Neural Network Modelling for Prediction of Zeta Potential
Abstract
:1. Introduction
2. Proposal for Experiments
2.1. Materials
2.2. Zeta Potential Measurements
2.3. Proposal of an Artificial Neural Network Architecture for Prediction
- Activation function is the hyperbolic tangent;
- Learning coefficient for the first 5000 learning cycles, then it decreases to ;
- Momentum .
3. Results and Discussions
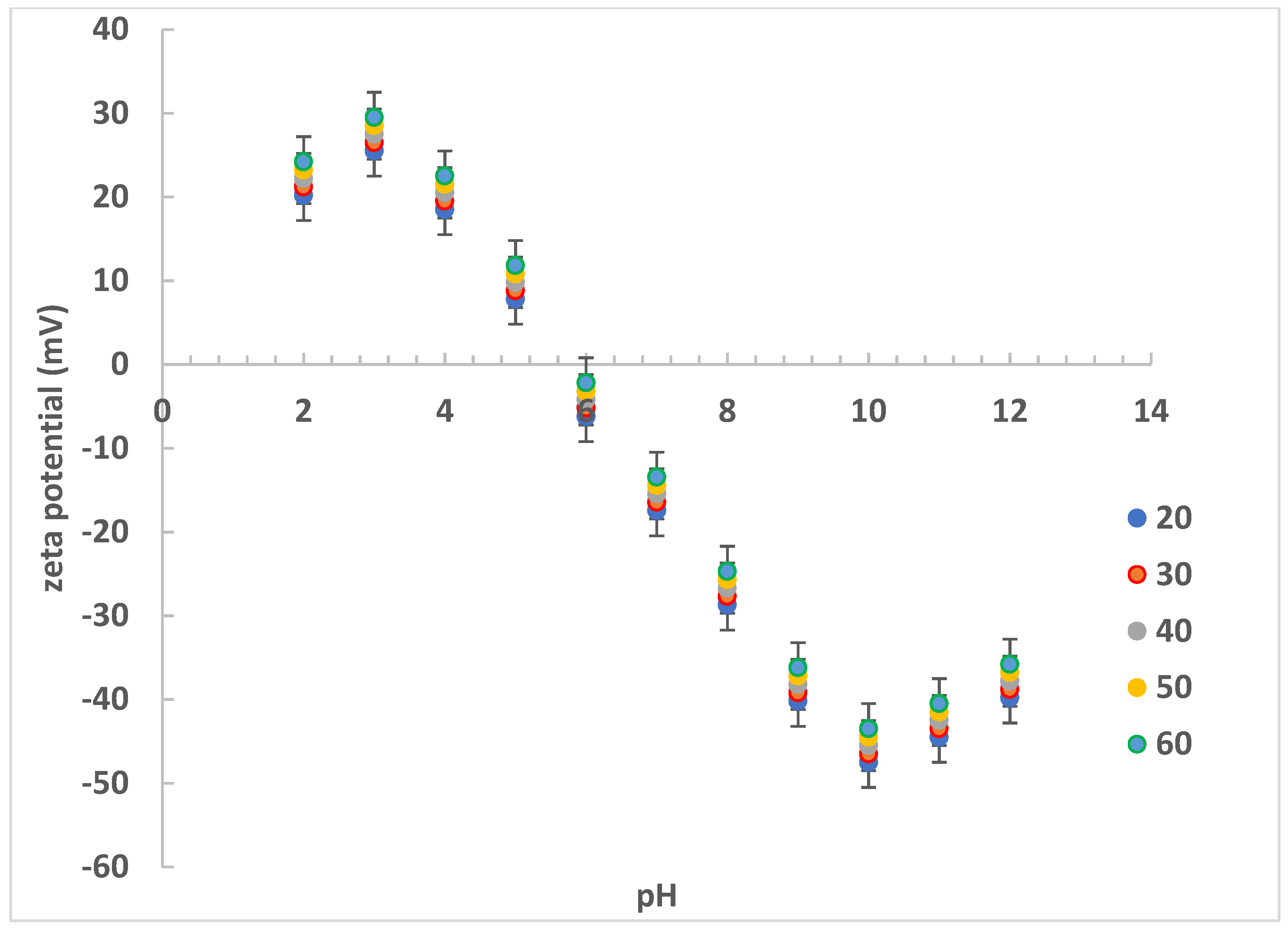
3.1. Experimental Zeta Potential
3.2. Artificial Neural Network
4. Comparison with Other Academic Works
5. Conclusions
6. Highlights
- The artificial neural network is a good tool for predicting the zeta potential of nanoparticles;
- The use of an artificial neural network allows for the determination of zeta potential values also for such conditions that cannot be realized;
- Prediction of zeta potential using an artificial neural network can save time and resources.
