Long-Acting Injectable Antipsychotics (LAIs) Prescribing Trends during the COVID-19 Pandemic in Romania
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Design
2.3. Statistical Analysis
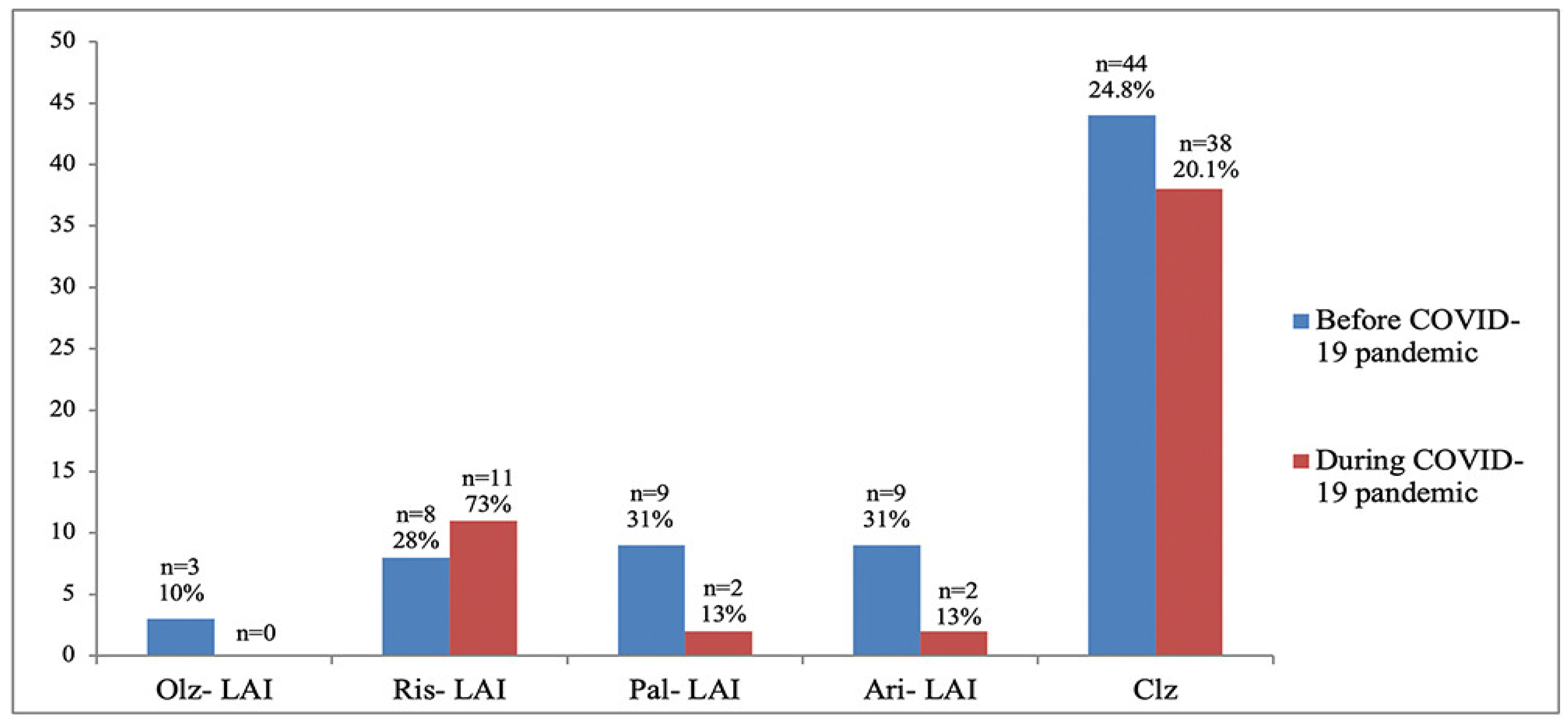
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LAI | long acting injectable |
| COVID-19 | coronavirus disease 2019 |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| FGA | first generation antipsychotics |
| SGA | second generation antipsychotic |
| WHO | World Health Organization |
| OA | oral antipsychotic |
| Olz-LAI | olanzapine long acting injectable |
| Ris-LAI | risperidone long acting injectable |
| Pal-LAI | paliperidone long acting injectable |
| Ari-LAI | aripiprazole long acting injectable |
| Clz | clozapine |
References
- Correll, C.U.; Citrome, L.; Haddad, P.M.; Lauriello, J.; Olfson, M.; Calloway, S.M.; Kane, J.M. The Use of Long-Acting Injectable Antipsychotics in Schizophrenia: Evaluating the Evidence. J. Clin. Psychiatry 2016, 77 (Suppl. S3), 1–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gannon, J.M.; Conlogue, J.; Sherwood, R.; Nichols, J.; Ballough, J.R.; Fredrick, N.M.; Chengappa, K.R. Long acting injectable antipsychotic medications: Ensuring care continuity during the COVID-19 pandemic restrictions. Schizophr. Res. 2020, 222, 532–533. [Google Scholar] [CrossRef] [PubMed]
- Tiihonen, J.; Mittendorfer-Rutz, E.; Majak, M.; Mehtälä, J.; Hoti, F.; Jedenius, E.; Enkusson, D.; Leval, A.; Sermon, J.; Tanskanen, A.; et al. Real-World Effectiveness of Antipsychotic Treatments in a Nationwide Cohort of 29 823 Patients with Schizophrenia. JAMA Psychiatry 2017, 74, 686–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keepers, G.A.; Fochtmann, L.J.; Anzia, J.M.; Benjamin, S.; Lyness, J.M.; Mojtabai, R.; Servis, M.; Walaszek, A.; Buckley, P.; Lenzenweger, M.F.; et al. The American Psychiatric Association Practice Guideline for the Treatment of Patients with Schizophrenia. Am. J. Psychiatry 2020, 177, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Kent, L.; O’Reilly, D.; Maguire, A. Impact of the COVID-19 pandemic on psychotropic medication uptake in Northern Ireland: A population-wide trend analysis. Lancet 2021, 398, S2. [Google Scholar] [CrossRef]
- Leong, C.; Kowalec, K.; Eltonsy, S.; Bolton, J.M.; Enns, M.W.; Tan, Q.; Yogendran, M.; Chateau, D.; Delaney, J.A.; Sareen, J.; et al. Psychotropic Medication Use Before and During COVID-19: A Population-Wide Study. Front. Pharmacol. 2022, 27, 886652. [Google Scholar] [CrossRef]
- Ifteni, P.; Dima, L.; Teodorescu, A. Long-acting injectable antipsychotics treatment during COVID-19 pandemic—A new challenge. Schizophr. Res. 2020, 220, 265–266. [Google Scholar] [CrossRef]
