The Actin-Binding Protein Cortactin Promotes Sepsis Severity by Supporting Excessive Neutrophil Infiltration into the Lung
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Induction of CLP (Cecal Ligation and Puncture) Sepsis
2.3. Histology
2.4. Haematology
2.5. Plasma Cytokines
2.6. Quantitative Real-Time RT-PCR (qRT-PCR)
2.7. Leukocyte Recruitment into the Lung
2.8. Peritoneal and Bronchoalveolar Lavage
2.9. Flow Cytometry
2.10. Bacterial Counts
2.11. Oxidative Stress Assay
2.12. Neutrophil Depletion
2.13. Statistical Analysis
3. Results
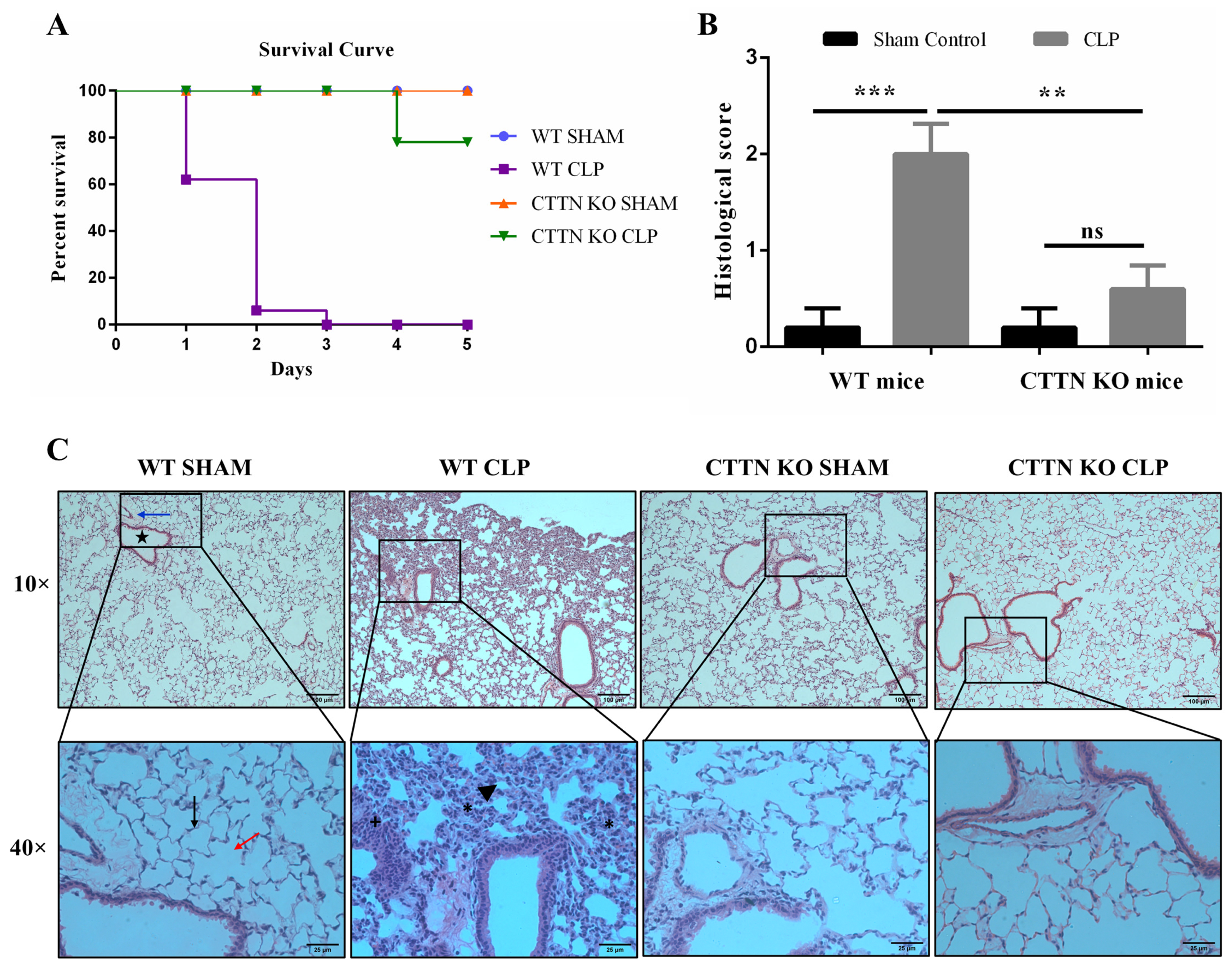
3.1. Cortactin Deficiency Improves Survival and Protects against Sepsis-Induced Lung Damage
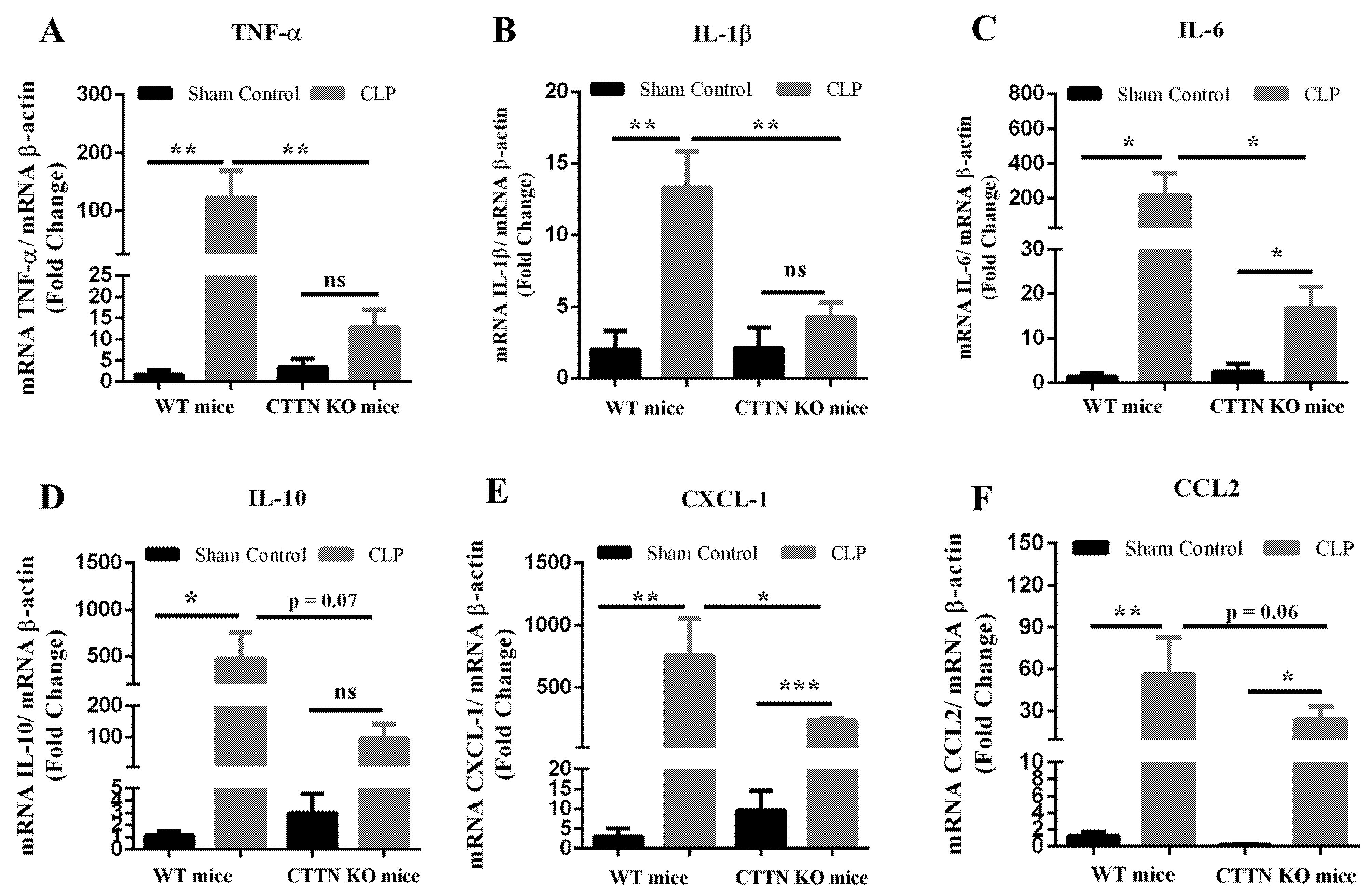
3.2. Cortactin Deficiency Attenuates the Inflammatory Response in the Lung during Sepsis
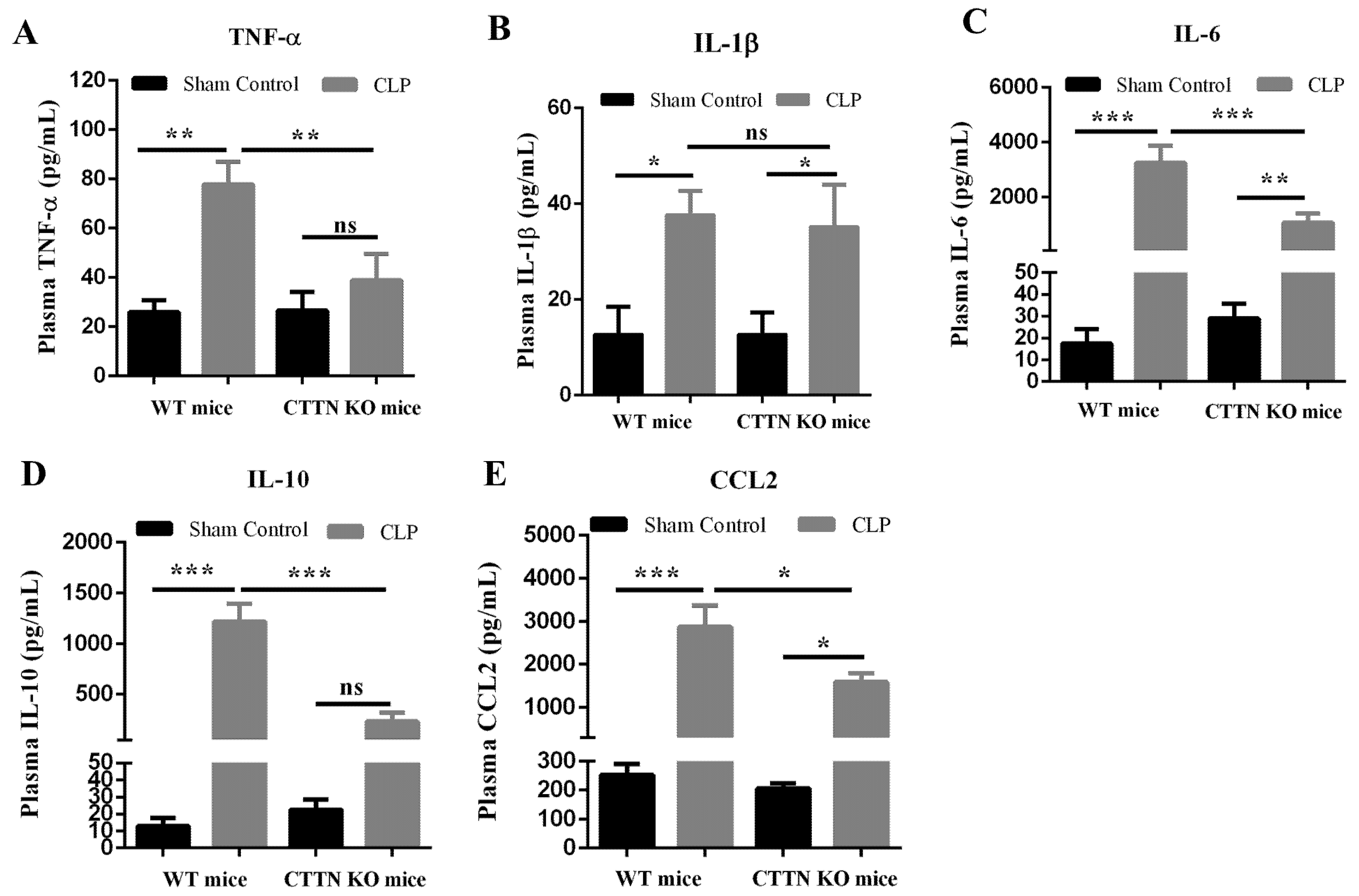
3.3. Genetic Disruption of Cortactin also Ameliorates Systemic Inflammation during Sepsis
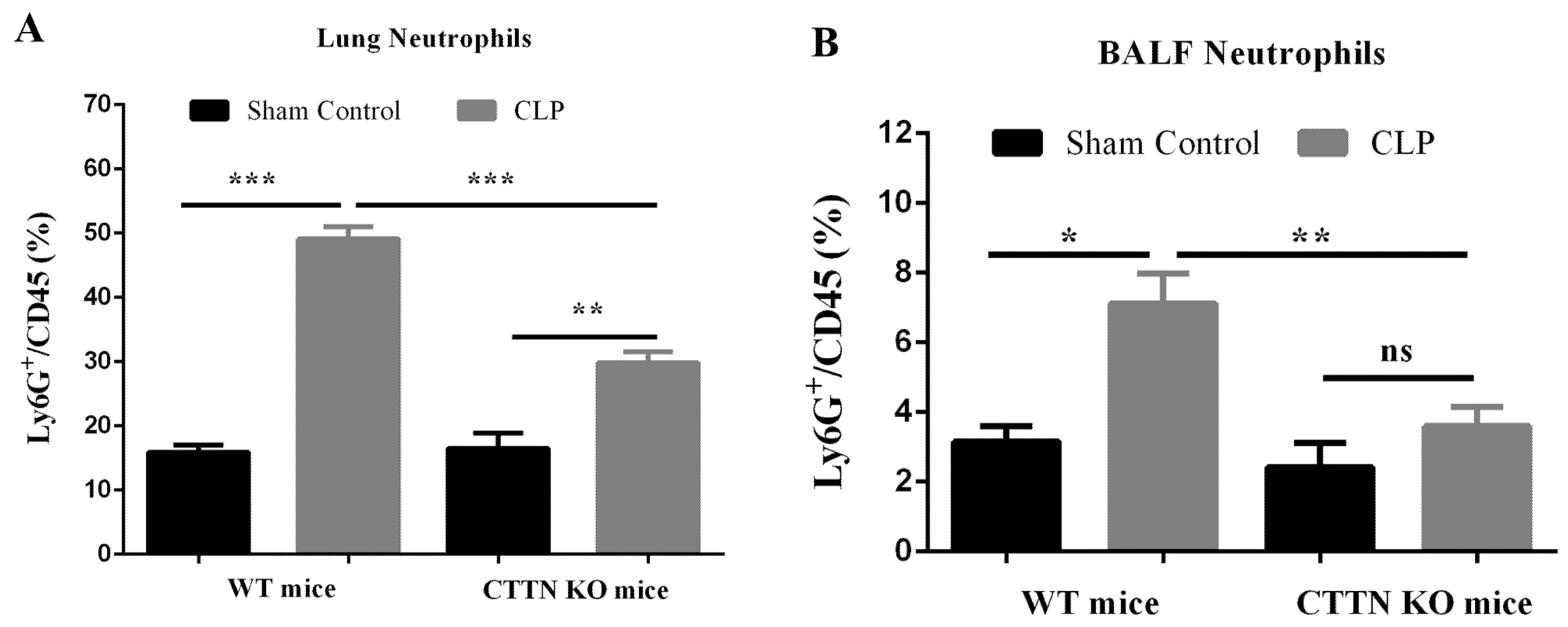
3.4. Septic CTTN KO Mice Show Reduced Lung Neutrophil Infiltration during Sepsis
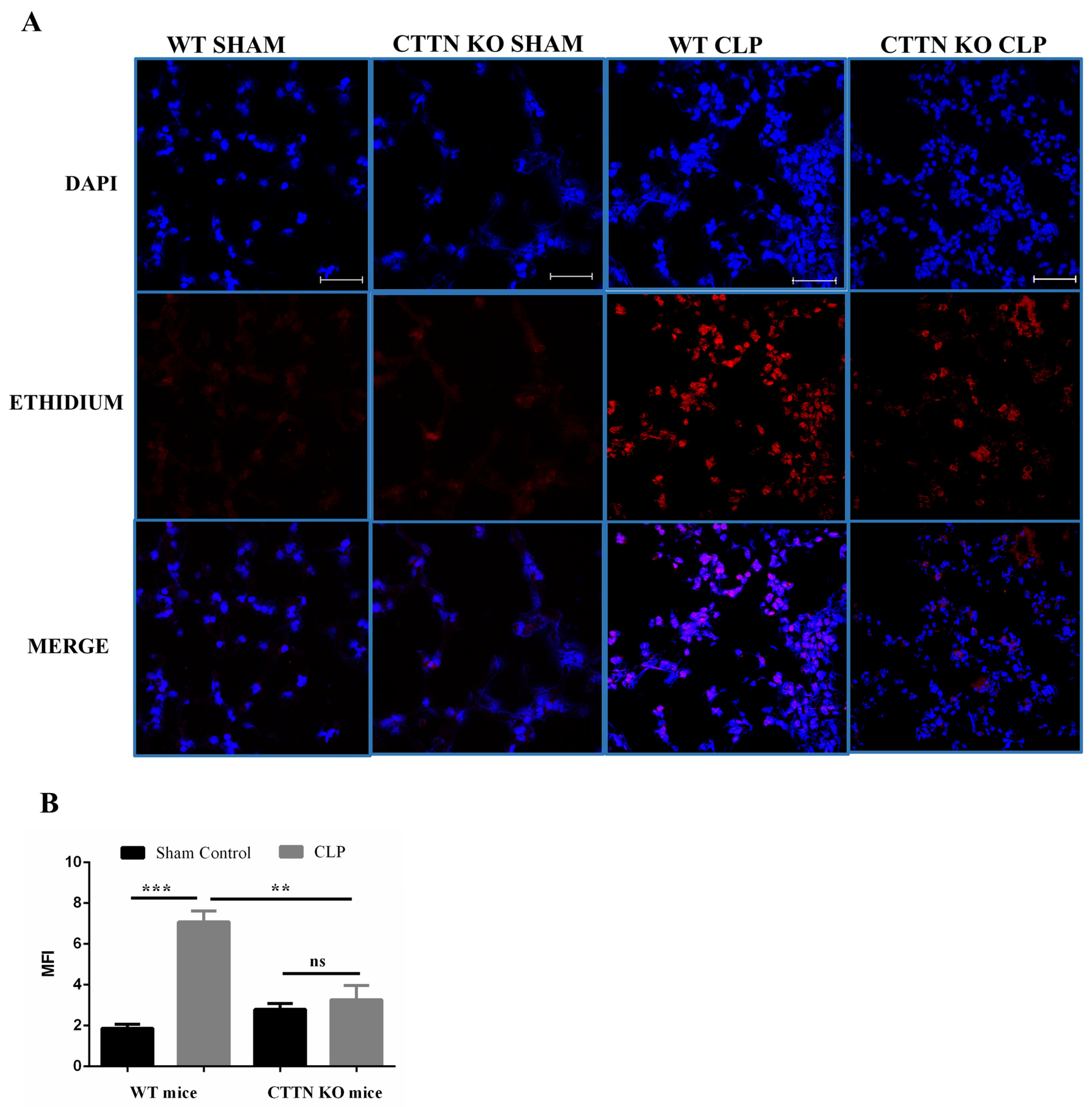
3.5. Oxidative Stress Is Ameliorated in the Lungs of Cortactin-Deficient Mice during Sepsis
3.6. Cortactin Deficiency Prevents Excessive Influx of Neutrophils into the Peritoneum without Affecting Bacteria Count
3.7. Septic Cortactin-Deficient Mice Do Not Develop Neutropenia
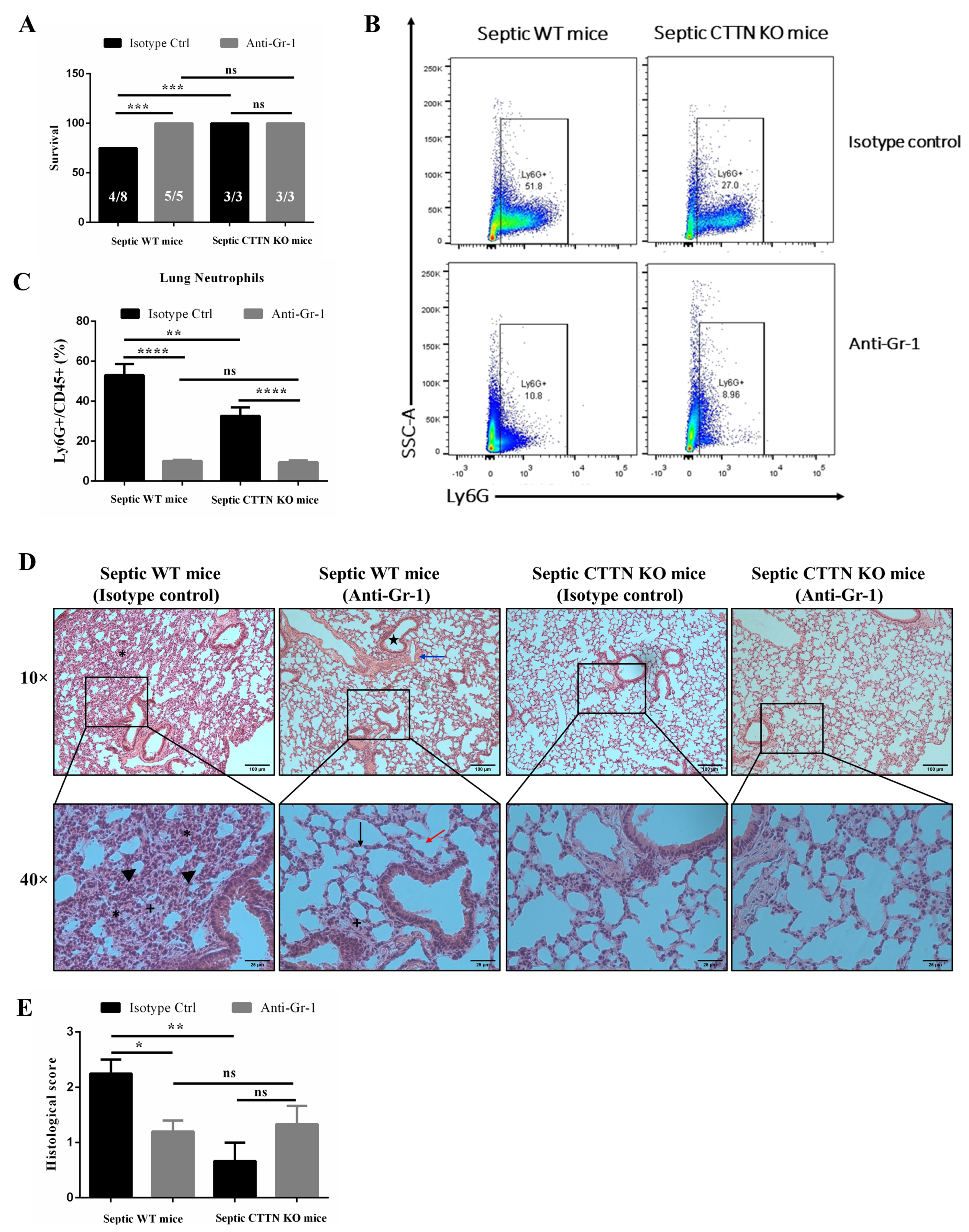
3.8. Delayed Neutrophil Depletion Provides No Additional Protective Effect in CTTN KO Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Angus, D.C.; Linde-Zwirble, W.T.; Lidicker, J.; Clermont, G.; Carcillo, J.; Pinsky, M.R. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001, 29, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.S.; Mannino, D.M.; Eaton, S.; Moss, M. The Epidemiology of Sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 2003, 348, 1546–1554. [Google Scholar] [CrossRef] [Green Version]
