Dynamic Analysis of the Fruit Sugar-Acid Profile in a Fresh-Sweet Mutant and Wild Type in ‘Shatangju’ (Citrus reticulata cv.)
Abstract
:1. Introduction
2. Results
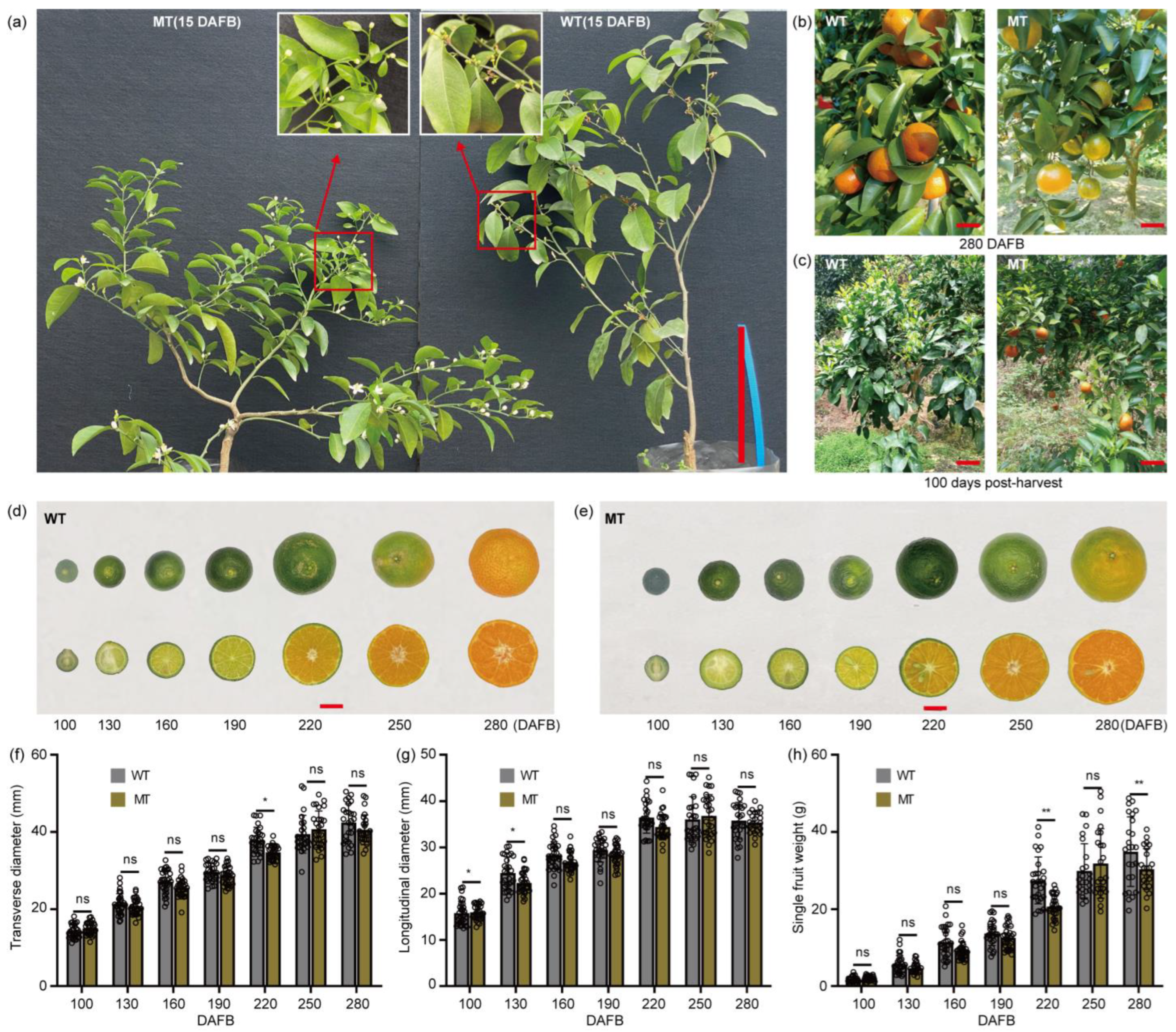
2.1. Morphological Characterization of Tree and Fruit in Normal ‘Shatangju’ (WT) and ‘Shatangju’ Mutant (MT)
2.2. Chromatogram of Organic Acids and Sugars
2.3. Dynamic Profiles of Organic Acids during the Development of WT and MT Fruits
2.4. Dynamic Profiles of the Soluble Sugars during the Development of WT and MT Fruits
3. Discussion
4. Materials and Methods
4.1. Experimental Materials and Measurements
4.2. Chemicals and Reagents
4.3. Sample Extraction
4.4. High-Performance Liquid Chromatography (HPLC) Analysis of Sugars and Organic Acids
4.5. Determination of Titratable Acidity Contents
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mao, Z.; Wang, Y.; Li, M.; Zhang, S.; Zhao, Z.; Xu, Q.; Liu, J.H.; Li, C. Vacuolar pro-teomic analysis reveals tonoplast transporters for accumulation of citric acid and sugar in citrus fruit. Hortic. Res. 2023, 11, uhad249. [Google Scholar] [CrossRef] [PubMed]
- Strazzer, P.; Spelt, C.E.; Li, S.; Bliek, M.; Federici, C.T.; Roose, M.L.; Koes, R.; Quattrocchio, F.M. Hyperacidification of Citrus fruits by a vacuolar proton-pumping P-ATPase complex. Nat. Commun. 2019, 26, 744. [Google Scholar] [CrossRef] [PubMed]
- Etienne, A.; Génard, M.; Lobit, P.; Mbeguié-A-Mbéguié, D.; Bugaud, C. What controls fleshy fruit acidity? a review of malate and citrate accumulation in fruit cells. J. Exp. Bot. 2013, 64, 1451–1469. [Google Scholar] [CrossRef] [PubMed]
- Finkemeier, I.; König, A.C.; Heard, W.; Nunes-Nesi, A.; Pham, P.A.; Leister, D.; Fernie, A.R.; Sweetlove, L.J. Transcriptomic analysis of the role of carboxylic acids in metabolite signaling in Arabidopsis leaves. Plant Physiol. 2013, 162, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Y.; Wang, C.K.; Zhao, Y.W.; Sun, C.H.; Hu, D.G. Mechanisms and regulation of organic acid accumulation in plant vacuoles. Hortic. Res. 2021, 8, 227. [Google Scholar] [CrossRef]
