Obstructive Sleep Apnea and Obesity Are Associated with Hypertension in a Particular Pattern: A Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Basic Characteristics
2.3. Anthropometric Measurements
2.4. Overnight PSG Parameters
2.5. Blood Pressure
2.6. Statistical Analysis
3. Results
3.1. Basic Characteristics and PSG Parameters Stratified by Obesity and Severe OSA
3.2. Blood Pressure Levels and Percentages of Abnormal Blood Pressure Stratified by Obesity and Severe OSA
3.3. Associations between Obesity and Severe OSA and Abnormal Blood Pressure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heinzer, R.; Vat, S.; Marques-Vidal, P.; Marti-Soler, H.; Andries, D.; Tobback, N.; Mooser, V.; Preisig, M.; Malhotra, A.; Waeber, G.; et al. Prevalence of sleep-disordered breathing in the general population: The HypnoLaus study. Lancet Respir. Med. 2015, 3, 310–318. [Google Scholar] [CrossRef] [Green Version]
- Nieto, F.J.; Young, T.B.; Lind, B.K.; Shahar, E.; Samet, J.M.; Redline, S.; D’Agostino, R.B.; Newman, A.B.; Lebowitz, M.D.; Pickering, T.G.; et al. Association of Sleep-Disordered Breathing, Sleep Apnea, and Hypertension in a Large Community-Based Study. JAMA 2000, 283, 1829–1836. [Google Scholar] [CrossRef] [Green Version]
- Lavie, P.; Herer, P.; Hoffstein, V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: Population study. BMJ 2000, 320, 479–482. [Google Scholar] [CrossRef] [Green Version]
- Young, T.; Peppard, P.; Palta, M.; Hla, K.M.; Finn, L.; Morgan, B.; Skatrud, J. Population-Based Study of Sleep-Disordered Breathing as a Risk Factor for Hypertension. Arch. Intern. Med. 1997, 157, 1746–1752. [Google Scholar] [CrossRef]
- Xia, Y.; You, K.; Xiong, Y. Relationships Between Cardinal Features of Obstructive Sleep Apnea and Blood Pressure: A Retrospective Study. Front. Psychiatry 2022, 13, 846275. [Google Scholar] [CrossRef]
