Analysis and Optimal Control of a Two-Strain SEIR Epidemic Model with Saturated Treatment Rate
Abstract
:1. Introduction
2. Model Formulation
3. Positivity and Boundedness of Solutions
3.1. Positivity of Solutions
3.2. Boundedness of Solutions
4. Basic Regeneration Number and Stability of Equilibrium
4.1. Basic Regeneration Number
4.2. Stability of Disease-Free Equilibrium
5. Optimal Control Strategy
5.1. Existence of an Optimal Control
5.2. Characterization of an Optimal Control
- (i)
- The control equation satisfaction:
- (ii)
- The following costate equation is satisfied:
- (iii)
- The minimum condition is the following:
- (iv)
- The Transversality conditions:
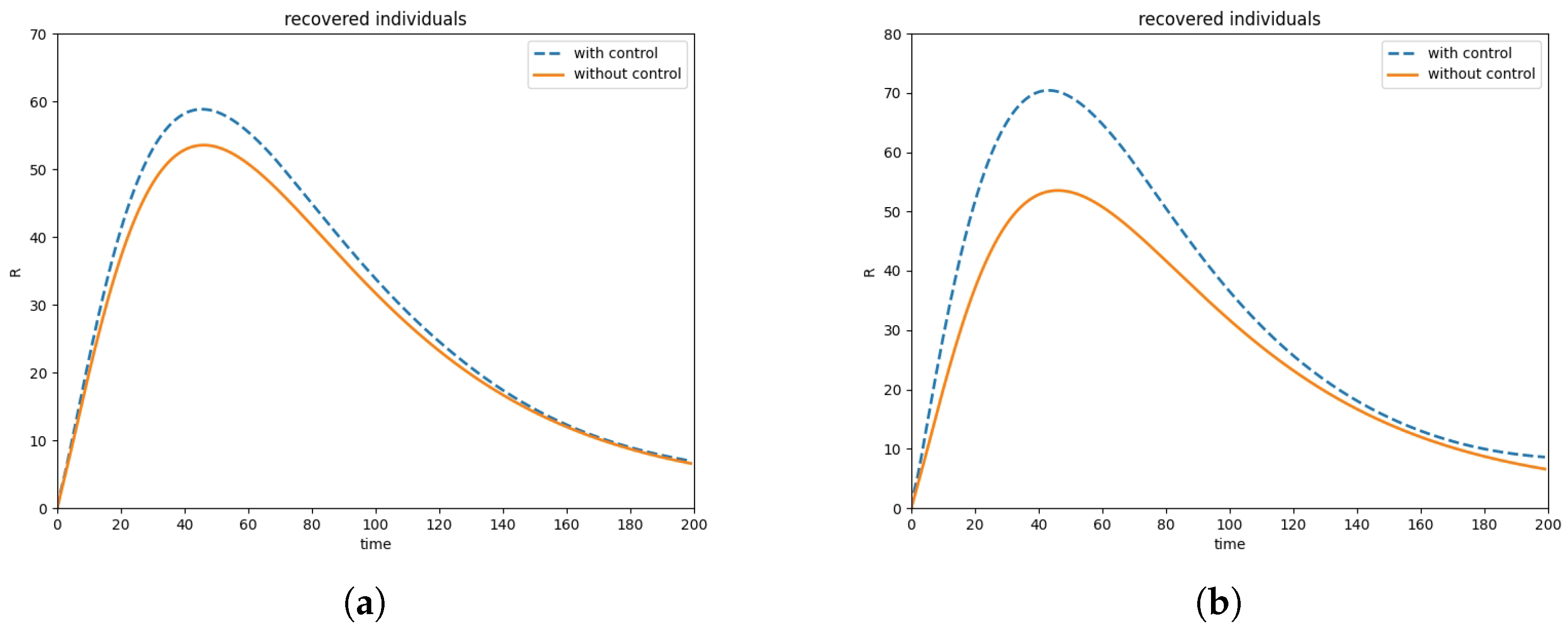
6. Numerical Simulations
6.1. Sensitivity Analysis
6.2. Optimal Control
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiao, J.; Liu, Z.; Cai, S. Dynamics of an SEIR model with infectivity in incubation period and homestead-isolation on the susceptible. Appl. Math. Lett. 2020, 107, 106442. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Lu, Z. Global asymptotic stability for the SEIRS models with varying total population size. Math. Biosci. 2018, 296, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-J.; Zhang, J.; Jin, Z. Analysis of an SIR model with bilinear incidence rate. Nonlinear Anal.-Real World Appl. 2010, 11, 2390–2402. [Google Scholar] [CrossRef]
- Zhou, X.; Guo, Z. Analysis of an influenza A (H1N1) epidemic model with vaccination. Arab. J. Math. 2012, 1, 267–282. [Google Scholar] [CrossRef]
- Gao, J.; Zhao, M. Stability and bifurcation of an epidemic model with saturated treatment function. In Computing and Intelligent Systems, Proceedings of the ICCIC 2011, Wuhan, China, 17–18 September 2011; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Ciric, L.; Ume, J.S.; Jesic, S.N. On random coincidence and fixed points for a pair of multivalued and single-valued mappings. J. Inequal. Appl. 2006, 2006, 1–12. [Google Scholar] [CrossRef]
- Li, J.-Q.; Ma, Z. Stability analysis for SIS epidemic models with vaccination and constant population size. Discrete Contin. Dyn. Syst.-B 2004, 4, 635–642. [Google Scholar] [CrossRef]
- Iannelli, M.; Martcheva, M.; Li, X. Strain replacement in an epidemic model with super-infection and perfect vaccination. Math. Biosci. 2005, 195, 23–46. [Google Scholar] [CrossRef] [PubMed]
- Kribs-Zaleta, C.; Velasco-Hernández, J.X. A simple vaccination model with multiple endemic states. Math. Biosci. 2000, 164, 183–201. [Google Scholar] [CrossRef] [PubMed]
- Wendi, W.; Ma, Z. Global dynamics of an epidemic model with time delay. Nonlinear Anal.-Real World Appl. 2002, 3, 365–373. [Google Scholar] [CrossRef]