- Singer, M.; Deutschman, C.S.; Seymour, C.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA J. Am. Med. Assoc. 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Schnoor, M.; García Ponce, A.; Vadillo, E.; Pelayo, R.; Rossaint, J.; Zarbock, A. Actin dynamics in the regulation of endothelial barrier functions and neutrophil recruitment during endotoxemia and sepsis. Cell. Mol. Life Sci. 2017, 74, 1985–1997. [Google Scholar] [CrossRef] [PubMed]
- Evans, T. Diagnosis and management of sepsis. Clin. Med. J. R. Coll. Physicians Lond. 2018, 18, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Rosales, C. Neutrophil: A cell with many roles in inflammation or several cell types? Front. Physiol. 2018, 9, 113. [Google Scholar] [CrossRef]
- Selders, G.S.; Fetz, A.E.; Radic, M.Z.; Bowlin, G.L. An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration. Regen. Biomater. 2017, 4, 55–68. [Google Scholar] [CrossRef]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef]
- Hoesel, L.M.; Neff, T.A.; Neff, S.B.; Younger, J.G.; Olle, E.W.; Gao, H.; Pianko, M.; Bernacki, K.D.; Sarma, J.V.; Ward, P.A. Harmful and protective roles of neutrophils in sepsis. Shock 2005, 24, 40–47. [Google Scholar] [CrossRef]
- Brown, K.; Brain, S.; Pearson, J.; Edgeworth, J.; Lewis, S.; Treacher, D. Neutrophils in development of multiple organ failure in sepsis. Lancet 2006, 368, 157–169. [Google Scholar] [CrossRef]
- Ayala, A.; Chung, C.S.; Lomas, J.L.; Song, G.Y.; Doughty, L.A.; Gregory, S.H.; Cioffi, W.G.; LeBlanc, B.W.; Reichner, J.; Simms, H.H.; et al. Shock-induced neutrophil mediated priming for acute lung injury in mice: Divergent effects of TLR-4 and TLR-4/FasL deficiency. Am. J. Pathol. 2002, 161, 2283–2294. [Google Scholar] [CrossRef]
