Nanocomposite Materials based on Metal Nanoparticles for the Electrochemical Sensing of Neurotransmitters
Abstract
:1. Introduction
2. Electrochemical Sensors
2.1. Conducting Polymers
2.2. Noble Metal NPs
3. Electrochemical Synthesis Methods of the Composite Material CPs-NPs
3.1. Conventional Methods
3.2. Innovative Methods
4. Electrochemical Sensors for Dopamine, Serotonin and Adrenaline Detection
4.1. Dopamine
4.2. Serotonin
4.3. Epinephrine/Adrenaline
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moon, J.M.; Thapliyal, N.; Hussain, K.K.; Goyal, R.N.; Shim, Y.B. Conducting polymer-based electrochemical biosensors for neurotransmitters: A review. Biosens. Bioelectron. 2018, 102, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Tavakolian-Ardakani, Z.; Hosu, O.; Cristea, C.; Mazloum-Ardakani, M.; Marrazza, G. Latest trends in electrochemical sensors for neurotransmitters: A review. Sensors 2019, 19, 2037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laughlin, S.B.; Sejnowski, T.J. Communication in neuronal networks. Science 2003, 301, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Herculano-Houzel, S. The human brain in numbers: A linearly scaled-up primate brain. Front. Hum. Neurosci. 2009, 3, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCoy, A.N.; Tan, S.Y. Otto Loewi (1873–1961): Dreamer and Nobel laureate. Singapore Med. J. 2014, 55, 3–4. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; McCracken, S.; Faruk Hossain, M.; Slaughter, G. Electrochemical Detection of Neurotransmitters. Biosensors 2020, 10, 101. [Google Scholar] [CrossRef]
- Niyonambaza, S.D.; Kumar, P.; Xing, P.; Mathault, J.; De Koninck, P.; Boisselier, E.; Boukadoum, M.; Miled, A. A Review of neurotransmitters sensing methods for neuro-engineering research. Appl. Sci. 2019, 9, 4719. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Bian, S.; Sawan, M. Real-time: In vivo detection techniques for neurotransmitters: A review. Analyst 2020, 145, 6193–6210. [Google Scholar] [CrossRef]
- Perry, M.; Li, Q.; Kennedy, R.T. Review of recent advances in analytical techniques for the determination of neurotransmitters. Anal. Chim. Acta 2010, 653, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Baluta, S.; Zając, D.; Szyszka, A.; Malecha, K.; Cabaj, J. Enzymatic platforms for sensitive neurotransmitter detection. Sensors 2020, 20, 423. [Google Scholar] [CrossRef] [Green Version]
- Suppiramaniam, V.; Bloemer, J.; Reed, M.; Bhattacharya, S. Neurotransmitter Receptors. Compr. Toxicol. Third Ed. 2018, 6–15, 174–201. [Google Scholar] [CrossRef]
- Marc, D.T.; Ailts, J.W.; Campeau, D.C.A.; Bull, M.J.; Olson, K.L. Neurotransmitters excreted in the urine as biomarkers of nervous system activity: Validity and clinical applicability. Neurosci. Biobehav. Rev. 2011, 35, 635–644. [Google Scholar] [CrossRef]
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G.; et al. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef] [Green Version]
- Sanghavi, B.J.; Wolfbeis, O.S.; Hirsch, T.; Swami, N.S. Nanomaterial-based electrochemical sensing of neurological drugs and neurotransmitters. Microchim. Acta 2015, 182, 1–41. [Google Scholar] [CrossRef] [Green Version]
- Rajarathinam, T.; Kang, M.; Hong, S.; Chang, S. Nanocomposite-Based Electrochemical Sensors for Neurotransmitters Detection in Neurodegenerative Diseases. Chemosensors 2023, 11, 103. [Google Scholar] [CrossRef]
- Kaur, H.; Siwal, S.S.; Saini, R.V.; Singh, N.; Thakur, V.K. Significance of an Electrochemical Sensor and Nanocomposites: Toward the Electrocatalytic Detection of Neurotransmitters and Their Importance within the Physiological System. ACS Nanosci. Au 2022, 3, 1–27. [Google Scholar] [CrossRef]
- Lakard, S.; Pavel, I.A.; Lakard, B. Electrochemical biosensing of dopamine neurotransmitter: A review. Biosensors 2021, 11, 179. [Google Scholar] [CrossRef]
- Hugo, V.; Castro, C.; Lucía, C.; Valenzuela, L.; Carlos, J.; Sánchez, S.; Peña, K.P.; Pérez, S.J.L.; Ibarra, J.O.; Villagrán, A.M. An Update of the Classical and Novel Methods Used for Measuring Fast Neurotransmitters during Normal and Brain Altered Function. Curr. Neuropharmacol. 2014, 12, 490–508. [Google Scholar] [CrossRef] [Green Version]