- Kribs-Zaleta, C.; Martcheva, M. Vaccination strategies and backward bifurcation in an age-since-infection structured model. Math. Biosci. 2002, 177–178, 317–332. [Google Scholar] [CrossRef]
- Kermack, W.O.; McKendrick, Á.G. A contribution to the mathematical theory of epidemics. Proc. Roy. Soc. A 1927, 115, 700–721. [Google Scholar]
- Rohith, G.; Devika, K.B. Dynamics and control of COVID-19 pandemic with nonlinear incidence rates. Nonlinear Dyn. 2020, 101, 2013–2026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zeb, A.; Egbelowo, O.F.; Erturk, V.S. Dynamics of a fractional order mathematical model for COVID-19 epidemic. Adv. Differ. Equ. 2020, 2020, 420. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xia, Y.; Qin, W. Study on SEAI Model of COVID-19 Based on Asymptomatic Infection. Axioms 2024, 13, 309. [Google Scholar] [CrossRef]
- Xu, C.; Qin, K. Analysis of epidemic situation in novel coronavirus based on SEIR model. Comput. Appl. Softw. 2021, 38, 87–90. [Google Scholar]
- Khyar, O.; Allali, K. Global dynamics of a multi-strain SEIR epidemic model with general incidence rates: Application to COVID-19 pandemic. Nonlinear Dyn. 2020, 102, 489–509. [Google Scholar] [CrossRef] [PubMed]
- Meskaf, A.; Khyar, O.; Danane, J.; Allali, K. Global stability analysis of a two-strain epidemic model with non-monotone incidence rates. Chaos Solitons Fractals 2020, 133, 109647. [Google Scholar] [CrossRef]
- Bhunu, C.P. Mathematical analysis of a three-strain tuberculosis transmission model. Appl. Math. Modell. 2011, 35, 4647–4660. [Google Scholar] [CrossRef]
- Saha, P.; Ghosh, U. Complex dynamics and control analysis of an epidemic model with non-monotone incidence and saturated treatment. Int. J. Dyn. Control 2022, 11, 301–323. [Google Scholar] [CrossRef]
- Jana, S.; Nandi, S.K.; Kar, T.K. Complex dynamics of an SIR epidemic model with saturated incidence rate and treatment. Acta Biotheor. 2016, 64, 65–84. [Google Scholar] [CrossRef]
- Khan, M.A.; Khan, Y.; Islam, S. Complex dynamics of an SEIR epidemic model with saturated incidence rate and treatment. Physica A 2018, 493, 210–227. [Google Scholar] [CrossRef]
- Saha, P.; Mondal, B.; Ghosh, U. Global dynamics and optimal control of a two-strain epidemic model with non-monotone incidence and saturated treatment. Iran. J. Sci. 2023, 47, 1575–1591. [Google Scholar] [CrossRef]
- Bentaleb, D.; Harroudi, S.; Amine, S.; Allali, K. Analysis and optimal control of a multistrain SEIR epidemic model with saturated incidence rate and treatment. Differ. Equ. Dyn. Syst. 2020, 31, 907–923. [Google Scholar] [CrossRef]
- Baba, I.A.; Hıncal, E. Global stability analysis of a two-strain epidemic model with bilinear and non-monotone incidence rates. Eur. Phys. J. Plus 2017, 132, 208. [Google Scholar] [CrossRef]