- Brealey, D.; Singer, M. Multi-organ dysfunction in the critically ill: Effects on different organs. J. R. Coll. Physicians Lond. 2000, 34, 428–431. [Google Scholar] [PubMed]
- Vestweber, D. How leukocytes cross the vascular endothelium. Nat. Rev. Immunol. 2015, 15, 692–704. [Google Scholar] [CrossRef]
- Daly, R.J. Cortactin signalling and dynamic actin networks. Biochem. J. 2004, 382, 13–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnoor, M.; Lai, F.P.L.; Zarbock, A.; Kläver, R.; Polaschegg, C.; Schulte, D.; Weich, H.A.; Oelkers, J.M.; Rottner, K.; Vestweber, D. Cortactin deficiency is associated with reduced neutrophil recruitment but increased vascular permeability in vivo. J. Exp. Med. 2011, 208, 1721–1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaefer, A.; te Riet, J.; Ritz, K.; Hoogenboezem, M.; Anthony, E.C.; Mul, F.P.J.; de Vries, C.J.; Daemen, M.J.; Figdor, C.G.; van Buul, J.D. Actin-binding proteins differentially regulate endothelial cell stiffness, ICAM-1 function and neutrophil transmigration. J. Cell Sci. 2014, 127, 4470–4482. [Google Scholar] [CrossRef] [Green Version]
- Toscano, M.G.; Ganea, D.; Gamero, M. Cecal ligation puncture procedure. J. Vis. Exp. 2011, 51, e2860. [Google Scholar] [CrossRef]
- Citalán-Madrid, A.F.; Vargas-Robles, H.; García-Ponce, A.; Shibayama, M.; Betanzos, A.; Nava, P.; Salinas-Lara, C.; Rottner, K.; Mennigen, R.; Schnoor, M. Cortactin deficiency causes increased RhoA/ROCK1-dependent actomyosin contractility, intestinal epithelial barrier dysfunction, and disproportionately severe DSS-induced colitis. Mucosal Immunol. 2017, 10, 1237–1247. [Google Scholar] [CrossRef] [Green Version]