- Labib, M.; Sargent, E.H.; Kelley, S.O. Electrochemical Methods for the Analysis of Clinically Relevant Biomolecules. Chem. Rev. 2016, 116, 9001–9090. [Google Scholar] [CrossRef]
- Hulanicki, A.; Stanislav, G.; Folke, I. Chemical sensors: Definitions and classification. Pure Appl. Chem. 1991, 63, 1247–1250. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Gatto, G.; Broncova, G.; Kumar, A. Electrochemical Sensors and Their Applications: A Review. Chemosensors 2022, 10, 363. [Google Scholar] [CrossRef]
- Diaz, A.F.; Castillo, J.I.; Logan, J.A.; Lee, W.-Y. Electrochemistry of conducting polypyrrole films. J. Electroanal. Chem. 1981, 129, 115–132. [Google Scholar] [CrossRef]
- Inzelt, G. Conducting polymers. A new era in electrochemistry. In Monographs in Electrochemistry, 2nd ed.; Scholz, F., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 978-3-642-27620-0. [Google Scholar]
- Lyutov, V.; Efimov, I.; Bund, A.; Tsakova, V. Electrochemical polymerization of 3,4-ethylenedioxythiophene in the presence of dodecylsulfate and polysulfonic anions—An acoustic impedance study. Electrochim. Acta 2014, 122, 21–27. [Google Scholar] [CrossRef]
- Lyutov, V.; Gruia, V.; Efimov, I.; Bund, A.; Tsakova, V. An acoustic impedance study of PEDOT layers obtained in aqueous solution. Electrochim. Acta 2016, 190, 285–293. [Google Scholar] [CrossRef]
- Tsakova, V.; Seeber, R. Conducting polymers in electrochemical sensing: Factors influencing the electroanalytical signal. Anal. Bioanal. Chem. 2016, 408, 7231–7241. [Google Scholar] [CrossRef]
- Darabdhara, G.; Das, M.R.; Singh, S.P.; Rengan, A.K.; Szunerits, S.; Boukherroub, R. Ag and Au nanoparticles/reduced graphene oxide composite materials: Synthesis and application in diagnostics and therapeutics. Adv. Colloid Interface Sci. 2019, 271, 101991. [Google Scholar] [CrossRef]
- Qiao, Z.; Zhang, J.; Hai, X.; Yan, Y.; Song, W.; Bi, S. Recent advances in templated synthesis of metal nanoclusters and their applications in biosensing, bioimaging and theranostics. Biosens. Bioelectron. 2021, 176, 112898. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, H.; Yan, L.; Li, N.; Shi, J.; Jiang, C. Recent Developments in Detection Using Noble Metal Nanoparticles. Crit. Rev. Anal. Chem. 2020, 50, 97–110. [Google Scholar] [CrossRef]
- Espino-López, I.E.; Romero-Romo, M.; de Oca-Yemha, M.G.M.; Morales-Gil, P.; Ramírez-Silva, M.T.; Mostany, J.; Palomar-Pardavé, M. Palladium Nanoparticles Electrodeposition onto Glassy Carbon from a Deep Eutectic Solvent at 298 K and Their Catalytic Performance toward Formic Acid Oxidation. J. Electrochem. Soc. 2019, 166, D3205–D3211. [Google Scholar] [CrossRef]
- Atta, N.F.; Galal, A.; El-Ads, E.H. Gold nanoparticles-coated poly(3,4-ethylene-dioxythiophene) for the selective determination of sub-nano concentrations of dopamine in presence of sodium dodecyl sulfate. Electrochim. Acta 2012, 69, 102–111. [Google Scholar] [CrossRef]
- Tian, J.; Peng, D.; Wu, X.; Li, W.; Deng, H.; Liu, S. Electrodeposition of Ag nanoparticles on conductive polyaniline/cellulose aerogels with increased synergistic effect for energy storage. Carbohydr. Polym. 2017, 156, 19–25. [Google Scholar] [CrossRef]
- Agrisuelas, J.; González-Sánchez, M.I.; Valero, E. Hydrogen peroxide sensor based on in situ grown Pt nanoparticles from waste screen-printed electrodes. Sens. Actuators B Chem. 2017, 249, 499–505. [Google Scholar] [CrossRef]
- Tertiș, M.; Cernat, A.; Lacatiș, D.; Florea, A.; Bogdan, D.; Suciu, M.; Săndulescu, R.; Cristea, C. Highly selective electrochemical detection of serotonin on polypyrrole and gold nanoparticles-based 3D architecture. Electrochem. Commun. 2017, 75, 43–47. [Google Scholar] [CrossRef]
- Mahalakshmi, S.; Sridevi, V. In Situ Electrodeposited Gold Nanoparticles on Polyaniline-Modified Electrode Surface for the Detection of Dopamine in Presence of Ascorbic Acid and Uric Acid. Electrocatalysis 2021, 12, 415–435. [Google Scholar] [CrossRef]
- Selvolini, G.; Lazzarini, C.; Marrazza, G. Electrochemical nanocomposite single-use sensor for dopamine detection. Sensors 2019, 19, 3097. [Google Scholar] [CrossRef] [Green Version]