- McKee, K.A.; Crocker, C.E.; Tibbo, P.G. Long-acting injectable antipsychotic (LAI) prescribing trends during COVID-19 restrictions in Canada: A retrospective observational study. BMC Psychiatry 2021, 21, 633. [Google Scholar] [CrossRef]
- Bojdani, E.; Rajagopalan, A.; Chen, A.; Gearin, P.; Olcott, W.; Shankar, V.; Cloutier, A.; Solomon, H.; Naqvi, N.Z.; Batty, N.; et al. COVID-19 Pandemic: Impact on psychiatric care in the United States. Psychiatry Res. 2020, 289, 113069. [Google Scholar] [CrossRef]
- Taipale, H.; Mittendorfer-Rutz, E.; Alexanderson, K.; Majak, M.; Mehtälä, J.; Hoti, F.; Jedenius, E.; Enkusson, D.; Leval, A.; Sermon, J.; et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr. Res. 2018, 197, 274–280. [Google Scholar] [CrossRef]
- American Psychiatric Association. Use of Long-Acting Injectables as a Clinically Necessary Treatment; American Psychiatric Association: Washington, DC, USA, 2020. [Google Scholar]
- Correll, C.U.; Chepke, C.; Gionfriddo, P.; Parks, J.; Foxworth, P.; Basu, A.; Brister, T.S.; Brown, D.; Clarke, C.; Hassoun, Y. The post COVID-19 healthcare landscape and the use of long-acting injectable antipsychotics for individuals with schizophrenia and bipolar I disorder: The importance of an integrated collaborative-care approach. BMC Psychiatry 2022, 22, 32. [Google Scholar] [CrossRef] [PubMed]
- Miron, A.-A.; Teodorescu, A.; Ifteni, P.; Irimie, C.A.; Dima, L.; Petric, P.-S. Switch from Olanzapine Long-Acting Injectable to its Oral Equivalent during COVID-19 Pandemic: A Real World Observational Study. Psychiatr. Q. 2022, 93, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Meyers, K.J.; Upadhyaya, H.P.; Landry, J.L.; Chhabra-Khanna, R.; Falk, D.M.; Rao, B.S.; Jones, M.E. Postinjection delirium/sedation syndrome in patients with schizophrenia receiving olanzapine long-acting injection: Results from a large observational study. BJPsych Open 2017, 3, 186–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parellada, E.; Bioque, M. Barriers to the Use of Long-Acting Injectable Antipsychotics in the Management of Schizophrenia. CNS Drugs 2016, 30, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Pinto, R. A survey of the attitudes of chronic psy-chiatric patients living in the community toward their medication. Acta Psychiatr. Scand. 1997, 95, 464–468. [Google Scholar] [CrossRef]
- Walburn, J.; Gray, R.; Gournay, K.; Quraishi, S.; David, A.S. Systematic review of patient and nurse attitudes to depot antipsy-chotic medication. Br. J. Psychiatry 2001, 179, 300–307. [Google Scholar] [CrossRef] [Green Version]
- Patel, M.; Haddad, P.; Chaudhry, I.; McLoughlin, S.; David, A. Psy-chiatrists’ use, knowledge and attitudes to first- and second-generation antipsychotic long-acting injections: Comparisonsover 5 years. J. Psychopharmacol. 2010, 24, 1473–1482. [Google Scholar] [CrossRef]
- Jaeger, M.; Rossler, W. Attitudes towards long-acting depotantipsychotics: A survey of patients, relatives and psychiatrists. Psychiatry Res. 2010, 175, 58–62. [Google Scholar] [CrossRef] [Green Version]