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Q.; Chen, Z.; Yang, Y. Study of the Air-Entraining Behavior Based on the Interactions between Cement Particles and Selected Cationic, Anionic and Nonionic Surfactants. Materials 2020, 13, 3514. [Google Scholar] [CrossRef]
- Ferrari, L.; Kaufmann, J.; Winnefeld, F.; Plank, J. Interaction of cement model systems with superplasticizers investigated by atomic force microscopy, zeta potential, and adsorption measurements. J. Colloid Interface Sci. 2010, 347, 15–24. [Google Scholar] [CrossRef]
- Plank, J.; Hirsch, C. Impact of zeta potential of early cement hydration phases on superplasticizer adsorption. Cem. Concr. Res. 2007, 37, 537–542. [Google Scholar] [CrossRef]
- Morfesis, A.; Jacobson, A.M.; Frollini, R.; Helgeson, M.; Billica, J.; Gertig, K.R. Role of Zeta (ζ) Potential in the Optimization of Water Treatment Facility Operations. Ind. Eng. Chem. Res. 2009, 48, 2305–2308. [Google Scholar] [CrossRef]
- Fazullin, D.D.; Mavrin, G.V.; Shaikhiev, I.G. Particle Size and Zeta Potential Changes in the Disperse Phase of Water-Emulsified Waste Waters in Different Treatment Stages. Chem. Technol. Fuels Oils 2015, 51, 501–505. [Google Scholar] [CrossRef]
- Fang, K.; Gao, H.; Sun, F.; Chen, W.; Xie, R.; Cai, Y. Effect of glass transition temperatures and zeta potentials of cationic copolymer latexes on pigment dyeing. Fibers Polym. 2017, 18, 2255–2260. [Google Scholar] [CrossRef]
- Abidi, N.; Semhi, K.; Trabelsi-Ayadi, M. Removal of anionic dye from textile industries’ effluents by using Tunisian clays as adsorbents. Ζeta potential and streaming-induced potential measurements. Comptes Rendus Chim. 2019, 22, 113–125. [Google Scholar] [CrossRef]
- Fuerstenau, D.W. Zeta potentials in the flotation of oxide and silicate minerals. Adv. Colloid Interface Sci. 2005, 114, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Multani, R.S.; Williams, H.; Johnson, B.; Li, R.; Waters, K.E. The effect of superstructure on the zeta potential, xanthate adsorption, and flotation response of pyrrhotite. Colloids Surf. A Physicochem. Eng. Asp. 2018, 551, 108–116. [Google Scholar] [CrossRef]
- Speers, R.A.; Durance, T.D.; Tung, M.A.; Tou, J. Colloidal properties of flocculent and nonflocculent brewing yeast suspensions. Biotechnol. Prog. 1993, 9, 267–272. [Google Scholar] [CrossRef]
- Li, J.; Joung, H.J.; Lee, I.W.; Chen, X.; Park, H.J. The influence of different water types and brewing durations on the colloidal properties of green tea infusion. Int. J. Food Sci. Technol. 2015, 50, 2483–2489. [Google Scholar] [CrossRef]
- Biriukov, D.; Fibich, P.; Předota, M. Zeta Potential Determination from Molecular Simulations. J. Phys. Chem. C 2020, 124, 3159–3170. [Google Scholar] [CrossRef]
- Ovanesyan, Z.; Aljzmi, A.; Almusaynid, M.; Khan, A.; Valderrama, E.; Nash, K.L.; Marucho, M. Ion–ion correlation, solvent excluded volume and pH effects on physicochemical properties of spherical oxide nanoparticles. J. Colloid Interface Sci. 2016, 462, 325–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinogradov, J.; Jackson, M.D.; Chamerois, M. Zeta potential in sandpacks: Effect of temperature, electrolyte pH, ionic strength and divalent cations. Colloids Surf. A Physicochem. Eng. Asp. 2018, 553, 259–271. [Google Scholar] [CrossRef]
