. [Google Scholar] [CrossRef]
- Rappsilber, J.; Mann, M. What does it mean to identify a protein in proteomics? Trends Biochem. Sci. 2002, 27, 74–78. [Google Scholar] [CrossRef]
- Budak, H.; Akpinar, B.A.; Unver, T.; Turktas, M. Proteome changes in wild and modern wheat leaves upon drought stress by two-dimensional electrophoresis and nanoLC-ESI–MS/MS. Plant Mol. Biol. 2013, 83, 89–103. [Google Scholar] [CrossRef]
- Uzlova, E.V.; Zimatkin, S.M. Cellular ATP synthase. Biol. Bull. Rev. 2021, 11, 134–142. [Google Scholar] [CrossRef]
- Tang, H.; Zhu, H. Specific changes in morphology and dynamics of plant mitochondria under abiotic Stress. Horticulturae 2022, 9, 11. [Google Scholar] [CrossRef]
- Khan, M.N.; Siddiqui, M.H.; AlSolami, M.A.; Siddiqui, Z.H. Melatonin-regulated heat shock proteins and mitochondrial ATP synthase induce drought tolerance through sustaining ROS homeostasis in H2S-dependent manner. Plant Physiol. Biochem. 2024, 206, 108231. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, J.; He, X.; Sun, H.; Zhang, G.; Wu, F. Comparative proteomic analysis of drought tolerance in the two contrasting Tibetan wild genotypes and cultivated genotype. BMC Genom. 2015, 16, 432. [Google Scholar] [CrossRef]
- Kikuchi, G.; Motokawa, Y.; Yoshida, T.; Hiraga, K. Glycine cleavage system: Reaction mechanism, physiological significance, and hyperglycinemia. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2008, 84, 246–263. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Fan, M.; Liu, Z.; Li, X.; Wang, H. Serine, glycine and one-carbon metabolism in cancer (Review). Int. J. Oncol. 2021, 58, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Ghatak, A.; Chaturvedi, P.; Bachmann, G.; Valledor, L.; Ramšak, Ž.; Bazargani, M.M.; Bajaj, P.; Jegadeesan, S.; Li, W.; Sun, X.; et al. Physiological and proteomic signatures reveal mechanisms of superior drought resilience in pearl millet compared to wheat. Front. Plant Sci. 2021, 11, 600278. [Google Scholar] [CrossRef]
- De Oliveira, H.O.; De Castro, G.L.S.; Correa, L.O.; Silvestre, W.V.D.; Nascimento, S.V.D.; Da Silva Valadares, R.B.; De Oliveira, G.C.; Santos, R.I.N.; Festucci-Buselli, R.A.; Pinheiro, H.A. Coupling physiological analysis with proteomic profile to understand the photosynthetic responses of young Euterpe oleracea palms to drought. Photosynth. Res. 2018, 140, 189–205. [Google Scholar] [CrossRef]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Dietz, K.J. Thiol-based peroxidases and ascorbate peroxidases: Why plants rely on multiple peroxidase systems in the photosynthesizing chloroplast? Mol. Cells 2016, 39, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G. Overview on peroxiredoxin. Mol. Cells 2016, 39, 1–5. [Google Scholar] [CrossRef]
- Kalinina, E.V.; Chernov, N.N.; Saprin, A.N. Involvement of thio-, peroxi-, and glutaredoxins in cellular redox-dependent processes. Biochemistry 2008, 73, 1493–1510. [Google Scholar] [CrossRef]
- Sudharsan, M.; Prasad, N.R.; Chakraborty, A.; Rajendrasozhan, S. Proteomic profiling of Deinococcus radiodurans with response to thioredoxin reductase inhibitor and ionizing radiation treatment. J. Proteom. 2022, 267, 104697. [Google Scholar] [CrossRef]
- Francoz, E.; Ranocha, P.; Nguyen-Kim, H.; Jamet, E.; Burlat, V.; Dunand, C. Roles of cell wall peroxidases in plant development. Phytochemistry 2015, 112, 15–21. [Google Scholar] [CrossRef]
- Kidwai, M.; Ahmad, I.Z.; Chakrabarty, D. Class III peroxidase: An indispensable enzyme for biotic/abiotic stress tolerance and a potent candidate for crop improvement. Plant Cell Rep. 2020, 39, 1381–1393. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.W.; Hwang, B.K. The pepper extracellular peroxidase CaPO2 is required for salt, drought and oxidative stress tolerance as well as resistance to fungal pathogens. Planta 2011, 235, 1369–1382. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wang, D.; Kang, S.; He, Z.; Li, X.; Tang, W.; Chen, K.; Pan, Y.; Zhou, Y.; Xu, Z.; et al. Ethylene regulates combined drought and low nitrogen tolerance in wheat: Proteomic analysis. Agronomy 2023, 13, 1950. [Google Scholar] [CrossRef]