- Lartey, N.L.; Valle-Reyes, S.; Vargas-Robles, H.; Jiménez-Camacho, K.E.; Guerrero-Fonseca, I.M.; Castellanos-Martínez, R.; Montoya-Garcia, A.; Garcia-Cordero, J.; Cedillo-Barron, L.; Nava, P. ADAM17/MMP inhibition prevents neutrophilia and lung injury in a mouse model of COVID-19. J. Leukoc. Biol. 2021, 1–12. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Xiao, G.; Qu, Z. Murine Bronchoalveolar Lavage. Bio-Protocol 2017, 7, e2287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erickson, S.E.; Martin, G.S.; Davis, J.L.; Matthay, M.A.; Eisner, M.D. Recent trends in acute lung injury mortality: 1996–2005. Crit. Care Med. 2009, 37, 1574–1579. [Google Scholar] [CrossRef] [PubMed]
- Sônego, F.; Castanheira, F.V.E.S.; Ferreira, R.G.; Kanashiro, A.; Leite, C.A.V.G.; Nascimento, D.C.; Colón, D.F.; Borges, V.D.F.; Alves-Filho, J.C.; Cunha, F.Q. Paradoxical roles of the neutrophil in sepsis: Protective and deleterious. Front. Immunol. 2016, 7, 155. [Google Scholar] [CrossRef] [Green Version]
- Chousterman, B.G.; Swirski, F.K.; Weber, G.F. Cytokine storm and sepsis disease pathogenesis. Semin. Immunopathol. 2017, 39, 517–528. [Google Scholar] [CrossRef]
- Asaduzzaman, M.; Zhang, S.; Lavasani, S.; Wang, Y.; Thorlacius, H. LFA-1 and Mac-1 mediate pulmonary recruitment of neutrophils and tissue damage in abdominal sepsis. Shock 2008, 30, 254–259. [Google Scholar] [CrossRef]
- Basit, A.; Reutershan, J.; Morris, M.A.; Solga, M.; Rose, C.E.; Ley, K. ICAM-1 and LFA-1 play critical roles in LPS-induced neutrophil recruitment into the alveolar space. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 291, L200–L207. [Google Scholar] [CrossRef] [Green Version]
- Czermak, B.J.; Breckwoldt, M.; Ravage, Z.B.; Huber-Lang, M.; Schmal, H.; Bless, N.M.; Fried, H.P.; Ward, P.A. Mechanisms of enhanced lung injury during sepsis. Am. J. Pathol. 1999, 154, 1057–1065. [Google Scholar] [CrossRef] [Green Version]
- Grommes, J.; Soehnlein, O. Contribution of neutrophils to acute lung injury. Mol. Med. 2011, 17, 293–307. [Google Scholar] [CrossRef]