- Liu, X.; Mäki-Arvela, P.; Aho, A.; Vajglova, Z.; Gun’ko, V.M.; Heinmaa, I.; Kumar, N.; Eränen, K.; Salmi, T.; Murzin, D.Y. Zeta Potential of Beta Zeolites: Influence of Structure, Acidity, pH, Temperature and Concentration. Molecules 2018, 23, 946. [Google Scholar] [CrossRef] [Green Version]
- Shih, Y.H.; Liu, W.S.; Su, Y.F. Aggregation of stabilized TiO2 nanoparticle suspensions in the presence of inorganic ions. Environ. Toxicol. Chem. 2012, 31, 1693–1698. [Google Scholar] [CrossRef]
- Marsalek, R. Particle Size and Zeta Potential of ZnO. APCBEE Procedia 2014, 9, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Suganthi, K.S.; Rajan, K.S. Temperature induced changes in ZnO–water nanofluid: Zeta potential, size distribution and viscosity profiles. Int. J. Heat Mass Transf. 2012, 55, 7969–7980. [Google Scholar] [CrossRef]
- Asadi, A.; Asadi, M.; Siahmargoi, M.; Asadi, T.; Andarati, M.G. The effect of surfactant and sonication time on the stability and thermal conductivity of water-based nanofluid containing Mg(OH)2 nanoparticles: An experimental investigation. Int. J. Heat Mass Transf. 2017, 108, 191–198. [Google Scholar] [CrossRef]
- Mucha, M.; Maršálek, R.; Bukáčková, M.; Zelenková, G. Interaction among clays and bovine serum albumin. RSC Adv. 2020, 10, 43927–43939. [Google Scholar] [CrossRef]
- Marsalek, R.; Pospisil, J.; Taraba, B. The influence of temperature on the adsorption of CTAB on coals. Colloids Surf. A Physicochem. Eng. Asp. 2011, 383, 80–85. [Google Scholar] [CrossRef]
- Peterson, K.L. Artificial Neural Networks and Their use in Chemistry. In Reviews in Computational Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 53–140. [Google Scholar]
- Duch, W.; Diercksen, G.H.F. Neural networks as tools to solve problems in physics and chemistry. Comput. Phys. Commun. 1994, 82, 91–103. [Google Scholar] [CrossRef]
- Zupan, J.; Novič, M.; Ruisánchez, I. Kohonen and counterpropagation artificial neural networks in analytical chemistry. Chemom. Intell. Lab. Syst. 1997, 38, 1–23. [Google Scholar] [CrossRef]
- Kazemi-Beydokhti, A.; Azizi Namaghi, H.; Asgarkhani, H.; Zeinali Heris, S. Prediction of stability and thermal conductivity of sno2nanofluid via statistical method and an artificial neural network. Braz. J. Chem. Eng. 2015, 32, 903–917. [Google Scholar] [CrossRef] [Green Version]
- Karimipour, A.; Ghasemi, S.; Darvanjooghi, M.H.K.; Abdollahi, A. A new correlation for estimating the thermal conductivity and dynamic viscosity of CuO/liquid paraffin nanofluid using neural network method. Int. Commun. Heat Mass Transf. 2018, 92, 90–99. [Google Scholar] [CrossRef]
- Kannaiyan, S.; Boobalan, C.; Nagarajan, F.C.; Sivaraman, S. Modeling of thermal conductivity and density of alumina/silica in water hybrid nanocolloid by the application of Artificial Neural Networks. Chin. J. Chem. Eng. 2019, 27, 726–736. [Google Scholar] [CrossRef]