- Li, C.; Ford, E.S.; Zhao, G.; Croft, J.B.; Balluz, L.S.; Mokdad, A.H. Prevalence of self-reported clinically diagnosed sleep apnea according to obesity status in men and women: National Health and Nutrition Examination Survey, 2005–2006. Prev. Med. 2010, 51, 18–23. [Google Scholar] [CrossRef]
- Newman, A.B.; Foster, G.; Givelber, R.; Nieto, F.J.; Redline, S.; Young, T. Progression and Regression of Sleep-Disordered Breathing With Changes in Weight. Arch. Intern. Med. 2005, 165, 2408–2413. [Google Scholar] [CrossRef] [Green Version]
- Grunstein, R.; Wilcox, I.; Yang, T.S.; Gould, Y.; Hedner, J. Snoring and sleep apnoea in men: Association with central obesity and hypertension. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 1993, 17, 533–540. [Google Scholar]
- Gasa, M.; Salord, N.; Fortuna, A.M.; Mayos, M.; Vilarrasa, N.; Dorca, J.; Montserrat, J.M.; Bonsignore, M.R.; Monasterio, C. Obstructive sleep apnoea and metabolic impairment in severe obesity. Eur. Respir. J. 2011, 38, 1089–1097. [Google Scholar] [CrossRef]
- Calhoun, D.A.; Jones, D.; Textor, S.; Goff, D.C.; Murphy, T.P.; Toto, R.D.; White, A.; Cushman, W.C.; White, W.; Sica, D.; et al. Resistant Hypertension: Diagnosis, Evaluation, and Treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 2008, 51, 1403–1419. [Google Scholar] [CrossRef] [Green Version]
- Persell, S.D. Prevalence of Resistant Hypertension in the United States, 2003–2008. Hypertension 2011, 57, 1076–1080. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.W.F.; D’Agostino, R.B.; Sullivan, L.; Parise, H.; Kannel, W.B. Overweight and Obesity as Determinants of Cardiovascular Risk. Arch. Intern. Med. 2002, 162, 1867–1872. [Google Scholar] [CrossRef] [Green Version]