- Hatchett, D.W.; Quy, T.; Goodwin, N.; Millick, N.M. In-Situ Reduction of Au, Pd, and Pt Metal Precursors in Polyaniline: Electrochemistry of Variable Metal Content Polymer/Metal Composites in Alkaline Solution. Electrochim. Acta 2017, 251, 699–709. [Google Scholar] [CrossRef]
- Sadanandhan, N.K.; Cheriyathuchenaaramvalli, M.; Devaki, S.J.; Ravindranatha Menon, A.R. PEDOT-reduced graphene oxide-silver hybrid nanocomposite modified transducer for the detection of serotonin. J. Electroanal. Chem. 2017, 794, 244–253. [Google Scholar] [CrossRef]
- Park, D.J.; Choi, J.H.; Lee, W.J.; Um, S.H.; Oh, B.K. Selective electrochemical detection of dopamine using reduced graphene oxide sheets-gold nanoparticles modified electrode. J. Nanosci. Nanotechnol. 2017, 17, 8012–8018. [Google Scholar] [CrossRef]
- Lupu, S.; Lete, C.; Balaure, P.C.; Del Campo, F.J.; Muñoz, F.X.; Lakard, B.; Hihn, J.Y. In Situ electrodeposition of biocomposite materials by sinusoidal voltages on microelectrodes array for tyrosinase based amperometric biosensor development. Sens. Actuators B Chem. 2013, 181, 136–143. [Google Scholar] [CrossRef]
- Lupu, S.; Lete, C.; Balaure, P.C.; Caval, D.I.; Mihailciuc, C.; Lakard, B.; Hihn, J.Y.; del Campo, F.J. Development of amperometric biosensors based on nanostructured tyrosinase-conducting polymer composite electrodes. Sensors 2013, 13, 6759–6774. [Google Scholar] [CrossRef] [Green Version]
- Lete, C.; Berger, D.; Matei, C.; Lupu, S. Electrochemical and microgravimetric studies of poly[3,4-ethylenedioxythiophene]-tyrosinase biocomposite material electrodeposited onto gold electrodes by a sinusoidal voltages method. J. Solid State Electrochem. 2016, 20, 3043–3051. [Google Scholar] [CrossRef]
- Lupu, S.; Lete, C.; JavierdelCampo, F. Dopamine Electroanalysis Using Electrochemical Biosensors Prepared by a Sinusoidal Voltages Method. Electroanalysis 2015, 27, 1649–1659. [Google Scholar] [CrossRef]
- Lete, C.; Lupu, S.; Lakard, B.; Hihn, J.Y.; Del Campo, F.J. Multi-analyte determination of dopamine and catechol at single-walled carbon nanotubes—Conducting polymer—Tyrosinase based electrochemical biosensors. J. Electroanal. Chem. 2015, 744, 53–61. [Google Scholar] [CrossRef]
- Lete, C.; Lakard, B.; Hihn, J.Y.; del Campo, F.J.; Lupu, S. Use of sinusoidal voltages with fixed frequency in the preparation of tyrosinase based electrochemical biosensors for dopamine electroanalysis. Sens. Actuators B Chem. 2017, 240, 801–809. [Google Scholar] [CrossRef]
- Lupu, S.; Del Campo, F.J.; Muñoz, F.X. Sinusoidal voltage electrodeposition and characterization of conducting polymers on gold microelectrode arrays. J. Electroanal. Chem. 2012, 687, 71–78. [Google Scholar] [CrossRef]
- Lupu, S.; Lakard, B.; Hihn, J.Y.; Dejeu, J. Novel in situ electrochemical deposition of platinum nanoparticles by sinusoidal voltages on conducting polymer films. Synth. Met. 2012, 162, 193–198. [Google Scholar] [CrossRef] [Green Version]
- Bottari, D.; Pigani, L.; Zanardi, C.; Terzi, F.; Paturca, S.V.; Grigorescu, S.D.; Matei, C.; Lete, C.; Lupu, S. Electrochemical sensing of caffeic acid using gold nanoparticles embedded in poly(3,4-ethylenedioxythiophene) layer by sinusoidal voltage procedure. Chemosensors 2019, 7, 65. [Google Scholar] [CrossRef] [Green Version]
- García-Guzmán, J.J.; López-Iglesias, D.; Cubillana-Aguilera, L.; Bellido-Milla, D.; Palacios-Santander, J.M.; Marin, M.; Grigorescu, S.D.; Lete, C.; Lupu, S. Silver nanostructures-poly(3,4-ethylenedioxythiophene) sensing material prepared by sinusoidal voltage procedure for detection of antioxidants. Electrochim. Acta 2021, 393, 139082. [Google Scholar] [CrossRef]
- García-Guzmán, J.J.; López-Iglesias, D.; Cubillana-Aguilera, L.; Lete, C.; Lupu, S.; Palacios-Santander, J.M.; Bellido-Milla, D. Assessment of the polyphenol indices and antioxidant capacity for beers and wines using a tyrosinase-based biosensor prepared by sinusoidal current method. Sensors 2019, 19, 66. [Google Scholar] [CrossRef] [Green Version]
- García Guzmán, J.J.; Aguilera, L.C.; Milla, D.B.; Rodríguez, I.N.; Lete, C.; Palacios Santander, J.M.; Lupu, S. Development of Sonogel-Carbon based biosensors using sinusoidal voltages and currents methods. Sens. Actuators B Chem. 2018, 255, 1525–1535. [Google Scholar] [CrossRef]