- Hashad, R.A.; Ishak, R.A.H.; Fahmy, S.; Mansour, S.; Geneidi, A.S. Chitosan-tripolyphosphate nanoparticles: Optimization of formulation parameters for improving process yield at a novel pH using artificial neural networks. Int. J. Biol. Macromol. 2016, 86, 50–58. [Google Scholar] [CrossRef]
- Tekeli, F.N. Application of artificial neural networks for Zeta potential of copolymer. Bulg. Chem. Commun. 2017, 49, 146–150. [Google Scholar]
- Yukselen, Y.; Erzin, Y. Artificial neural networks approach for zeta potential of Montmorillonite in the presence of different cations. Environ. Geol. 2008, 54, 1059–1066. [Google Scholar] [CrossRef]
- Asadi, A.; Moayedi, H.; Huat, B.B.; Boroujeni, F.Z.; Parsaie, A.; Sojoudi, S. Prediction of Zeta Potential for Tropical Peat in the presence of different Cations using Artificial Neural Networks. Int. J. Electrochem. Sci. 2011, 6, 1146–1158. [Google Scholar]
- Li, H.; Chen, F.; Cheng, K.; Zhao, Z.; Yang, D. Prediction of Zeta Potential of Decomposed Peat via Machine Learning: Comparative Study of Support Vector Machine and Artificial Neural Networks. Int. J. Electrochem. Sci. 2015, 10, 6044–6056. [Google Scholar]
- Imanparast, F.; Faramarzi, M.A.; Paknejad, M.; Kobarfard, F.; Amani, A.; Doosti, M. Preparation, optimization, and characterization of simvastatin nanoparticles by electrospraying: An artificial neural networks study. J. Appl. Polym. Sci. 2016, 133, 43602. [Google Scholar] [CrossRef]
- Erzin, Y.; Yukselen, Y. The Use of Neural Networks for the Prediction of Zeta Potential of Kaolinite. Math. Geosci. 2009, 41, 779–797. [Google Scholar] [CrossRef]
- Zhao, F.; Lu, J.; Jin, X.; Wang, Z.; Sun, Y.; Gao, D.; Li, X.; Liu, R. Comparison of response surface methodology and artificial neural network to optimize novel ophthalmic flexible nano-liposomes: Characterization, evaluation, in vivo pharmacokinetics and molecular dynamics simulation. Colloids Surf. B Biointerfaces 2018, 172, 288–297. [Google Scholar] [CrossRef]
- Liu, H.H.; Lanphere, J.; Walker, S.; Cohen, Y. Effect of hydration repulsion on nanoparticle agglomeration evaluated via a constant number Monte–Carlo simulation. Nanotechnology 2015, 26, 045708. [Google Scholar] [CrossRef] [PubMed]
- Bouhaik, I.S.; Leroy, P.; Ollivier, P.; Azaroual, M.; Mercury, L. Influence of surface conductivity on the apparent zeta potential of TiO2 nanoparticles: Application to the modeling of their aggregation kinetics. J. Colloid Interface Sci. 2013, 406, 75–85. [Google Scholar] [CrossRef] [Green Version]
- Bukackova, M.; Rusnok, P.; Marsalek, R. Mathematical Methods in the Calculation of the Zeta Potential of BSA. J. Solut. Chem. 2018, 47, 1942–1952. [Google Scholar] [CrossRef]
- Jiang, Y.; Sulgani, M.T.; Ranjbarzadeh, R.; Karimipour, A.; Nguyen, T.K. Hybrid GMDH-type neural network to predict fluid surface tension, shear stress, dynamic viscosity & sensitivity analysis based on empirical data of iron(II) oxide nanoparticles in light crude oil mixture. Phys. A Stat. Mech. Its Appl. 2019, 526, 120948. [Google Scholar]