- He, J.; Sun, J.; Huang, Y.; Wang, L.; Liu, S.; Jiang, Z.; Wang, X.; Xu, Q. Transcriptome analysis reveals the common and specific pathways of citric acid accumulation in different citrus species. Hortic. Plant J. 2024, in press. [Google Scholar] [CrossRef]
- Huang, D.; Zhao, Y.; Cao, M.; Qiao, L.; Zheng, Z.L. Integrated systems biology analysis of transcriptomes reveals candidate genes for acidity control in developing fruits of sweet orange (Citrus sinensis L. Osbeck). Front. Plant Sci. 2016, 7, 7486. [Google Scholar] [CrossRef]
- Li, S.; Yin, X.; Xie, X.; Allan, A.C.; Ge, H.; Shen, S.; Chen, K. The citrus transcription factor, CitERF13, regulates citric acid accumulation via a protein-protein interaction with the vacuolar proton pump, CitVHA-c4. Sci. Rep. 2016, 3, 6. [Google Scholar] [CrossRef]
- Fürtauer, L.; Küstner, L.; Weckwerth, W.; Heyer, A.G.; Nägele, T. Resolving subcellular plant metabolism. Plant J. 2019, 100, 438–455. [Google Scholar] [CrossRef]
- Liu, S.; Liu, X.; Gou, B.; Wang, D.; Liu, C.; Sun, J.; Yin, X.; Grierson, D.; Li, S.; Chen, K. The interaction between CitMYB52 and CitbHLH2 negatively regulates citrate accumulation by activating CitALMT in citrus fruit. Front. Plant Sci. 2022, 13, 848869. [Google Scholar] [CrossRef]
- Li, S.J.; Wang, W.L.; Ma, Y.C.; Liu, S.C.; Grierson, D.; Yin, X.R.; Chen, K.S. Citrus CitERF6 contributes to citric acid degradation via upregulation of CitAclα1, Encoding ATP-Citrate Lyase Subunit α. J. Agric. Food Chem. 2020, 68, 10081–10087. [Google Scholar] [CrossRef] [PubMed]
- Butelli, E.; Licciardello, C.; Ramadugu, C.; Durand-Hulak, M.; Celant, A.; Recupero, G.R.; Froelicher, Y.; Martin, C. Noemi controls production of flavonoid pigments and fruit acidity and illustrates the domestication routes of modern citrus varieties. Curr. Biol. 2019, 29, 158–164.e2. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.J.; Zuo, H.; Xu, Q. Genomic insights into citrus domestication and its important agronomic traits. Plant Commun. 2020, 2, 100138. [Google Scholar] [CrossRef]