- Brissos, S.; Veguilla, M.R.; Taylor, D.; Balanza-Martinez, V. Therole of long-acting injectable antipsychotics in schizophrenia: Acritical appraisal. Ther. Adv. Psychopharmacol. 2014, 4, 198–219. [Google Scholar] [CrossRef] [Green Version]
- Iyer, S.; Banks, N.; Roy, M.A.; Tibbo, P.; Williams, R.; Manchanda, R.; Chue, P.; Malla, A. A qualitative study of experiences with and perceptions regarding long-acting injectable antipsychotics: Part I-patient perspectives. Can. J. Psychiatry 2013, 58 (Suppl. S1), 14S–22S. [Google Scholar] [CrossRef]
- Das, A.K.; Malik, A.; Haddad, P.M. A qualitative study of the atti-tudes of patients in an early intervention service towardsantipsychotic long-acting injections. Ther. Adv. Psychopharmacol. 2014, 4, 179–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirschner, M.; Theodoridou, A.; Fusar-Poli, P.; Kaiser, S.; Jäger, M. Patients’ and clinicians’ attitude towards long-acting depot antipsychotics in subjects with a first episode of psychosis. Ther. Adv. Psychopharmacol. 2012, 3, 89–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potkin, S.; Bera, R.; Zubek, D.; Lau, G. Patient and prescriber per-spectives on long-acting injectable (LAI) antipsychotics and analysis of in-office discussion regarding LAI treatment for schizophrenia. BMC Psychiatry 2013, 13, 261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baser, O.; Xie, L.; Pesa, J.; Durkin, M. Healthcare utilization andcosts of veterans health administration patients with schizophrenia treated with paliperidone palmitate long-acting injection or oral atypical antipsychotics. J. Med. Econ. 2015, 18, 357–365. [Google Scholar] [CrossRef]
- Asseburg, C.; Willis, M.; Lothgren, M.; Seppala, N.; Hakala, M.; Persson, U. Hospitalisation utilisation and costs in schizophrenia patients in Finland before and after initiation of risperidone long-acting injection. Schizophr. Res. Treat. 2012, 2012, 791468. [Google Scholar] [CrossRef] [Green Version]
- Ifteni, P.; Petric, P.-S.; Teodorescu, A. Rating Opportunity for Long-Acting Injectable Antipsychotic Initiation Index (ROLIN). Front. Psychiatry 2021, 12, 767756. [Google Scholar] [CrossRef]
- Kane, J.M.; Peters-Strickland, T.; Baker, R.A.; Hertel, P.; Eramo, A.; Jin, N.; Perry, P.P.; Gara, M.; McQuade, R.D.; Carson, W.H.; et al. Aripiprazole once-monthly in the acute treatment of schizophrenia: Findings from a 12-week, randomized, double-blind, placebo-controlled study. J. Clin. Psychiatry 2014, 75, 1254–1260. [Google Scholar] [CrossRef] [Green Version]
- Moga, S.; Teodorescu, A.; Ifteni, P.; Petric, P.S.; Miron, A.A. Clozapine and Neutropenia in Patients with Schizophrenia and SARS-CoV-2 Infection. Neuropsychiatr. Dis. Treat. 2022, 18, 977–983. [Google Scholar] [CrossRef]