- Hasani Bijarbooneh, F.; Zhao, Y.; Kim, J.H.; Sun, Z.; Malgras, V.; Aboutalebi, S.H.; Heo, Y.U.; Ikegami, M.; Dou, S.X. Aqueous Colloidal Stability Evaluated by Zeta Potential Measurement and Resultant TiO2 for Superior Photovoltaic Performance. J. Am. Ceram. Soc. 2013, 96, 2636–2643. [Google Scholar] [CrossRef]
- Gómez-Merino, A.I.; Rubio-Hernández, F.J.; Velázquez-Navarro, J.F.; Aguiar, J. Assessment of ζ-potential in TiO2 aqueous suspensions: A comparative study based on thermodynamic and rheological methods. Ceram. Int. 2015, 41, 5331–5340. [Google Scholar] [CrossRef]
- Wang, N.; Hsu, C.; Zhu, L.; Tseng, S.; Hsu, J.P. Influence of metal oxide nanoparticles concentration on their zeta potential. J. Colloid Interface Sci. 2013, 407, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Masters, T. Practical Neural Network in C++; Academic Press: Cambridge, MA, USA, 1993. [Google Scholar]
- Rojas, R. Neural Networks; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]





| Quantity | Values | NN Representation | Number of Neurons | |
|---|---|---|---|---|
| Input vector | pH | range 2–12.29 | Transformed to interval [−1, 1] | 1 |
| 1-pH | Transformed to interval [−1, 1] | 1 | ||
| T | 20, 30, 40, 50, 60 | Binarized, values 0 or 1 | 5 | |
| I | 0.01; 0.05; 0.1; 0.5; 1 | Binarized, values 0 or 1 | 5 | |
| c | 10; 50; 100; 250; 500 | Binarized, values 0 or 1 | 5 | |
| Output vector | Zeta_NN | [−60, 70] | Transformed from interval [−1, 1] | 1 |
| Correlation coefficient R | 0.99907 |
| The coefficient of determination R2 | 0.99814 |
| Number of measurements | 250 |
| SS (Sum of Squares) | MS (Mean Square) | F | Significance of F | |
|---|---|---|---|---|
| Regression | 190,807.8 | 190,807.8 | 133,178.7566 | 0 |
| Residues | 355.3144 | 1.432719 | ||
| Total | 191,163.1 |
| Model (Test Set) | Neural Network | The Coefficient of Determination R2 | |
|---|---|---|---|
| Our approach | TiO2 | MLP | 0.998 |
| Yukselen, Y.; Erzin, Y. [30] | Montmorillonite—Salt | MLP | 0.947 |
| Montmorillonite—Heavy Metal | 0.902 | ||
| Asadi, A. et al. [19] | Peat | MLP | 0.9504 |
| Lia, H. et al. [32] | Decomposed Peat | SVM | 0.923 |
| GRNN | 0.923 | ||
| Erzin, Y.; Yukselen, Y. [34] | the kaolinite—Salt | MLP | 0.992 |
| the kaolinite—Heavy Metal | 0.996 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marsalek, R.; Kotyrba, M.; Volna, E.; Jarusek, R. Neural Network Modelling for Prediction of Zeta Potential. Mathematics 2021, 9, 3089. https://doi.org/10.3390/math9233089
Marsalek R, Kotyrba M, Volna E, Jarusek R. Neural Network Modelling for Prediction of Zeta Potential. Mathematics. 2021; 9(23):3089. https://doi.org/10.3390/math9233089
Chicago/Turabian StyleMarsalek, Roman, Martin Kotyrba, Eva Volna, and Robert Jarusek. 2021. "Neural Network Modelling for Prediction of Zeta Potential" Mathematics 9, no. 23: 3089. https://doi.org/10.3390/math9233089