- Guo, R.F.; Ward, P.A. Role of oxidants in lung injury during sepsis. Antioxid. Redox Signal. 2007, 9, 1991–2002. [Google Scholar] [CrossRef]
- Craciun, F.L.; Schuller, E.R.; Remick, D.G. Early Enhanced Local Neutrophil Recruitment in Peritonitis-Induced Sepsis Improves Bacterial Clearance and Survival. J. Immunol. 2010, 185, 6930–6938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganopolsky, J.G.; Castellino, F.J. A protein C deficiency exacerbates inflammatory and hypotensive responses in mice during polymicrobial sepsis in a cecal ligation and puncture model. Am. J. Pathol. 2004, 165, 1433–1446. [Google Scholar] [CrossRef] [Green Version]
- Bauer, P.; Lush, C.W.; Kvietys, P.R.; Russell, J.M.; Granger, D.N. Role of endotoxin in the expression of endothelial selectins after cecal ligation and perforation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 278, R1140–R1147. [Google Scholar] [CrossRef] [PubMed]
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The multifaceted functions of neutrophils. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 181–218. [Google Scholar] [CrossRef] [Green Version]
- Cilloniz, C.; Peroni, H.J.; Gabarrús, A.; García-Vidal, C.; Pericàs, J.M.; Bermejo-Martin, J.; Torres, A. Lymphopenia Is Associated with Poor Outcomes of Patients with Community-Acquired Pneumonia and Sepsis. Open Forum Infect. Dis. 2021, 8, ofab169. [Google Scholar] [CrossRef]
- Girardot, T.; Rimmelé, T.; Venet, F.; Monneret, G. Apoptosis-induced lymphopenia in sepsis and other severe injuries. Apoptosis 2017, 22, 295–305. [Google Scholar] [CrossRef]
- Vardon Bounes, F.; Mémier, V.; Marcaud, M.; Jacquemin, A.; Hamzeh-Cognasse, H.; Garcia, C.; Minville, V.; Gratacap, M.P.; Payrastre, B. Platelet activation and prothrombotic properties in a mouse model of peritoneal sepsis. Sci. Rep. 2018, 8, 13536. [Google Scholar] [CrossRef]