- Huang, Y.; He, J.; Xu, Y.; Zheng, W.; Wang, S.; Chen, P.; Zeng, B.; Yang, S.; Jiang, X.; Liu, Z.; et al. Pangenome analysis provides insight into the evolution of the orange subfamily and a key gene for citric acid accumulation in citrus fruits. Nat. Genet. 2023, 55, 1964–1975. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Tan, F.Q.; Fan, Y.J.; Wang, T.T.; Song, X.; Xie, K.D.; Wu, X.M.; Zhang, F.; Deng, X.-X.; Grosser, J.W.; et al. Acetylome reprograming participates in the establishment of fruit metabolism during polyploidization in citrus. Plant Physiol. 2022, 190, 2519–2538. [Google Scholar] [CrossRef]
- Zeng, J.; Gao, C.; Deng, G.; Jiang, B.; Yi, G.; Peng, X.; Zhong, Y.; Zhou, B.; Liu, K. Transcriptome analysis of fruit development of a citrus late-ripening mutant by microarray. Sci. Hortic. 2012, 134, 32–39. [Google Scholar] [CrossRef]
- Ban, S.; Jung, J.H. Somatic mutations in fruit trees: Causes, detection methods, and molecular mechanisms. Plants 2023, 12, 1316. [Google Scholar] [CrossRef]
- Gulsen, O.; Roose, M.L. Lemons: Diversity and relationships with selected citrus genotypes as measured with nuclear genome markers. J. Am. Soc. Hortic. Sci. Jashs 2001, 126, 309–317. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, A.; Chai, L.; Zhou, W.; Yu, K.; Ding, J.; Xu, J.; Deng, X. Transcriptome analysis of a spontaneous mutant in sweet orange [Citrus sinensis (L.) Osbeck] during fruit development. J. Exp. Bot. 2009, 60, 801–813. [Google Scholar] [CrossRef]
- Zingaretti, L.M.; Monfort, A.; Pérez-Enciso, M. Automatic fruit morphology phenome and genetic analysis: An application in the octoploid strawberry. Plant Phenom. 2021, 2021, 9812910. [Google Scholar] [CrossRef]
- Fan, Z.; Xiong, H.; Luo, Y.; Wang, Y.; Zhao, H.; Li, W.; He, X.; Wang, J.; Shi, X.; Zhang, Y. Fruit yields depend on biomass and nutrient accumulations in new shoots of citrus trees. Agronomy 2020, 10, 1988. [Google Scholar] [CrossRef]
- Tocmo, R.; Pena-Fronteras, J.; Calumba, K.F.; Mendoza, M.; Johnson, J.J. Valorization of pomelo (Citrus grandis Osbeck) peel: A review of current utilization, phytochemistry, bioactivities, and mechanisms of action. Compr. Rev. Food. Sci. Food Saf. 2020, 19, 1969–2012. [Google Scholar] [CrossRef] [PubMed]
- Emmanouilidou, M.G.; Kyriacou, M.C. Rootstock-modulated yield performance, fruit maturation and phytochemical quality of ‘Lane Late’ and ‘Delta’ sweet orange. Sci. Hortic. 2017, 225, 112–121. [Google Scholar] [CrossRef]