- Kim, A.M.; Keenan, B.T.; Nicholas, J.; Chan, E.L.; Bethany, S.; Harish, P.; Torigian, D.A.; Pack, A.I.; Schwab, R.J. Tongue Fat and its Relationship to Obstructive Sleep Apnea. Sleep 2014, 37, 1639–1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salome, C.M.; King, G.G.; Berend, N. Physiology of obesity and effects on lung function. J. Appl. Physiol. 2010, 108, 206–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drager, L.F.; Jun, J.C.; Polotsky, V.Y. Metabolic consequences of intermittent hypoxia: Relevance to obstructive sleep apnea. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 843–851. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Zhong, Y.; Li, J.-Q.; Zhang, L.-L.; Xiong, Y.-P.; Zhang, Z.-Y.; Xia, Y.-Y. The Interaction of Severe Obstructive Sleep Apnea Hypopnea Syndrome and Abdominal Obesity on Cognitive Function. J. Integr. Neurosci. 2022, 21, 85. [Google Scholar] [CrossRef]
- Xia, Y.; You, K.; Xiong, Y. Interaction effects between characteristics of obstructive sleep apnea and obesity on dyslipidemia. Auris Nasus Larynx 2021, 49, 437–444. [Google Scholar] [CrossRef]
- Kaw, R.; El Zarif, S.; Wang, L.; Bena, J.; Blackstone, E.H.; Mehra, R. Obesity as an Effect Modifier in Sleep-Disordered Breathing and Postcardiac Surgery Atrial Fibrillation. Chest 2017, 151, 1279–1287. [Google Scholar] [CrossRef]
- Hypertension WGoCGftMo. 2018 Chinese guidelines for the management of hypertension. Chin. J. Cardiovasc. Med. 2019, 19, 1–44. [Google Scholar] [CrossRef]
- Chen, C.; Lu, F.C. Department of Disease Control Ministry of Health, PR China The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed. Environ. Sci. 2004, 17, 1–36. [Google Scholar]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for Scoring Respiratory Events in Sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef] [Green Version]