- Leau, S.A.; Lete, C.; Marin, M.; Javier, F.; Diaconu, I.; Lupu, S. Electrochemical sensors based on antimony tin oxide-Prussian blue screen-printed electrode and PEDOT-Prussian blue for potassium ion detection. J. Solid State Electrochem. 2023. [Google Scholar] [CrossRef]
- Yoon, H. Current trends in sensors based on conducting polymer nanomaterials. Nanomaterials 2013, 3, 524–549. [Google Scholar] [CrossRef] [Green Version]
- Juárez Olguín, H.; Calderón Guzmán, D.; Hernández García, E.; Barragán Mejía, G. The role of dopamine and its dysfunction as a consequence of oxidative stress. Oxid. Med. Cell. Longev. 2016, 2016, 9730467. [Google Scholar] [CrossRef] [Green Version]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell. Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef]
- Daubner, S.C.; Le, T.; Wang, S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch. Biochem. Biophys. 2011, 508, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, J.; Faisal, M.; Alsareii, S.A.; Jalalah, M.; Harraz, F.A. A novel gold-decorated porous silicon-poly(3-hexylthiophene) ternary nanocomposite as a highly sensitive and selective non-enzymatic dopamine electrochemical sensor. J. Alloys Compd. 2022, 931, 167403. [Google Scholar] [CrossRef]
- Harsini, M.; Widyaningrum, B.A.; Fitriany, E.; Ayuparamita, D.R.; Farida, A.N.; Farida, A.; Kurniawan, F.; Wibawasakti, S.C. Electrochemical synthesis of Polymelamine/gold nanoparticle modified carbon paste electrode as voltammetric sensor of dopamine. Chinese J. Anal. Chem. 2022, 50, 100052. [Google Scholar] [CrossRef]
- Chu, W.; Zhou, Q.; Li, S.; Zhao, W.; Li, N.; Zheng, J. Oxidation and sensing of ascorbic acid and dopamine on self-Assembled gold nanoparticles incorporated within polyaniline film. Appl. Surf. Sci. 2015, 353, 425–432. [Google Scholar] [CrossRef]
- Inagaki, C.S.; Oliveira, M.M.; Bergamini, M.F.; Marcolino-Junior, L.H.; Zarbin, A.J.G. Facile synthesis and dopamine sensing application of three component nanocomposite thin films based on polythiophene, gold nanoparticles and carbon nanotubes. J. Electroanal. Chem. 2019, 840, 208–217. [Google Scholar] [CrossRef]
- Khudaish, E.A.; Al-Nofli, F.; Rather, J.A.; Al-Hinaai, M.; Laxman, K.; Kyaw, H.H.; Al-Harthy, S. Sensitive and selective dopamine sensor based on novel conjugated polymer decorated with gold nanoparticles. J. Electroanal. Chem. 2016, 761, 80–88. [Google Scholar] [CrossRef]
- Pan, J.; Liu, M.; Li, D.; Zheng, H.; Zhang, D. Overoxidized poly(3,4-ethylenedioxythiophene)–gold nanoparticles–graphene-modified electrode for the simultaneous detection of dopamine and uric acid in the presence of ascorbic acid. J. Pharm. Anal. 2021, 11, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Paulraj, P.; Umar, A.; Rajendran, K.; Manikandan, A.; Kumar, R.; Manikandan, E.; Pandian, K.; Mahnashi, M.H.; Alsaiari, M.A.; Ibrahim, A.A.; et al. Solid-state synthesis of Ag-doped PANI nanocomposites for their end-use as an electrochemical sensor for hydrogen peroxide and dopamine. Electrochim. Acta 2020, 363, 137158. [Google Scholar] [CrossRef]
- Xu, G.; Liang, S.; Zhang, M.; Fan, J.; Feng, J.; Yu, X. Studies on the electrochemical and dopamine sensing properties of AgNP-modified carboxylated cellulose nanocrystal-doped poly(3,4-ethylenedioxythiophene). Ionics Kiel 2017, 23, 3211–3218. [Google Scholar] [CrossRef]
- Pandian, P.; Kalimuthu, R.; Arumugam, S.; Kannaiyan, P. Solid phase mechanochemical synthesis of Poly(o-anisidine) protected Silver nanoparticles for electrochemical dopamine sensor. Mater. Today Commun. 2021, 26, 102191. [Google Scholar] [CrossRef]
- Ulubay, Ş.; Dursun, Z. Cu nanoparticles incorporated polypyrrole modified GCE for sensitive simultaneous determination of dopamine and uric acid. Talanta 2010, 80, 1461–1466. [Google Scholar] [CrossRef]
- Atta, N.F.; El-Kady, M.F.; Galal, A. Palladium nanoclusters-coated polyfuran as a novel sensor for catecholamine neurotransmitters and paracetamol. Sens. Actuators B Chem. 2009, 141, 566–574. [Google Scholar] [CrossRef]