- Zarbock, A.; Polanowska-Grabowska, R.K.; Ley, K. Platelet-neutrophil-interactions: Linking hemostasis and inflammation. Blood Rev. 2007, 21, 99–111. [Google Scholar] [CrossRef]
- Luo, S.; Wang, Y.; An, Q.; Chen, H.; Zhao, J.; Zhang, J.; Meng, W.; Du, L. Platelets protect lung from injury induced by systemic inflammatory response. Sci. Rep. 2017, 7, 42080. [Google Scholar] [CrossRef] [Green Version]
- Jimenez, M.F.; Watson, R.W.G.; Parodo, J.; Evans, D.; Foster, D.; Steinberg, M.; Rotstein, O.D.; Marshall, J.C. Dysregulated expression of neutrophil apoptosis in the systemic inflammatory response syndrome. Arch. Surg. 1997, 132, 1263–1270. [Google Scholar] [CrossRef]
- Angus, D.C. The search for effective therapy for sepsis: Back to the drawing board? JAMA J. Am. Med. Assoc. 2011, 306, 2614–2615. [Google Scholar] [CrossRef] [PubMed]
- Henes, J.; Schmit, M.A.; Morote-Garcia, J.C.; Mirakaj, V.; Köhler, D.; Glover, L.; Eldh, T.; Walter, U.; Karhausen, J.; Colgan, S.P.; et al. Inflammation-associated repression of vasodilator-stimulated phosphoprotein (VASP) reduces alveolar-capillary barrier function during acute lung injury. FASEB J. 2009, 23, 4244–4255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aird, W.C. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ. Res. 2007, 100, 158–173. [Google Scholar] [CrossRef] [PubMed]
- Hack, C.E.; Aarden, L.A.; Thijs, L.G. Role of cytokines in sepsis. Adv. Immunol. 1997, 66, 101–195. [Google Scholar]
- Chaudhry, H.; Zhou, J.; Zhong, Y.; Ali, M.M.; Mcguire, F.; Nagarkatti, P.S.; Nagarkatti, M. Role of cytokines as a double-edged sword in sepsis. In Vivo 2013, 27, 669–684. [Google Scholar]
- Schulte, W.; Bernhagen, J.; Bucala, R. Cytokines in sepsis: Potent immunoregulators and potential therapeutic targets—An updated view. Mediat. Inflamm. 2013, 2013, 165974. [Google Scholar] [CrossRef]