- Tarzia, P.; Lanza, G.A.; Sestito, A.; Villano, A.; Russo, G.; Figliozzi, S.; Lamendola, P.; De Vita, A.; Crea, F. Long-term effects of bariatric surgery on peripheral endothelial function and coronary microvascular function. Obes. Res. Clin. Pract. 2017, 11, 114–117. [Google Scholar] [CrossRef]
- Koç, A.K.; Koçak, H.E.; Erdoğan, B.; Ulusoy, H.A.; Yiğitbay, M.; Bilece, Z.T.; Elbistanlı, M.S.; Kaya, K.H. Severe OSAS causes systemic microvascular dysfunction: Clinical evaluation of ninety-eight OSAS patients. Clin. Otolaryngol. 2019, 44, 412–415. [Google Scholar] [CrossRef]
- Ghadami, M.R. Obstructive Sleep Apnea and Hypertension: Systolic Versus Diastolic Blood Pressure. Obesity 2018, 26, 1249–1250. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Jin, X.; Chen, C.; Zhang, P.; Li, D.; Su, Q.; Yin, G.; Hang, Y. Diastolic Blood Pressure Rises with the Exacerbation of Obstructive Sleep Apnea in Males. Obesity 2017, 25, 1980–1987. [Google Scholar] [CrossRef] [Green Version]
- Tryfon, S.; Stanopoulos, I.; Dascalopoulou, E.; Argyropoulou, P.; Bouros, D.; Mavrofridis, E. Sleep Apnea Syndrome and Diastolic Blood Pressure Elevation during Exercise. Respiration 2004, 71, 499–504. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, R.; Zhong, X.; Xiao, Y. Cardiovascular Consequences of Repetitive Arousals over the Entire Sleep Duration. BioMed Res. Int. 2017, 2017, 4213861. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, Q.J. The Prevalence of Prehypertension and Hypertension Among US Adults According to the New Joint National Committee Guidelines: New challenges of the old problem. Arch. Intern. Med. 2004, 164, 2126–2134. [Google Scholar] [CrossRef] [Green Version]
- Neter, J.E.; Stam, B.E.; Kok, F.J.; Grobbee, D.E.; Geleijnse, J.M. Influence of Weight Reduction on Blood Pressure: A meta-analysis of randomized controlled trials. Hypertension 2003, 42, 878–884. [Google Scholar] [CrossRef] [Green Version]