- Atta, N.F.; El-Kady, M.F. Novel poly(3-methylthiophene)/Pd, Pt nanoparticle sensor: Synthesis, characterization and its application to the simultaneous analysis of dopamine and ascorbic acid in biological fluids. Sens. Actuators B Chem. 2010, 145, 299–310. [Google Scholar] [CrossRef]
- Atta, N.F.; El-Kady, M.F.; Galal, A. Simultaneous determination of catecholamines, uric acid and ascorbic acid at physiological levels using poly(N-methylpyrrole)/Pd-nanoclusters sensor. Anal. Biochem. 2010, 400, 78–88. [Google Scholar] [CrossRef]
- Ahmed, J.; Faisal, M.; Harraz, F.A.; Jalalah, M.; Alsareii, S.A. Development of an amperometric biosensor for dopamine using novel mesoporous silicon nanoparticles fabricated via a facile stain etching approach. Phys. E Low-Dimens. Syst. Nanostruct. 2022, 135, 114952. [Google Scholar] [CrossRef]
- Amidi, S.; Ardakani, Y.H.; Amiri-Aref, M.; Ranjbari, E.; Sepehri, Z.; Bagheri, H. Sensitive electrochemical determination of rifampicin using gold nanoparticles/poly-melamine nanocomposite. RSC Adv. 2017, 7, 40111–40118. [Google Scholar] [CrossRef] [Green Version]
- Cao, Q.; Puthongkham, P.; Venton, B.J. Review: New insights into optimizing chemical and 3D surface structures of carbon electrodes for neurotransmitter detection. Anal. Methods 2019, 11, 247–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wani, I.A. Review—Recent Advances in Biogenic Silver Nanoparticles & NanoComposite Based Plasmonic-Colorimetric and Electrochemical Sensors. ECS J. Solid State Sci. Technol. 2021, 10, 047003. [Google Scholar] [CrossRef]
- Ankitha, M.; Arjun, A.M.; Shabana, N.; Rasheed, P.A. A Mini Review on Recent Advances in MXene Based Electrochemical Wearable Sensing Devices. Biomed. Mater. Devices 2022. [Google Scholar] [CrossRef]
- Ni, M.; Chen, J.; Wang, C.; Wang, Y.; Huang, L.; Xiong, W.; Zhao, P.; Xie, Y.; Fei, J. A high-sensitive dopamine electrochemical sensor based on multilayer Ti3C2 MXene, graphitized multi-walled carbon nanotubes and ZnO nanospheres. Microchem. J. 2022, 178, 107410. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, B.; Ding, A.; Weng, B.; Chen, J. Synthesis of MXene/DNA/Pd/Pt nanocomposite for sensitive detection of dopamine. J. Electroanal. Chem. 2018, 816, 189–194. [Google Scholar] [CrossRef]
- Ankitha, M.; Shabana, N.; Mohan Arjun, A.; Muhsin, P.; Abdul Rasheed, P. Ultrasensitive electrochemical detection of dopamine from human serum samples by Nb2CTx-MoS2 hetero structures. Microchem. J. 2023, 187, 108424. [Google Scholar] [CrossRef]
- Kondziella, D. The Top 5 Neurotransmitters from a Clinical Neurologist’s Perspective. Neurochem. Res. 2017, 42, 1767–1771. [Google Scholar] [CrossRef]
- Lv, J.; Liu, F. The role of serotonin beyond the central nervous system during embryogenesis. Front. Cell. Neurosci. 2017, 11, 1–7. [Google Scholar] [CrossRef]
- Chung, S.; Akhtar, M.H.; Benboudiaf, A.; Park, D.S.; Shim, Y.B. A Sensor for Serotonin and Dopamine Detection in Cancer Cells Line Based on the Conducting Polymer−Pd Complex Composite. Electroanalysis 2020, 32, 520–527. [Google Scholar] [CrossRef]
- Li, J.; Lin, X. Simultaneous determination of dopamine and serotonin on gold nanocluster/overoxidized-polypyrrole composite modified glassy carbon electrode. Sens. Actuators B Chem. 2007, 124, 486–493. [Google Scholar] [CrossRef]
- Sadanandhan, N.K.; Devaki, S.J. Gold nanoparticle patterned on PANI nanowire modified transducer for the simultaneous determination of neurotransmitters in presence of ascorbic acid and uric acid. J. Appl. Polym. Sci. 2017, 134, 1–9. [Google Scholar] [CrossRef]
- Xue, C.; Wang, X.; Zhu, W.; Han, Q.; Zhu, C.; Hong, J.; Zhou, X.; Jiang, H. Electrochemical serotonin sensing interface based on double-layered membrane of reduced graphene oxide/polyaniline nanocomposites and molecularly imprinted polymers embedded with gold nanoparticles. Sens. Actuators B Chem. 2014, 196, 57–63. [Google Scholar] [CrossRef]
- Selvarajan, S.; Suganthi, A.; Rajarajan, M. A novel highly selective and sensitive detection of serotonin based on Ag/polypyrrole/Cu2O nanocomposite modified glassy carbon electrode. Ultrason. Sonochem. 2018, 44, 319–330. [Google Scholar] [CrossRef]
- Cesarino, I.; Galesco, H.V.; Machado, S.A.S. Determination of serotonin on platinum electrode modified with carbon nanotubes/polypyrrole/silver nanoparticles nanohybrid. Mater. Sci. Eng. C 2014, 40, 49–54. [Google Scholar] [CrossRef]