- Ohlsson, K.; Björk, P.; Bergenfeldt, M.; Hageman, R.; Thompson, R.C. Interleukin-1 receptor antagonist reduces mortality from endotoxin shock. Nature 1990, 348, 550–552. [Google Scholar] [CrossRef]
- Riedemann, N.C.; Neff, T.A.; Guo, R.-F.; Bernacki, K.D.; Laudes, I.J.; Sarma, J.V.; Lambris, J.; Ward, P.A. Protective Effects of IL-6 Blockade in Sepsis Are Linked to Reduced C5a Receptor Expression. J. Immunol. 2003, 170, 503–507. [Google Scholar] [CrossRef] [Green Version]
- Tracey, K.J.; Fong, Y.; Hesse, D.G.; Manogue, K.R.; Lee, A.T.; Kuo, G.C.; Lowry, S.F.; Cerami, A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature 1987, 330, 662–664. [Google Scholar] [CrossRef]
- Li, Q.; Verma, I.M. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.F.; Malik, A.B. NF-κB activation as a pathological mechanism of septic shock and inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 290, L622–L645. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Lei, X.; Wu, L.; Liu, L. The role of endothelial cell adhesion molecules P-selectin, E-selectin and intercellular adhesion molecule-1 in leucocyte recruitment induced by exogenous methylglyoxal. Immunology 2012, 137, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Samus, M.; Li, Y.T.; Sorokin, L.; Rottner, K.; Vestweber, D. Actin-binding protein cortactin promotes pathogenesis of experimental autoimmune encephalomyelitis by supporting leukocyte infiltration into the central nervous system. J. Neurosci. 2020, 40, 1389–1404. [Google Scholar] [CrossRef]
- Tegtmeyer, N.; Soltan Esmaeili, D.; Sharafutdinov, I.; Knorr, J.; Naumann, M.; Alter, T.; Backert, S. Importance of cortactin for efficient epithelial NF-ĸB activation by Helicobacter pylori, Salmonella enterica and Pseudomonas aeruginosa, but not Campylobacter spp. Eur. J. Microbiol. Immunol. 2022, 11, 95–103. [Google Scholar] [CrossRef]