- Patel, M.S.; Korotchkina, L.G. The biochemistry of the pyruvate dehydrogenase complex. Biochem. Mol. Biol. Educ. 2003, 31, 5–15. [Google Scholar] [CrossRef]
- Sutendra, G.; Kinnaird, A.; Dromparis, P.; Paulin, R.; Stenson, T.H.; Haromy, A.; Hashimoto, K.; Zhang, N.; Flaim, E.; Michelakis, E.D. A nuclear pyruvate dehydrogenase complex is important for the generation of Acetyl-COA and histone acetylation. Cell 2014, 158, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Z.; Wang, P.; Jiang, B.; Lei, X.; Wu, J.; Dong, W.; Gao, C. A 2-Cys peroxiredoxin gene from Tamarix hispida improved salt stress tolerance in plants. BMC Plant Biol. 2020, 20, 360. [Google Scholar] [CrossRef]
- Reddy, B.M.; Johnson, A.M.A.; Kumar, N.J.; Venkatesh, B.; Jayamma, N.; Pandurangaiah, M.; Sudhakar, C. De novo transcriptome analysis of drought-adapted cluster bean (Cultivar RGC-1025) reveals the Wax regulatory genes involved in drought resistance. Front. Plant Sci. 2022, 13, 868142. [Google Scholar] [CrossRef]
- Chakrabarti, P.; Chakravarty, D. Intrinsically disordered proteins/regions and insight into their biomolecular interactions. Biophys. Chem. 2022, 283, 106769. [Google Scholar] [CrossRef]
- Holehouse, A.S.; Kragelund, B.B. The molecular basis for cellular function of intrinsically disordered protein regions. Nat. Rev. Mol. Cell Biol. 2023, 25, 187–211. [Google Scholar] [CrossRef]
- Hsiao, A.S. Plant protein disorder: Spatial regulation, broad specificity, switch of signaling and physiological status. Front. Plant Sci. 2022, 13, 904446. [Google Scholar] [CrossRef]
- Viera, A.J.; Garrett, J.M. Understanding interobserver agreement: The kappa statistic. Fam. Med. 2005, 37, 360–363. [Google Scholar] [PubMed]
- Mandrekar, J.N. Measures of interrater agreement. J. Thorac. Oncol. 2011, 6, 6–7. [Google Scholar] [CrossRef] [PubMed]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.-H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef] [PubMed]
- ClueGO. Available online: http://www.ici.upmc.fr/cluego/ClueGODocumentation2019.pdf (accessed on 2 April 2024).
- Huang, D.; Sherman, B.T.; Tan, Q.; Collins, J.R.; Alvord, W.G.; Roayaei, J.; Stephens, R.; Baseler, M.W.; Lane, H.C.; Lempicki, R.A. The DAVID gene functional classification tool: A novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biol. 2007, 8, R183. [Google Scholar] [CrossRef]
- Lamba, K.; Kumar, M.; Singh, V.; Chaudhary, L.; Sharma, R.; Yashveer, S.; Dalal, M.S. Heat stress tolerance indices for identification of the heat tolerant wheat genotypes. Sci. Rep. 2023, 13, 10842. [Google Scholar] [CrossRef]
- Bukhari, M.A.; Shah, A.N.; Fahad, S.; Iqbal, J.; Nawaz, F.; Manan, A.; Baloch, M.S. Screening of wheat (Triticum aestivum L.) genotypes for drought tolerance using polyethylene glycol. Arab. J. Geosci. 2021, 14, 2808. [Google Scholar] [CrossRef]
- Haque, M.; Husna, M.T.; Uddin, M.; Hossain, M.; Sarwar, A.K.M.G.; Ali, O.M.; Abdel Latef, A.A.H.; Hossain, A. Heat stress at early reproductive stage differentially alters several physiological and biochemical traits of three tomato cultivars. Horticulturae 2021, 7, 330. [Google Scholar] [CrossRef]
- Kaur, H.; Kaur, R.; Manchanda, G.; Bindra, S.; Sharma, A. Screening of Cicer arietinum L. genotypes under combined presence of NaCl and anthracene using membership function value of stress tolerance. Plant Gene 2022, 31, 100371. [Google Scholar] [CrossRef]
- Kaur, S.; Suhalia, A.; Sarlach, R.S.; Shamshad, M.; Singh, P.; Grover, G.; Brar, A.; Sharma, A. Uncovering the Iranian wheat landraces for salinity stress tolerance at early stages of plant growth. Cereal Res. Commun. 2022, 50, 895–904. [Google Scholar] [CrossRef]
- Gokkuş, M.K.; Dumlupinar, Z.; Degirmenci, H. Drought resistance, quality characteristics and water-yield relationships of some wheat (Triticum aestivum L.) lines and varieties. J. Agron. Crop Sci. 2023, 210, e12678. [Google Scholar] [CrossRef]
- Arnon, D.I. Localization of polyphenoloxidase in the chloroplasts of Beta vulgaris. Nature 1948, 162, 341–342. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; pp. 121–126. [Google Scholar] [CrossRef]
- Primer 3. Available online: http://bioinfo.ut.ee/primer3-0.4.0 (accessed on 1 December 2023).