- Moga, S.; Teodorescu, A.; Ifteni, P.; Gavris, C.; Petric, P.S. Inflammatory Response in SARS-CoV-2 Infection of Patients with Schizophrenia and Long-Term Antipsychotic Treatment. Neuropsychiatr. Dis. Treat. 2021, 17, 3053–3060. [Google Scholar] [CrossRef]


| Antipsychotic | LAI Formulation | Oral Formulation | Available in Romania |
|---|---|---|---|
| Flupenthixol | √ | - | √ |
| Zuclopenthixol | √ | - | √ |
| Haloperidol | √ | √ | √ |
| Fluphenazine | √ | √ | - |
| Olanzapine | √ | √ | √ |
| Risperidone | √ | √ | √ |
| Aripiprazole | √ | √ | √ |
| Paliperidone | √ | √ | √ |
| LAIs Initiation | |||
|---|---|---|---|
| Before COVID-19 Pandemic | During COVID-19 Pandemic | p Value | |
| n = 29 | n = 15 | ||
| Gender male (n, %) | 14 (48.2%) | 4 (26.6%) | 0.0728 |
| Age (years, SD) | 42.3 (7.8) | 44.4 (8.3) | 0.4121 |
| Age of onset (years, SD) | 28.6 (8.6) | 25.2 (6.6) | 0.1881 |
| Duration of illness (years, SD) | 13.8 (9.2) | 19.2 (6.2) | 0.0476 |
| Length of stay (days, SD) | 14.1 (8.6) | 16.7 (6.6) | 0.3120 |
| LAI type | |||
| aripiprazole (n, %) | 9 (31%) | 2 (13%) | 0.1955 |
| olanzapine (n, %) | 3 (10%) | 0 | - |
| risperidone (n, %) | 8 (28%) | 11 (73%) | 0.2036 |
| paliperidone (n, %) | 9 (31%) | 2 (13%) | 0.1955 |
| 2019 | 2020 | p Value | |
|---|---|---|---|
| OAs (n, %) | 177 (79%) | 189 (86.7%) | 0.0322 |
| olanzapine (n, %) | 61 (34.5%) | 69 (36.5%) | 0.6899 |
| quetiapine (n, %) | 18 (10.2%) | 24 (12.7%) | 0.4542 |
| risperidone (n, %) | 18 (10.2%) | 23 (12.2%) | 0.5454 |
| paliperidone (n, %) | 3 (1.7%) | 7 (3.7%) | 0.2415 |
| aripiprazole (n, %) | 8 (4.5%) | 6 (3.2%) | 0.5178 |
| amisulpride (n, %) | 14 (7.9%) | 10 (5.3%) | 0.3160 |
| haloperidol (n, %) | 11 (6.2%) | 12 (6.3%) | 0.9685 |
| clozapine (n, %) | 44 (24.8%) | 38 (20.1%) | 0.2816 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miron, A.A.; Ifteni, P.I.; Teodorescu, A.; Petric, P.S. Long-Acting Injectable Antipsychotics (LAIs) Prescribing Trends during the COVID-19 Pandemic in Romania. Healthcare 2022, 10, 1265. https://doi.org/10.3390/healthcare10071265
Miron AA, Ifteni PI, Teodorescu A, Petric PS. Long-Acting Injectable Antipsychotics (LAIs) Prescribing Trends during the COVID-19 Pandemic in Romania. Healthcare. 2022; 10(7):1265. https://doi.org/10.3390/healthcare10071265
Chicago/Turabian StyleMiron, Ana A., Petru I. Ifteni, Andreea Teodorescu, and Paula S. Petric. 2022. "Long-Acting Injectable Antipsychotics (LAIs) Prescribing Trends during the COVID-19 Pandemic in Romania" Healthcare 10, no. 7: 1265. https://doi.org/10.3390/healthcare10071265
APA StyleMiron, A. A., Ifteni, P. I., Teodorescu, A., & Petric, P. S. (2022). Long-Acting Injectable Antipsychotics (LAIs) Prescribing Trends during the COVID-19 Pandemic in Romania. Healthcare, 10(7), 1265. https://doi.org/10.3390/healthcare10071265