- Landsberg, L.; Aronne, L.J.; Beilin, L.J.; Burke, V.; Igel, L.I.; Lloyd-Jones, D.; Sowers, J. Obesity-Related Hypertension: Pathogenesis, Cardiovascular Risk, and Treatment: A position paper of The Obesity Society and the American Society of Hypertension. J. Clin. Hypertens. 2012, 15, 14–33. [Google Scholar] [CrossRef]
- Efe, F.K.; Tek, M.; Hastanesi, T.E. Increased ambulatory arterial stiffness index and blood pressure load in normotensive obese patients. Afr. Health Sci. 2021, 21, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
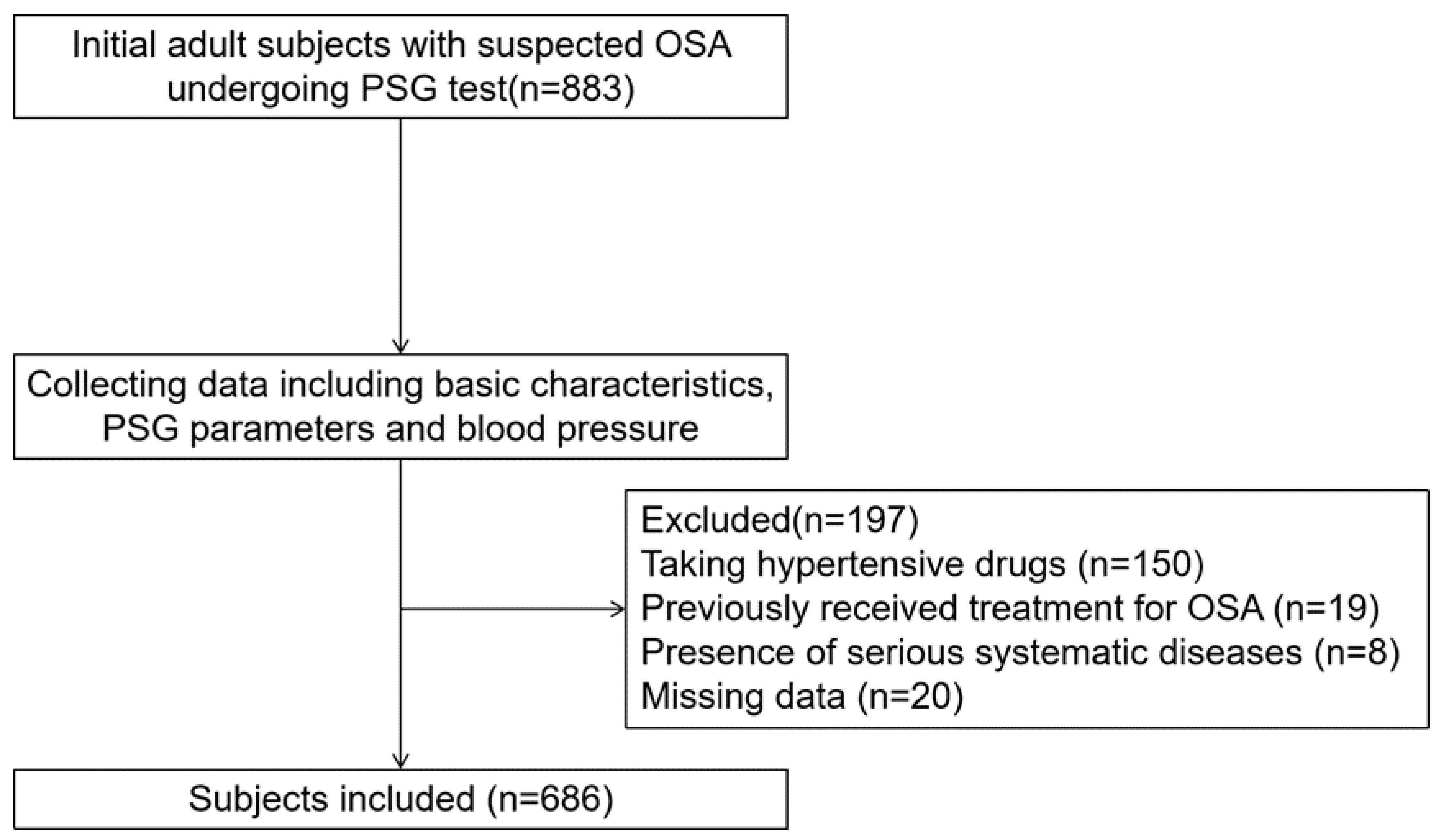
| Whole Subjects (N = 686) | Non-Obese Category (N = 433) | Obese Category (N = 253) | |||||
|---|---|---|---|---|---|---|---|
| Non-Severe OSA Group (Group a, N = 232) | Severe OSA Group (Group b, N = 201) | p | Non-Severe OSA Group (Group c, N = 61) | Severe OSA Group (Group d, N = 192) | p | ||
| Basic characteristics | |||||||
| Male, N (%) | 589 (85.9) | 177 (76.3) | 182 (90.5) | <0.001 | 50 (82.0) | 180 (93.8) | 0.005 |
| Age | 42.0 (32.0, 51.0) | 46.0 (33.3, 54.0) | 43.0 (32.0, 51.0) | 0.281 | 44.0 (30.5, 51.0) | 37.0 (30.0, 45.0) | 0.320 |
| BMI | 26.9 (24.6, 29.4) | 24.8 (22.9, 26.4) | 25.4 (24.2, 26.9) | 0.005 | 29.8 (29.0, 31.3) | 30.5 (29.3, 32.7) | 0.409 |
| Smoking, N (%) | 257 (37.5) | 72 (31.0) | 68 (33.8) | 0.535 | 28 (45.9) | 89 (46.4) | 0.951 |
| Drinking, N (%) | 106 (15.5) | 31 (13.4) | 32 (15.9) | 0.451 | 11 (18.0) | 32 (16.7) | 0.805 |
| Diabetes, N (%) | 26 (3.8) | 7 (3.0) | 5 (2.5) | 0.738 | 3 (4.9) | 11 (5.7) | 1.000 |
| Glucose | 5.17 (4.68, 5.76) | 5.13 (4.63, 5.56) | 5.06 (4.59, 5.57) | 0.992 | 5.17 (4.62, 6.21) | 5.39 (4.85, 6.02) | 1.000 |
| PSG parameters | |||||||
| AHI | 37.1 (16.7, 61.7) | 10.9 (4.7, 21.3) | 56.6 (41.4, 65.7) | <0.001 | 16.9 (6.1, 22.0) | 64.8 (48.7, 64.8) | <0.001 |