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using Real-Time Quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Damerval, C.; De Vienne, D.; Zivy, M.; Thiellement, H. Technical improvements in two-dimensional electrophoresis increase the level of genetic variation detected in wheat-seedling proteins. Electrophoresis 1986, 7, 52–54. [Google Scholar] [CrossRef]
- Albayrak, M.G.B.; Simsek, T.; Kasap, M.; Akpinar, G.; Canturk, N.Z.; Guler, S.A. Tissue proteome analysis revealed an association between cancer, immune system response, and the idiopathic granulomatous mastitis. Med. Oncol. 2022, 39, 238. [Google Scholar] [CrossRef]
- UniProt Knowledgebase. Available online: https://www.uniprot.org (accessed on 2 April 2024).
- Gene Ontology Browser. Available online: https://www.informatics.jax.org (accessed on 2 April 2024).
- How to Calculate Cohen’s Kappa in Excel. Available online: https://www.statology.org/cohens-kappa-excel/ (accessed on 2 April 2024).
- Nguyen, N.T.; Lindsey, M.L.; Jin, Y.F. Systems analysis of gene ontology and biological pathways involved in post-myocardial infarction responses. BMC Genom. 2015, 16, S18. [Google Scholar] [CrossRef]
- Index of /src/base/R-4. Available online: https://cran.r-project.org/src/base/R-4/ (accessed on 1 March 2024).


| Group | Number of Spots in Control Group | Number of Spots in PEG-Treated Group | Number of Shared Spots |
|---|---|---|---|
| Parental line | 193 | 105 | 62 |
| Mutant line 4 | 177 | 106 | 66 |
| Mutant line 5 | 318 | 281 | 145 |
| Total | 688 | 492 | 273 |
| Spot | ID | Name | Amino Acids | Molecular Weight (kDa) | pI | Detected in |
|---|---|---|---|---|---|---|
| A4 | A0A3B6DE81 | ATP synthase subunit beta | 498 | 53.9 | 5.16 | Mutant line 4 |
| A7 | A0A0C4BJ55 | Thioredoxin-dependent peroxiredoxin | 258 | 27.9 | 6.79 | Mutant line 4 |
| A8 | P11383 | Ribulose bisphosphate carboxylase large chain | 477 | 52.8 | 6.68 | Mutant line 4 |
| B1 | A0A3B6CFK1 | Aminomethyltransferase | 415 | 44.4 | 8.57 | Mutant line 5 |
| B2 | A0A3B6CFK1 | Aminomethyltransferase | 415 | 44.4 | 8.57 | Mutant line 5 |
| B4 | A0A3B6LWV1 | Uncharacterized protein | 148 | 15.6 | 9.70 | Mutant line 5 |
| C5 | A0A3B6TLZ2 | Peroxidase | 358 | 37.7 | 6.77 | Parental line |
| Spot | ID | Name | Amino Acids | Molecular Weight (kDa) | pI | Detected in |
|---|---|---|---|---|---|---|
| A2 | P11383 | Ribulose bisphosphate carboxylase large chain | 477 | 52.8 | 6.68 | Mutant line 4 |
| A3 | P11383 | Ribulose bisphosphate carboxylase large chain | 477 | 52.8 | 6.68 | Mutant line 4 |
| A5 | P11383 | Ribulose bisphosphate carboxylase large chain | 477 | 52.8 | 6.68 | Mutant line 4 |
| A6 | A0A3B6LWF0 | Dihydrolipoamide acetyltransferase component of PDC | 475 | 48.8 | 8.91 | Mutant line 4 |
| C4 | A0A3B6LWV1 | Uncharacterized protein | 148 | 15.6 | 9.70 | Parental line |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şen, A.; Gümüş, T.; Temel, A.; Öztürk, İ.; Çelik, Ö. Biochemical and Proteomic Analyses in Drought-Tolerant Wheat Mutants Obtained by Gamma Irradiation. Plants 2024, 13, 2702. https://doi.org/10.3390/plants13192702
Şen A, Gümüş T, Temel A, Öztürk İ, Çelik Ö. Biochemical and Proteomic Analyses in Drought-Tolerant Wheat Mutants Obtained by Gamma Irradiation. Plants. 2024; 13(19):2702. https://doi.org/10.3390/plants13192702
Chicago/Turabian StyleŞen, Ayşe, Tamer Gümüş, Aslıhan Temel, İrfan Öztürk, and Özge Çelik. 2024. "Biochemical and Proteomic Analyses in Drought-Tolerant Wheat Mutants Obtained by Gamma Irradiation" Plants 13, no. 19: 2702. https://doi.org/10.3390/plants13192702