- Rouseff, R.; Ruiz Pérez-Cacho, P.; Jabalpurwala, F.A. Historical review of citrus flavor research during the past 100 years. J. Agric. Food. Chem. 2009, 57, 8115–8124. [Google Scholar] [CrossRef]
- Wang, L.; Huang, Y.; Liu, Z.; He, J.; Jiang, X.; He, F.; Lu, Z.; Yang, S.; Chen, P.; Yu, H.; et al. Somatic variations led to the selection of acidic and acidless orange cultivars. Nat. Plants 2021, 7, 954–965. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, C.; Tan, Q.; Zheng, C.; Gui, H.; Zeng, W.; Sun, X.; Zhao, X. Plant nutrition status, yield and quality of satsuma mandarin (Citrus unshiu Marc.) under soil application of Fe-EDDHA and combination with zinc and manganese in calcareous soil. Sci. Hortic. 2014, 174, 46–53. [Google Scholar] [CrossRef]
- Moing, A.; Rothan, C.; Svanella, L.; Just, D.; Diakou, P.; Raymond, P.; Gaudillère, J.; Monet, R. Role of phosphoenolpyruvate carboxylase in organic acid accumulation during peach fruit development. Physiol. Plant. 2000, 108, 1–10. [Google Scholar] [CrossRef]
- Sedaghat, S.; Rahemi, M. Enzyme activity regarding sugar and organic acid changes during developmental stages in rainfed fig (Ficus carica L.cv Sabz). Int. J. Fruit. Sci. 2018, 18, 14–28. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, S.; Ma, Y.; Liu, Z.; Tu, H.; Wang, H.; Zhang, J.; Chen, Q.; He, W.; Li, M.; et al. Soluble sugar and organic acid composition and flavor evaluation of Chinese cherry fruits. Food Chem. X 2023, 20, 100953. [Google Scholar] [CrossRef]
- Albertini, M.; Carcouet, E.; Pailly, O.; Gambotti, C.; Luro, F.; Berti, L. Changes in organic acids and sugars during early stages of development of acidic and acidless citrus fruit. J. Agric. Food. Chem. 2006, 54, 8335–8339. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J. Chemical composition and antioxidant capacity of different anatomical parts of pear (Pyrus communis L.). Food Chem. 2016, 203, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Liu, S.; Zheng, T. Chemotaxonomic identification of key taste and nutritional components in ‘Shushanggan Apricot’ fruits by widely targeted metabolomics. Molecules 2022, 27, 3870. [Google Scholar] [CrossRef] [PubMed]