| ODI | 35.4 (14.4, 63.9) | 10.0 (3.6, 20.7) | 53.9 (40.1, 67.7) | <0.001 | 16.0 (6.5, 26.3) | 67.3 (49.9, 84.7) | <0.001 |
| LSpO2 | 77.0 (65.0, 85.0) | 86.0 (81.0, 90.0) | 71.0 (61.0, 78.0) | <0.001 | 84.0 (79.0, 88.0) | 65.5 (54.3, 73.0) | <0.001 |
| Whole Subjects (N = 686) | Non-Obese Category (N = 433) | Obese Category (N = 253) | p1 | Main Effect, p2 | Interaction, p3 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Non-Severe OSA Group (Group a, N = 232) | Severe OSA Group (Group b, N = 201) | Non-Severe OSA Group (Group c, N = 61) | Severe OSA Group (Group d, N = 192) | Obesity | Severe OSA | ||||
| Blood pressure levels | |||||||||
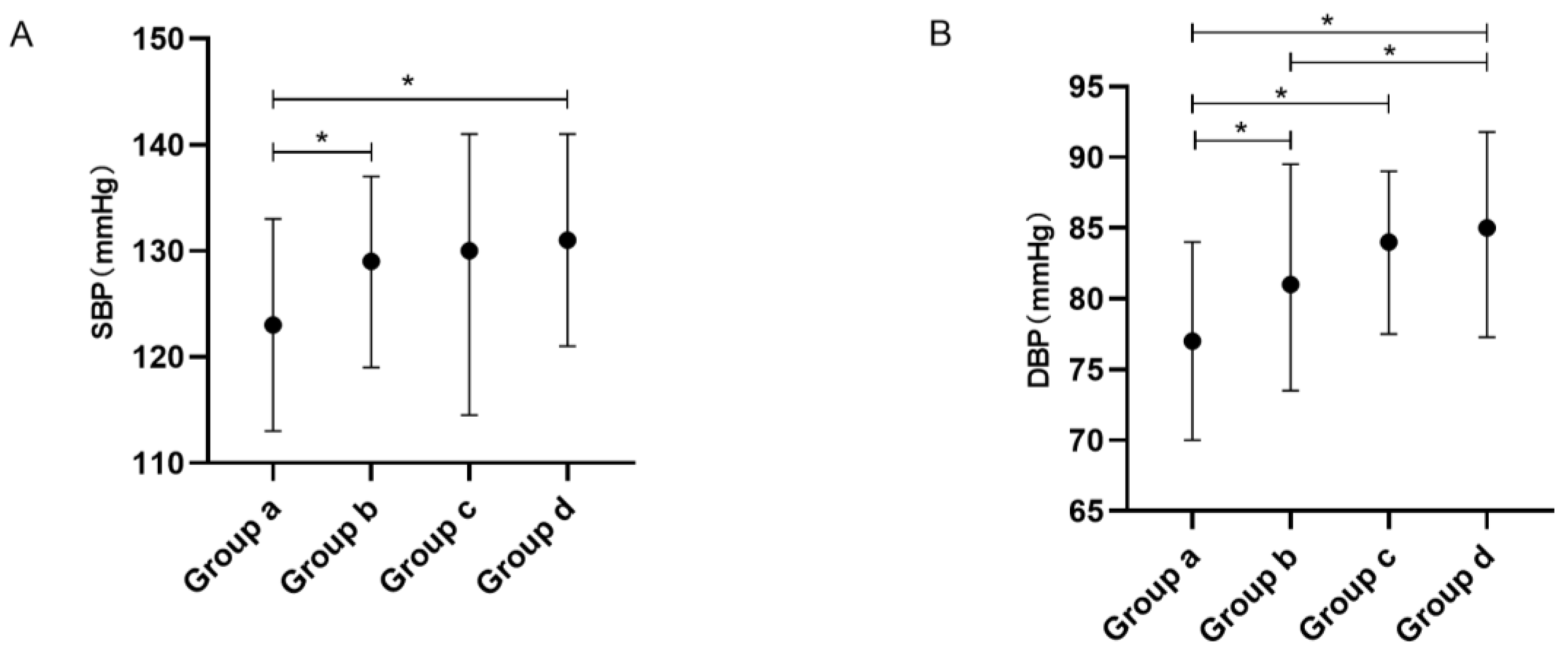
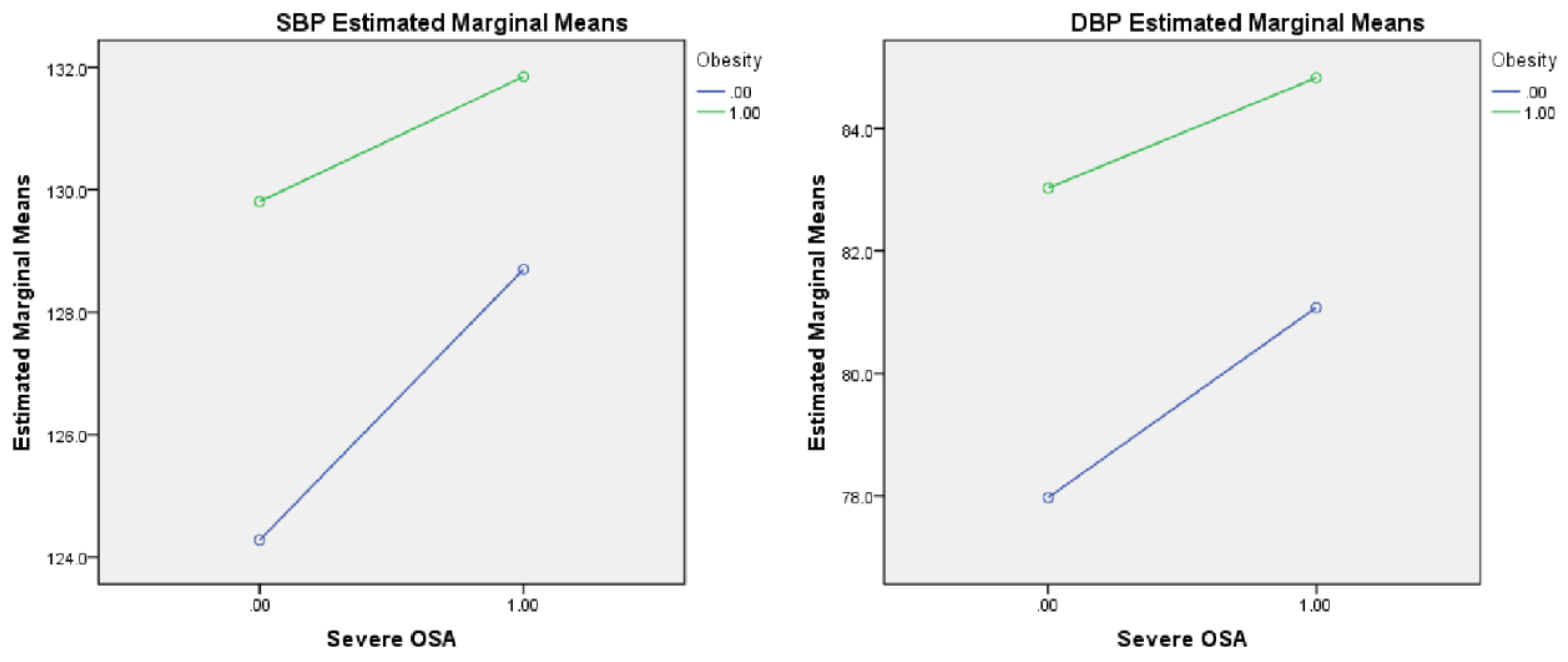
| SBP | 128.0 (117.0, 138.0) | 123.0 (113.0, 133.0) ab, ad | 129.0 (119.0, 137.0) | 130.0 (114.5, 141.0) | 131.0 (121.0, 141.0) | <0.001 | 0.002 | 0.020 | 0.382 |
| DBP | 81.0 (73.0, 89.0) | 77.0 (70.0, 84.0) ab,ac,ad | 81.0 (73.5, 89.5) bd | 84.0 (77.5, 89.0) | 85.0 (77.3, 91.8) | <0.001 | <0.001 | 0.017 | 0.518 |
| Whole Subjects (N = 686) | Non-obese Category (N = 433) | Obese Category (N = 253) | p1 | |||
|---|---|---|---|---|---|---|
| Non-Severe OSA Group (Group a, N = 232) | Severe OSA Group (Group b, N = 201) | Non-Severe OSA Group (Group c, N = 61) | Severe OSA Group (Group d, N = 192) | |||
| Percentages of abnormal blood pressure | ||||||
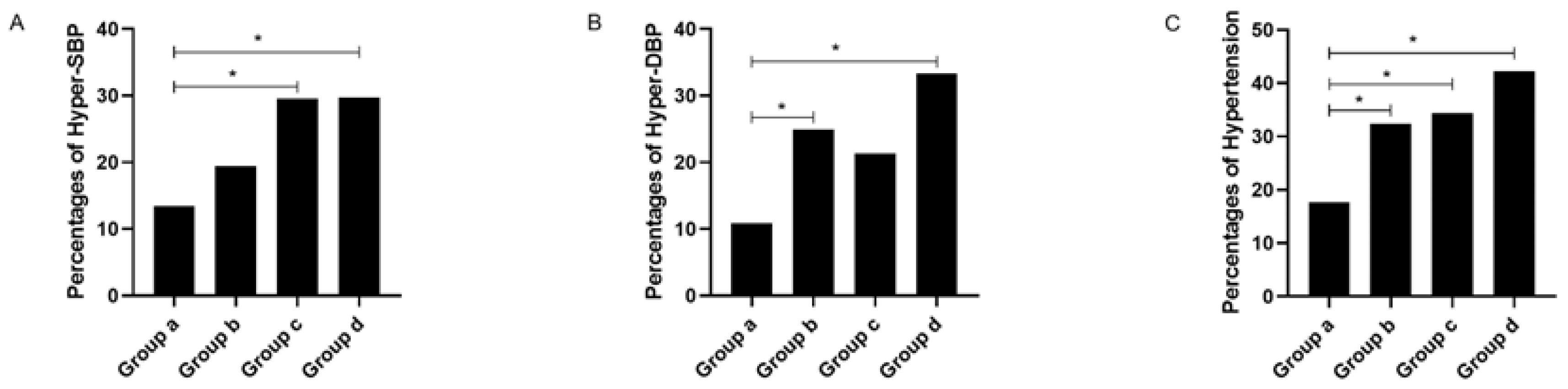
| Hyper-SBP, N (%) | 145 (21.1) | 31 (13.4) ac,ad | 39 (19.4) | 18 (29.5) | 57 (29.7) | <0.001 |
| Hyper-DBP, N (%) | 152 (22.2) | 25 (10.8) ab,ad | 50 (24.9) | 13 (21.3) | 64 (33.3) | <0.001 |
| Hypertension, N (%) | 208 (30.3) | 41 (17.7) ab,ac,ad | 65 (32.3) | 21 (34.4) | 81 (42.2) | <0.001 |
| Hyper-SBP | Hyper-DBP | Hypertension | ||||
|---|---|---|---|---|---|---|
| OR (95%CI) | p | OR (95%CI) | p | OR (95%CI) | p | |
| Gender | 0.647 (0.330, 1.271) | 0.206 | 0.843 (0.439, 1.621) | 0.609 | 0.747 (0.420, 1.331) | 0.322 |