- Ghanbari, K.; Bonyadi, S. An electrochemical sensor based on Pt nanoparticles decorated over-oxidized polypyrrole/reduced graphene oxide nanocomposite for simultaneous determination of two neurotransmitters dopamine and 5-Hydroxy tryptamine in the presence of ascorbic acid. Int. J. Polym. Anal. Charact. 2020, 25, 105–125. [Google Scholar] [CrossRef]
- Khan, M.Z.H.; Liu, X.; Tang, Y.; Zhu, J.; Hu, W.; Liu, X. A glassy carbon electrode modified with a composite consisting of gold nanoparticle, reduced graphene oxide and poly(L-arginine) for simultaneous voltammetric determination of dopamine, serotonin and L-tryptophan. Microchim. Acta 2018, 185, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, G.; Asif, M.; Aziz, A.; Iftikhar, T.; Liu, H. Rice-Spikelet-like Copper Oxide Decorated with Platinum Stranded in the CNT Network for Electrochemical in Vitro Detection of Serotonin. ACS Appl. Mater. Interfaces 2021, 13, 6023–6033. [Google Scholar] [CrossRef] [PubMed]
- Kausar, A. Polymeric nanocomposites reinforced with nanowires: Opening doors to future applications. J. Plast. Film Sheeting 2019, 35, 65–98. [Google Scholar] [CrossRef]
- Soloducho, J.; Cabaj, J. Conducting Polymers in Sensor Design. In Conducting Polymers; Faris, Y., Ed.; IntechOpen: London, UK, 2016. [Google Scholar]
- Malathi, S.; Pakrudheen, I.; Kalkura, S.N.; Webster, T.J.; Balasubramanian, S. Disposable biosensors based on metal nanoparticles. Sens. Int. 2022, 3, 100169. [Google Scholar] [CrossRef] [PubMed]
- Deroco, P.B.; Giarola, J.d.F.; Wachholz Júnior, D.; Arantes Lorga, G.; Tatsuo Kubota, L. Paper-Based Electrochemical Sensing Devices, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2020; Volume 89. [Google Scholar]
- Ellis, L.; Farrington, D.P.; Hoskin, A.W. Handbook of Crime Correlates, 2nd ed.; Ellis, L., Farrington, D.P., Hoskin, A.W., Eds.; Academic Press: San Diego, CA, USA, 2019; ISBN 9780128044179. [Google Scholar]
- Teleanu, R.I.; Niculescu, A.G.; Roza, E.; Vladâcenco, O.; Grumezescu, A.M.; Teleanu, D.M. Neurotransmitters—Key Factors in Neurological and Neurodegenerative Disorders of the Central Nervous System. Int. J. Mol. Sci. 2022, 23, 5954. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, K.; Walker, P.; McLean, L.; Carrive, P. Fear and the Defence Cascade: Clinical Implications and Management; Harvard Review of Psychiatry; Harvard Medical School: Boston, MA, USA, 2015; ISBN 9780128033579. [Google Scholar]
- Liu, F.; Kan, X. Conductive imprinted electrochemical sensor for epinephrine sensitive detection and double recognition. J. Electroanal. Chem. 2019, 836, 182–189. [Google Scholar] [CrossRef]
- Ghanbari, K.; Hajian, A. Electrochemical characterization of Au/ZnO/PPy/RGO nanocomposite and its application for simultaneous determination of ascorbic acid, epinephrine, and uric acid. J. Electroanal. Chem. 2017, 801, 466–479. [Google Scholar] [CrossRef]
- Mao, H.; Zhang, H.; Jiang, W.; Liang, J.; Sun, Y.; Zhang, Y.; Wu, Q.; Zhang, G.; Song, X.M. Poly(ionic liquid) functionalized polypyrrole nanotubes supported gold nanoparticles: An efficient electrochemical sensor to detect epinephrine. Mater. Sci. Eng. C 2017, 75, 495–502. [Google Scholar] [CrossRef]
- Zou, L.; Li, Y.; Cao, S.; Ye, B. Gold nanoparticles/polyaniline Langmuir-Blodgett Film modified glassy carbon electrode as voltammetric sensor for detection of epinephrine and uric acid. Talanta 2013, 117, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, Y.; Du, J.; Zhou, X.; Xue, Z.; Liu, X.; Wang, Z. A novel nanocomposites sensor for epinephrine detection in the presence of uric acids and ascorbic acids. Electrochim. Acta 2011, 56, 7261–7266. [Google Scholar] [CrossRef]
- Li, J.; Lin, X.Q. Electrodeposition of gold nanoclusters on overoxidized polypyrrole film modified glassy carbon electrode and its application for the simultaneous determination of epinephrine and uric acid under coexistence of ascorbic acid. Anal. Chim. Acta 2007, 596, 222–230. [Google Scholar] [CrossRef]