- Tanase, K.; Shiratake, K.; Mori, H.; Yamaki, S. Changes in the phosphorylation state of sucrose synthase during development of Japanese pear fruit. Physiol. Plant 2002, 114, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, C.; Liu, H.; Liu, J.; Jiao, Z. Profiles of sugar and organic acid of fruit juices: A comparative study and implication for authentication. J. Food Qual. 2020, 1, 7236534. [Google Scholar] [CrossRef]
- Cercós, M.; Soler, G.; Iglesias, D.J.; Gadea, J.; Forment, J.; Talón, M. Global analysis of gene expression during development and ripening of citrus fruit flesh. A proposed mechanism for citric Acid utilization. Plant Mol. Biol. 2006, 62, 513–527. [Google Scholar] [CrossRef]
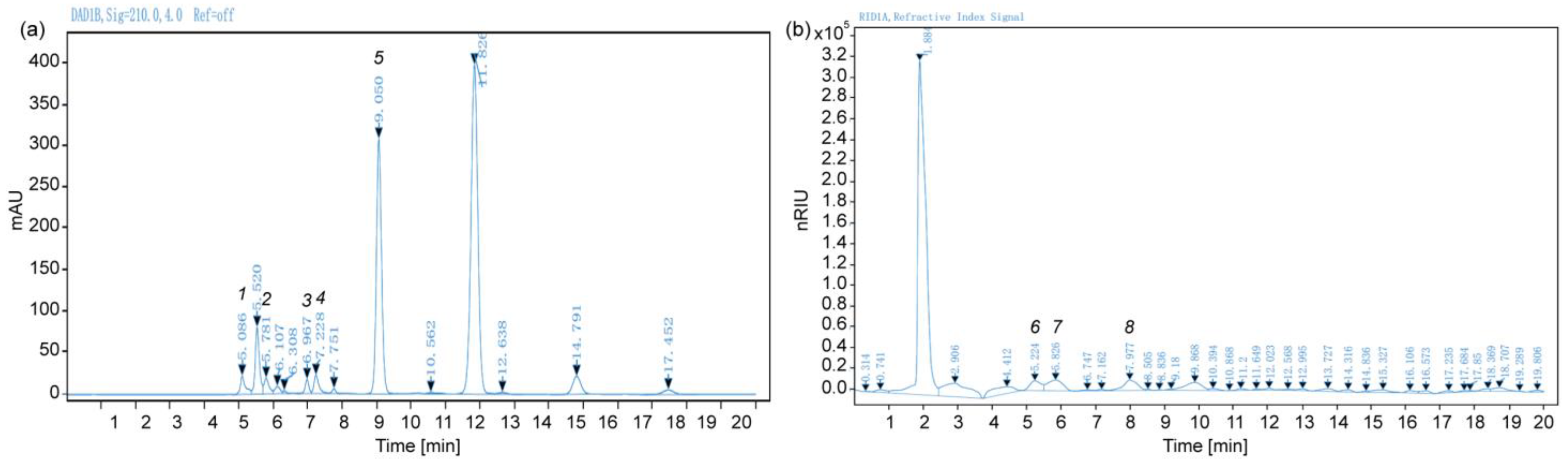


| Compound | Peak Time (min) | Linear Equation (Integrator Response to Concentration (mg mL−1), n = 3) | Correlation Coefficient (R2) |
|---|---|---|---|
| Glucose | 5.826 | y = 5 × 10−6 x + 0.0046 | 0.999 |
| Fructose | 5.224 | y = 6 ×10−6 x − 0.0433 | 0.997 |
| Sucrose | 7.977 | y = 6 × 10−6 x − 0.0691 | 0.992 |
| Citrate | 9.050 | y = 8 × 10−4 x + 0.0096 | 0.9999 |
| L-Malate | 6.967 | y = 1 × 10−3 x + 0.0001 | 1.000 |
| Quinate | 5.781 | y = 1.5 × 10−3 x − 0.0151 | 0.9994 |
| Vitamin C | 7.228 | y = 9 × 10−5 x + 0.1075 | 0.997 |
| Oxalate | 5.086 | y = 5 × 10−5 x − 0.0087 | 0.9995 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zeng, Y.; Wang, T.; Jiang, B.; Liao, M.; Lv, Y.; Li, J.; Zhong, Y. Dynamic Analysis of the Fruit Sugar-Acid Profile in a Fresh-Sweet Mutant and Wild Type in ‘Shatangju’ (Citrus reticulata cv.). Plants 2024, 13, 2722. https://doi.org/10.3390/plants13192722
Li X, Zeng Y, Wang T, Jiang B, Liao M, Lv Y, Li J, Zhong Y. Dynamic Analysis of the Fruit Sugar-Acid Profile in a Fresh-Sweet Mutant and Wild Type in ‘Shatangju’ (Citrus reticulata cv.). Plants. 2024; 13(19):2722. https://doi.org/10.3390/plants13192722
Chicago/Turabian StyleLi, Xiangyang, Yuan Zeng, Ting Wang, Bo Jiang, Mingjing Liao, Yuanda Lv, Juan Li, and Yun Zhong. 2024. "Dynamic Analysis of the Fruit Sugar-Acid Profile in a Fresh-Sweet Mutant and Wild Type in ‘Shatangju’ (Citrus reticulata cv.)" Plants 13, no. 19: 2722. https://doi.org/10.3390/plants13192722