| Age | 1.031 (1.014, 1.049) | <0.001 | 1.006 (0.990, 1.023) | 0.436 | 1.022 (1.007, 1.037) | 0.004 |
| Smoking | 1.061 (0.692, 1.628) | 0.785 | 1.297 (0.856, 1.965) | 0.219 | 1.162 (0.792, 1.703) | 0.443 |
| Drinking | 1.299 (0.774, 2.181) | 0.322 | 0.929 (0.547, 1.576) | 0.784 | 1.077 (0.668, 1.737) | 0.762 |
| Glucose | 1.284 (1.105, 1.491) | 0.001 | 1.198 (1.038, 1.382) | 0.013 | 1.250 (1.083, 1.441) | 0.002 |
| Obesity | 2.695 (1.335, 5.440) | 0.006 | 2.023 (0.949, 4.313) | 0.068 | 2.365 (1.233, 4.535) | 0.010 |
| Severe OSA | 1.596 (0.936, 2.721) | 0.086 | 2.702 (1.585, 4.607) | <0.001 | 2.285 (1.438, 3.631) | <0.001 |
| Severe OSA*Obesity | 0.670 (0.288, 1.558) | 0.352 | 0.694 (0.290, 1.659) | 0.412 | 0.636 (0.294, 1.373) | 0.249 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Y.; Liang, C.; Kang, J.; You, K.; Xiong, Y. Obstructive Sleep Apnea and Obesity Are Associated with Hypertension in a Particular Pattern: A Retrospective Study. Healthcare 2023, 11, 402. https://doi.org/10.3390/healthcare11030402
Xia Y, Liang C, Kang J, You K, Xiong Y. Obstructive Sleep Apnea and Obesity Are Associated with Hypertension in a Particular Pattern: A Retrospective Study. Healthcare. 2023; 11(3):402. https://doi.org/10.3390/healthcare11030402
Chicago/Turabian StyleXia, Yunyan, Caihong Liang, Junxin Kang, Kai You, and Yuanping Xiong. 2023. "Obstructive Sleep Apnea and Obesity Are Associated with Hypertension in a Particular Pattern: A Retrospective Study" Healthcare 11, no. 3: 402. https://doi.org/10.3390/healthcare11030402
APA StyleXia, Y., Liang, C., Kang, J., You, K., & Xiong, Y. (2023). Obstructive Sleep Apnea and Obesity Are Associated with Hypertension in a Particular Pattern: A Retrospective Study. Healthcare, 11(3), 402. https://doi.org/10.3390/healthcare11030402