- Dorraji, P.S.; Jalali, F. Novel sensitive electrochemical sensor for simultaneous determination of epinephrine and uric acid by using a nanocomposite of MWCNTs-chitosan and gold nanoparticles attached to thioglycolic acid. Sens. Actuators B Chem. 2014, 200, 251–258. [Google Scholar] [CrossRef]
- Zhan, S.; Xu, C.; Chen, J.; Xiao, Q.; Zhou, Z.; Xing, Z.; Gu, C.; Yin, Z.; Liu, H. A novel epinephrine biosensor based on gold nanoparticles coordinated polydopamine-functionalized acupuncture needle microelectrode. Electrochim. Acta 2022, 437, 141468. [Google Scholar] [CrossRef]
- Fouad, D.M.; El-Said, W.A. Selective Electrochemical Detection of Epinephrine Using Gold Nanoporous Film. J. Nanomater. 2016, 2016, 6194230. [Google Scholar] [CrossRef] [Green Version]
- Yousif, N.M.; Attia, R.M.; Balboul, M.R. Adrenaline biosensors based on r Go/Ag nanocomposites functionalized textiles using advanced electron beam irradiation technique. J. Organomet. Chem. 2022, 972, 122392. [Google Scholar] [CrossRef]
- Thanh, T.D.; Balamurugan, J.; Tuan, N.T.; Jeong, H.; Lee, S.H.; Kim, N.H.; Lee, J.H. Enhanced electrocatalytic performance of an ultrafine AuPt nanoalloy framework embedded in graphene towards epinephrine sensing. Biosens. Bioelectron. 2017, 89, 750–757. [Google Scholar] [CrossRef]
- Jain, R.; Jadon, N.; Pawaiya, A. Polypyrrole based next generation electrochemical sensors and biosensors: A review. TrAC-Trends Anal. Chem. 2017, 97, 363–373. [Google Scholar] [CrossRef]
- Kaur, G.; Adhikari, R.; Cass, P.; Bown, M.; Gunatillake, P. Electrically conductive polymers and composites for biomedical applications. RSC Adv. 2015, 5, 37553–37567. [Google Scholar] [CrossRef]
- Marcu, M.; Spataru, T.; Calderon-Moreno, J.M.; Osiceanu, P.; Preda, L.; Spataru, N. Anodic Voltammetry of Epinephrine at Graphene-Modified Conductive Diamond Electrodes and Its Analytical Application. J. Electrochem. Soc. 2018, 165, B523–B529. [Google Scholar] [CrossRef]
- Scott, K. Electrochemical Principles and Characterization of Bioelectrochemical Systems; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; ISBN 9781782423966. [Google Scholar]
- Borgus, J.R.; Wang, Y.; Discenza, D.J.; Venton, B.J. Spontaneous Adenosine and Dopamine Cotransmission in the Caudate-Putamen Is Regulated by Adenosine Receptors. ACS Chem. Neurosci. 2021, 12, 4371–4379. [Google Scholar] [CrossRef]
- Venton, B.J.; Cao, Q. Fundamentals of fast-scan cyclic voltammetry for dopamine detection. Analyst 2020, 145, 1158–1168. [Google Scholar] [CrossRef]
- Cao, Q.; Shin, M.; Lavrik, N.V.; Venton, B.J. 3D-Printed Carbon Nanoelectrodes for In Vivo Neurotransmitter Sensing. Nano Lett. 2020, 20, 6831–6836. [Google Scholar] [CrossRef]
- Hao, S.W.; Jin, Q.H. Association between the +104T/C polymorphism in the 5′UTR of GDF5 and susceptibility to knee osteoarthritis: A meta-analysis. Mol. Med. Rep. 2013, 7, 485–488. [Google Scholar] [CrossRef] [Green Version]
- Berni, A.; Lahcen, A.A.; Salama, K.N.; Amine, A. 3D-porous laser-scribed graphene decorated with overoxidized polypyrrole as an electrochemical sensing platform for dopamine. J. Electroanal. Chem. 2022, 919, 116529. [Google Scholar] [CrossRef]
- Shabana, N.; Arjun, A.M.; Ankitha, M.; Mohandas, S.A.; Gangadharan, P.; Rasheed, P.A. A flexible and sensitive electrochemical sensing platform based on dimethyl sulfoxide modified carbon cloth: Towards the detection of dopamine and carvedilol. Anal. Methods 2023, 15, 685–692. [Google Scholar] [CrossRef]
- Bai, Q.; Luo, H.; Yi, X.; Shi, S.; Wang, L.; Liu, M.; Du, F.; Yang, Z.; Sui, N. Nitrogen-Doped Graphdiyne Quantum-dots as an Optical-Electrochemical sensor for sensitive detection of dopamine. Microchem. J. 2022, 179, 107521. [Google Scholar] [CrossRef]
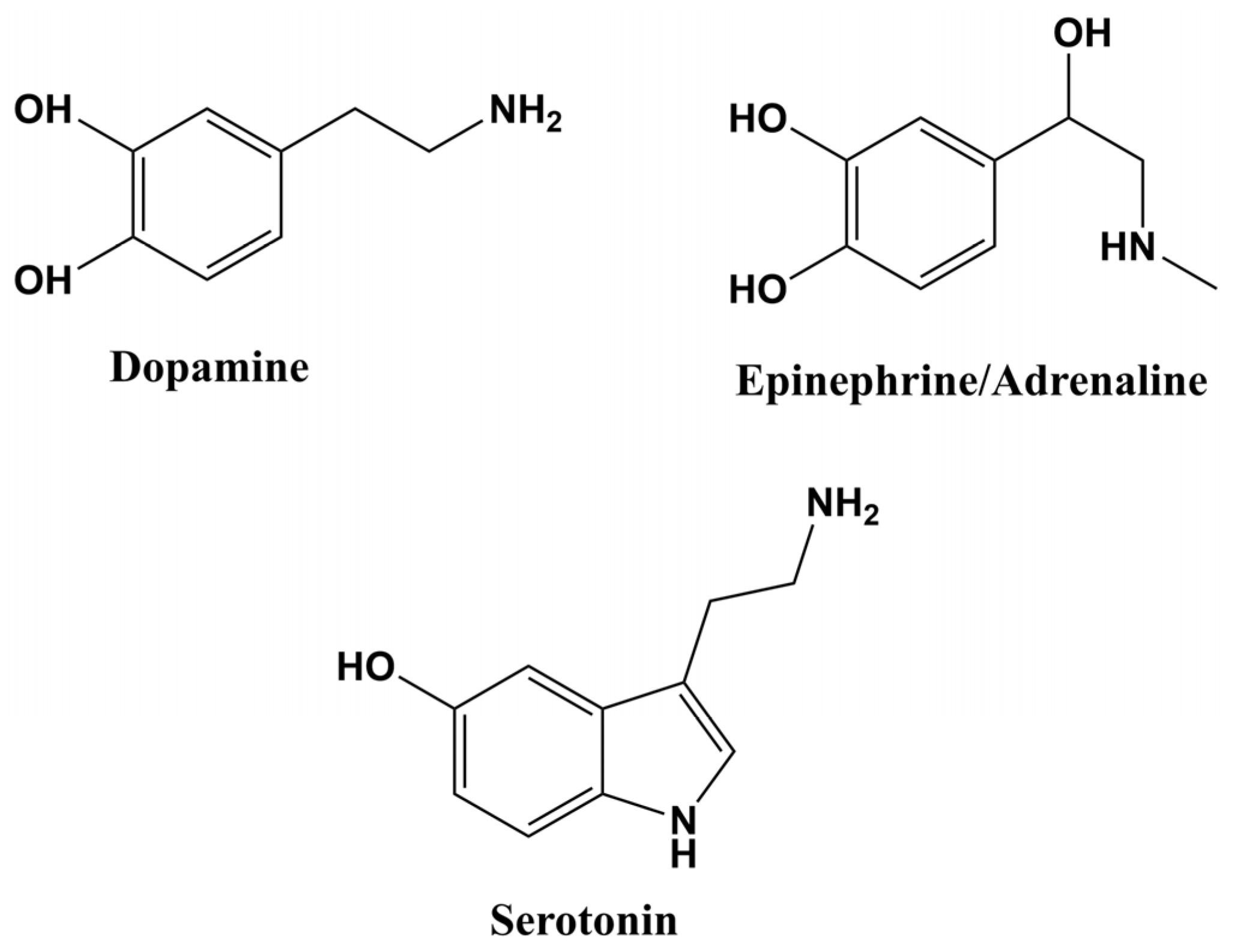
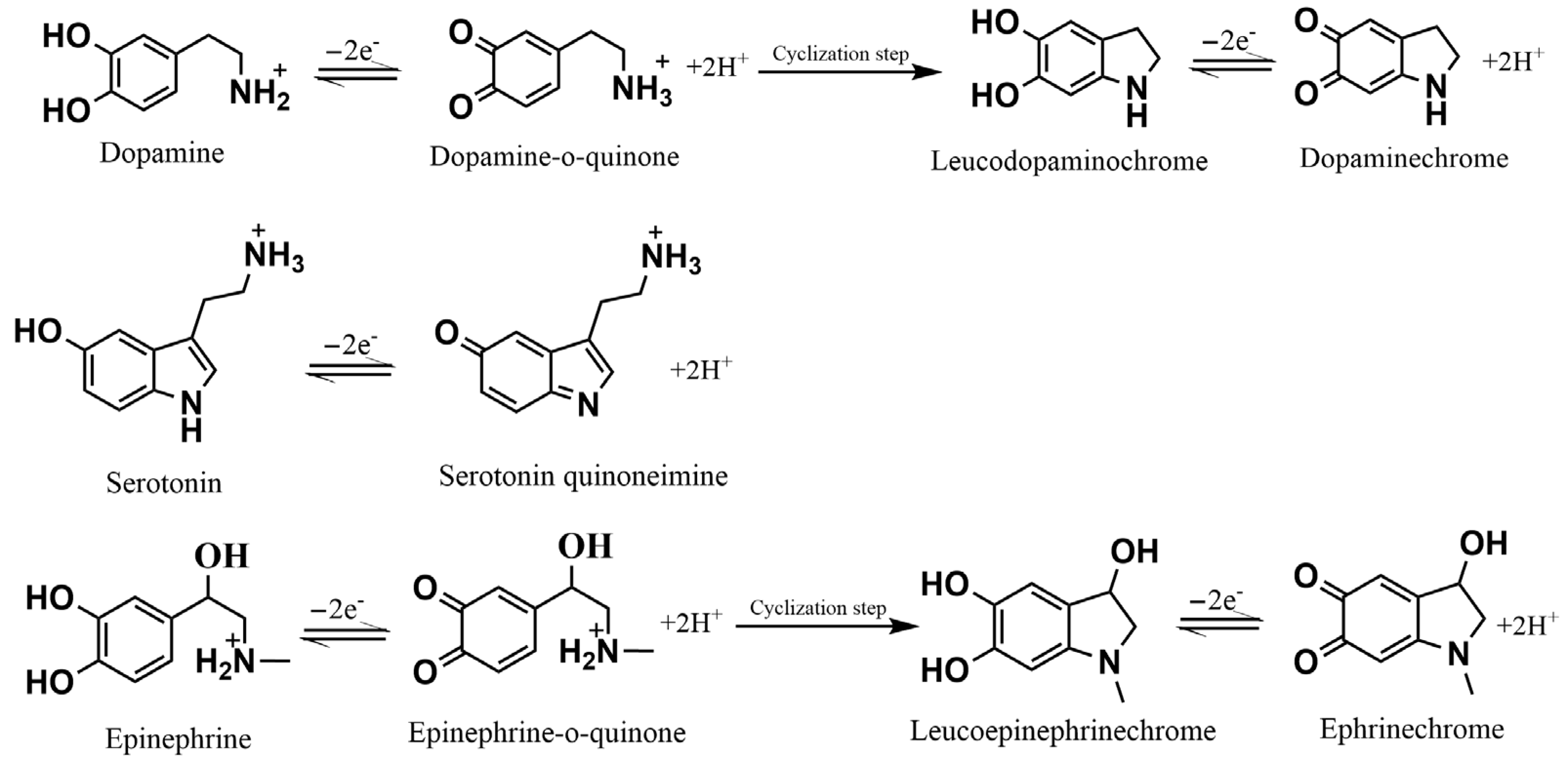
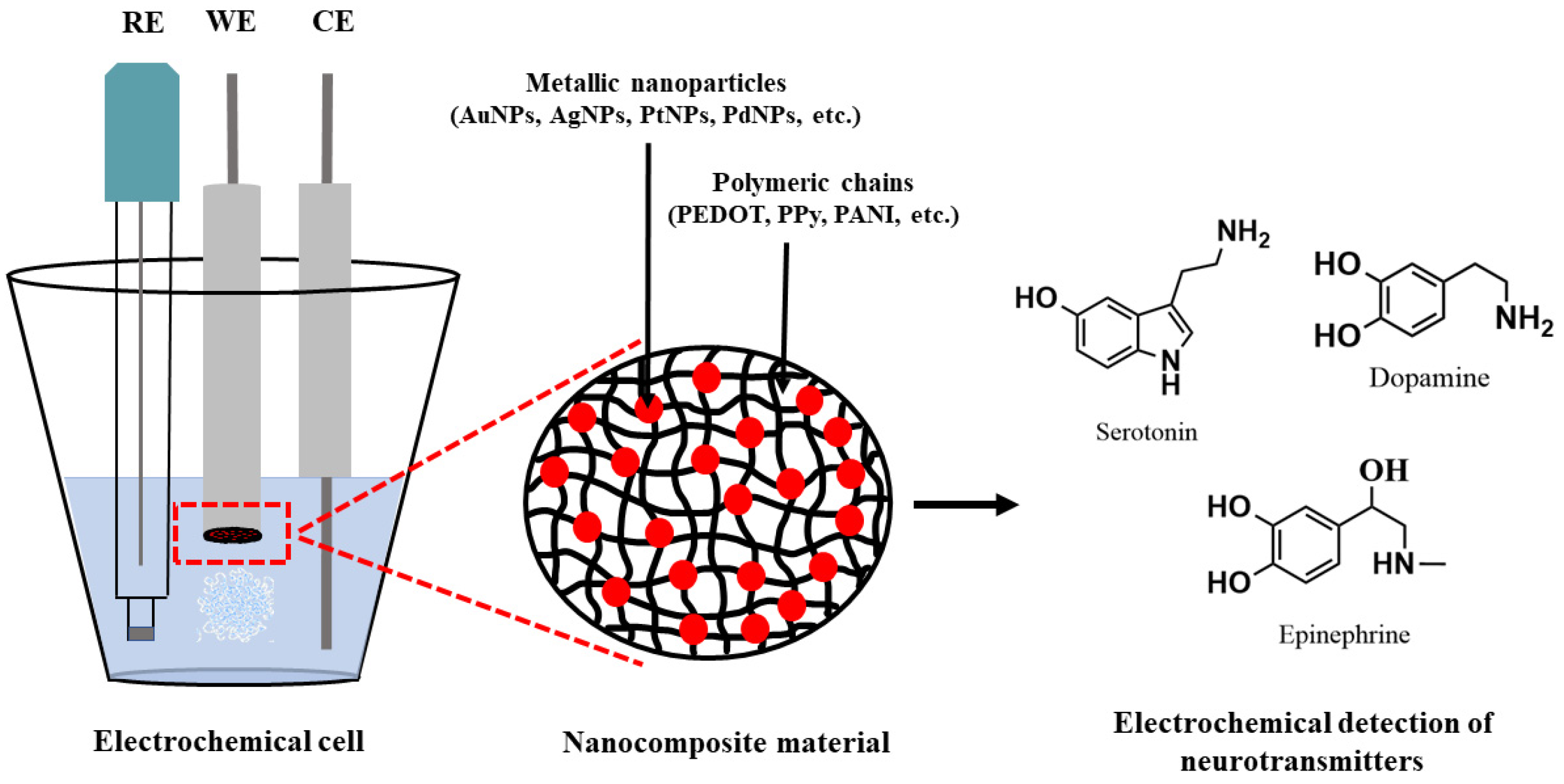
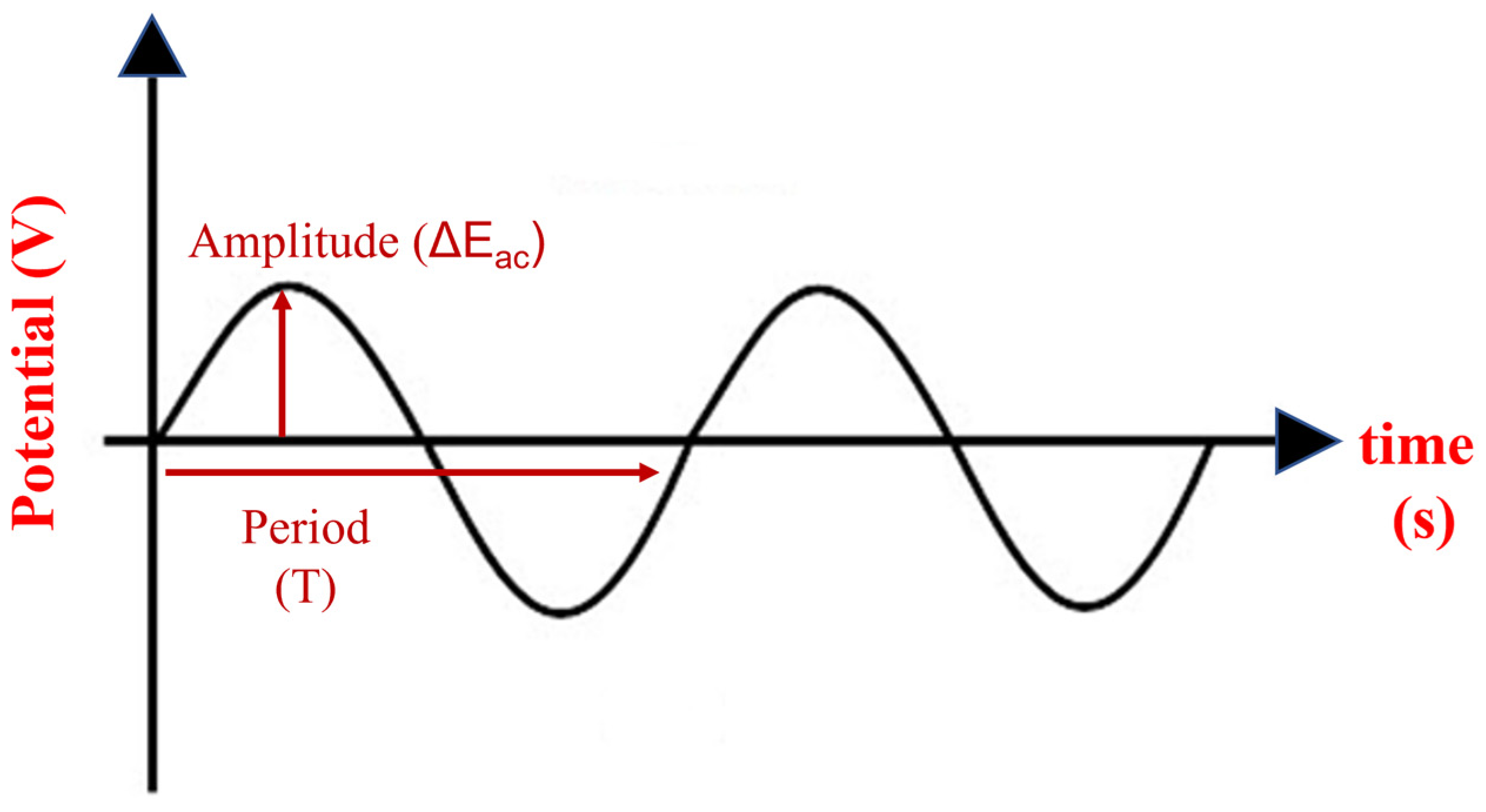
| Electrode | Detection Method | LOD (µM) | Linear Response Range (µM) | Sensitivity (µA µM−1) | Sample Matrix | Refs. |
|---|---|---|---|---|---|---|
| Au@PSi-P3HT/GCE | CA | 6.3 × 10−1 | 1–460 | 0.5112 | 0.1 M PBS (pH = 7) | [58] |
| AuNPs/PM/CPE | DPV | 6.7 × 10−2 | 2 × 10−1–11 | - | 0.1 M PBS (pH = 6) | [59] |
| AuNPs/PAN/ITO | CA | 9.1 × 10−1 | 1–102 | 0.0928 | 0.1 M H2SO4 (pH = 1) | [60] |