- Bentaleb, D.; Amine, S. Lyapunov function and global stability for a two-strain SEIR model with bilinear and non-monotone incidence. Int. J. Biomath. 2019, 12, 1950001. [Google Scholar] [CrossRef]
- Liu, W.-M.; Levin, S.A.; Iwasa, Y. Influence of nonlinear incidence rates upon the behavior of SIRS epidemiological models. J. Math. Biol. 1986, 23, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, X. Backward bifurcation of an epidemic model with saturated treatment function. J. Math. Anal. Appl. 2008, 348, 433–443. [Google Scholar] [CrossRef]
- Lakshmikantham, V.; Leela, S.; Martynyuk, A.A. Stability Analysis of Nonlinear Systems; Dekker: New York, NY, USA, 1989. [Google Scholar]
- van den Driessche, P.; Watmough, J. Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math. Biosci. 2002, 180, 29–48. [Google Scholar] [CrossRef]
- Kamrujjaman, M.; Saha, P.; Islam, M.S.; Ghosh, U. Dynamics of SEIR model: A case study of COVID-19 in Italy. Results Control Optim. 2020, 7, 2666–7207. [Google Scholar] [CrossRef]
- ellman, R.; Pontryagin, L.S.; Boltyanskii, V.G.; Gamkrelidze, R.V.; Mischenko, E.F. Mathematical Theory of Optimal Processes. 1962. Available online: https://api.semanticscholar.org/CorpusID:118166571 (accessed on 1 August 2024).
- Berhe, H.W.; Makinde, O.D.; Theuri, D.M. Parameter estimation and sensitivity analysis of dysentery diarrhea epidemic model. J. Appl. Math. 2019, 2019, 8465747. [Google Scholar] [CrossRef]
- Rodrigues, H.S.; Monteiro, M.T.; Torres, D. Optimal control and numerical software: An overview. arXiv 2014, arXiv:1401.7279. [Google Scholar]




| Parameters | Biological Meanings |
|---|---|
| the enrolling rate | |
| the infection rate of strain i from S to E | |
| the isolation rate of strain i | |
| the infection effect of the exposed in incubation period | |
| the transition rate of strain i from E to I | |
| the natural death rate | |
| the cure rate of strain i | |
| the infected being delayed for treatment for strain i | |
| the transition rate of strain i from I to R | |
| K | the psychological or inhibitory effect of the population |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Wang, H.; Jiang, S. Analysis and Optimal Control of a Two-Strain SEIR Epidemic Model with Saturated Treatment Rate. Mathematics 2024, 12, 3026. https://doi.org/10.3390/math12193026
Hu Y, Wang H, Jiang S. Analysis and Optimal Control of a Two-Strain SEIR Epidemic Model with Saturated Treatment Rate. Mathematics. 2024; 12(19):3026. https://doi.org/10.3390/math12193026
Chicago/Turabian StyleHu, Yudie, Hongyan Wang, and Shaoping Jiang. 2024. "Analysis and Optimal Control of a Two-Strain SEIR Epidemic Model with Saturated Treatment Rate" Mathematics 12, no. 19: 3026. https://doi.org/10.3390/math12193026