| PT/Au/CNT/ITO/glass | DPV | 6.9 × 10−1 | 1–10 | 19.492 | 1 mM acetate buffer (pH = 4) | [61] |
| Au/PEDOT-Aunano…SDS | LSV | 3.9 × 10−4 | 5 × 10−1–20 | 0.0381 | 0.1 M PBS (pH = 7.4) | [31] |
| AuNPs/PTAP/GCE | DPV | 1.7 × 10−2 | 15 × 10−2–15 × 10−1 | 6.580 | 0.1 M PBS (pH = 7.2) | [62] |
| OPEDOT/AuNPs/ERGO/GCE | SWV | 1 | 4–102 | - | 10 mM PBS (pH = 7.4) | [63] |
| Ag/PANI/GCE | CA | 1.9 | 10–90 | 0.102 | 0.1 M PBS (pH = 6) | [64] |
| PEDOT/AgNPs/CNCC/GCE | CA | 1.7 × 10−2 | 5 × 10−2–782 | - | 0.2 M PBS (pH = 7.4) | [65] |
| POA@Ag/GCE | CA | 8.3 × 10−1 | 5–45 | - | 0.1 M PBS (pH = 6) | [66] |
| Cu/PPy/GCE | DPV | 8.5 × 10−4 | 10−3–10−1 | - | 0.1 M PBS (pH = 7) | [67] |
| Pt/PF (BE)/Pd (CV) | DPV | 4.8 × 10−2 | 5 × 10−1–102 | 0.478 | 0.1 M H2SO4 | [68] |
| Pt/PMT/Pdnano | DPV | 9 × 10−3 | 5 × 10−2–1 | 1.37 | 0.1 M PBS (pH = 7.4) | [69] |
| Pt/PMPy/Pdnano | DPV | 1.2 × 10−2 | 10−1–10 | 0.71 | 0.1 M PBS (pH = 7.4) | [70] |
| Electrode | Detection Method | LOD (µM) | Linear Response Range (µM) | Sensitivity (µA µM−1) | Sample Matrix | Refs. |
|---|---|---|---|---|---|---|
| AuNPs@PPy/GSPE | SWV | 33.22 × 10−3 | 10−1–15 | 0.3316 | 0.02 M PBS (pH = 7.4) | [34] |
| AuNPs@rGO/pTBA-Pd (C2H4N2S2)2/NF | SWV | 2.5 × 10−3 | 2 × 10−2–2 × 102 | - | 0.1 M PBS (pH = 7.4) | [81] |
| nano-Au/PPyox/GCE | DPV | 1 × 10−3 | 7 × 10−3–22 × 10−1 | - | 0.1 M PBS (pH = 7) | [82] |
| PANIS/Au/GCE | DPV | 25 × 10−3 | 3 × 10−1–103 | - | 0.1 M PBS (pH = 7.4) | [83] |
| rGO/PANI/AuNPs@MIPs | DPV | 11.7 × 10−3 | 2 × 10−1–10 | - | 0.1 M PBS (pH = 7.5) | [84] |
| Ag/PPy/Cu2O/GCE | DPV | 124 × 10−4 | 10−2–250 | - | 0.1 M PBS (pH = 7.2) | [85] |
| Pt/MWCNT/PPy/AgNPs | DPV | 15 × 10−2 | 5 × 10−1–5 | - | 0.2 M PBS (pH = 8) | [86] |
| PEDOTNTs/rGO/AgNPs/GCE | DPV | 1 × 10−4 | 10−3–5 × 10−2 | 14.304 | 0.1 M PBS (pH = 8) | [38] |
| PtNPs/OPPy/rGO/GCE | DPV | 106 × 10−3 | 10–470 | - | 0.1 M PBS (pH = 7) | [87] |
| P-Arg/ErGO/AuNP/GCE | DPV | 30 × 10−3 | 10−2–5 × 10−1 | 5.97 | 0.1 M PBS (pH = 7) | [88] |
| CNTs-Cu2O-CuO@Pt | CA | 3 × 10−3 | 10–5 × 102 | - | 0.1 M PBS (pH = 7.4) | [89] |
| Electrode | Detection Method | LOD (µM) | Linear Response Range (µM) | Sensitivity (µA µM−1) | Sample Matrix | Refs. |
|---|---|---|---|---|---|---|
| MIP/AuNPs/GCE | DPV | 7.6 × 10−2 | 9 × 10−2–102 | - | 0.1 M PBS (pH = 7) | [97] |
| Au/ZnO/PPy/RGO/GCE | DPV | 6 × 10−2 | 6 × 10−1–5 × 102 | - | 0.1 M PBS (pH = 7) | [98] |
| Au/PILs/PPyNTs/GCE | DPV | 298.9 × 10−3 | 35–960 | 42.7799 | 0.05 M PBS (pH = 7.4) | [99] |
| GNPs/Pan-LB/GCE | SWV | 8 × 10−2 | 4 × 10−1–10 | - | 0.2 M PBS (pH = 6) | [100] |
| PPy/AuNPs/SWCNTs-AuE | DPV | 2 × 10−3 | 4 × 10−3–10−1 | - | 0.05 M PBS (pH = 7) | [101] |
| Nano-Au/PPyox/GCE | DPV | 3 × 10−2 | 3 × 10−1–21 | - | 0.1 M PBS (pH = 7) | [102] |
| AuNPs/TGA/CS-MWCNTs | CA | 60 × 10−3 | 4 × 10−1–11 | 2.31 | 0.1 M PBS (pH = 7) | [103] |
| AuNPs/PDA/AN | DPV | 0.26 | 1–103 | - | 0.1 M PBS (pH = 7.4) | [104] |
| Au nanoporous film/AuE | CV | 19 | 50–103 | - | 0.01 M PBS (pH = 7) | [105] |
| rGO/AgNPs cotton and rGO/AgNPs/polyester | SWV DPV | 9.73 × 10−3 3.05 × 10−3 | 0.5–40 1–30 | - | 0.1 M PBS (pH = 7) | [106] |
| AuPt@GR | CA | 0.9 × 10−3 | 15 × 10−4–96 × 10−1 | 1628 | 0.1 M PBS (pH = 7.4) | [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leau, S.-A.; Lete, C.; Lupu, S. Nanocomposite Materials based on Metal Nanoparticles for the Electrochemical Sensing of Neurotransmitters. Chemosensors 2023, 11, 179. https://doi.org/10.3390/chemosensors11030179
Leau S-A, Lete C, Lupu S. Nanocomposite Materials based on Metal Nanoparticles for the Electrochemical Sensing of Neurotransmitters. Chemosensors. 2023; 11(3):179. https://doi.org/10.3390/chemosensors11030179
Chicago/Turabian StyleLeau, Sorina-Alexandra, Cecilia Lete, and Stelian Lupu. 2023. "Nanocomposite Materials based on Metal Nanoparticles for the Electrochemical Sensing of Neurotransmitters" Chemosensors 11, no. 3: 179. https://doi.org/10.3390/chemosensors11030179
APA StyleLeau, S. -A., Lete, C., & Lupu, S. (2023). Nanocomposite Materials based on Metal Nanoparticles for the Electrochemical Sensing of Neurotransmitters. Chemosensors, 11(3), 179. https://doi.org/10.3390/chemosensors11030179