- Hildebrand, F.; Pape, H.C.; Harwood, P.; Müller, K.; Hoevel, P.; Pütz, C.; Siemann, A.; Krettek, C.; van Griensven, M. Role of adhesion molecule ICAM in the pathogenesis of polymicrobial sepsis. Exp. Toxicol. Pathol. 2005, 56, 281–290. [Google Scholar] [CrossRef]
- De Oliveira, S.; Rosowski, E.E.; Huttenlocher, A. Neutrophil migration in infection and wound repair: Going forward in reverse. Nat. Rev. Immunol. 2016, 16, 378–391. [Google Scholar] [CrossRef] [Green Version]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Jin, L.; Batra, S.; Douda, D.N.; Palaniyar, N.; Jeyaseelan, S. CXCL1 Contributes to Host Defense in Polymicrobial Sepsis via Modulating T Cell and Neutrophil Functions. J. Immunol. 2014, 193, 3549–3558. [Google Scholar] [CrossRef]
- Vermont, C.L.; Hazelzet, J.A.; de Kleijn, E.D.; Van den Dobbelsteen, G.P.J.M.; de Groot, R. CC and CXC chemokine levels in children with meningococcal sepsis accurately predict mortality and disease severity. Crit. Care. 2006, 10, R33. [Google Scholar] [CrossRef] [Green Version]
- Bozza, F.A.; Salluh, J.I.; Japiassu, A.M.; Soares, M.; Assis, E.F.; Gomes, R.N.; Bozza, M.T.; Castro-Faria-Neto, H.C.; Bozza, P.T. Cytokine profiles as markers of disease severity in sepsis: A multiplex analysis. Crit. Care. 2007, 11, R49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souto, F.O.; Alves-Filho, J.C.; Turato, W.M.; Auxiliadora-Martins, M.; Basile-Filho, A.; Cunha, F.Q. Essential role of CCR2 in neutrophil tissue infiltration and multiple organ dysfunction in sepsis. Am. J. Respir. Crit. Care Med. 2011, 183, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Cebinelli, G.C.M.; de Lima, K.A.; Silva Castanheira, F.V.E.; Hiroki, C.H.; Monteiro, V.V.S.; de Lima, M.H.F.; Nascimento, D.C.B.; Alves Filho, J.C.; Cunha, T.M.; Cunha, F.D.Q. CCR2-deficient mice are protected to sepsis by the disruption of the inflammatory monocytes emigration from the bone marrow. J. Leukoc. Biol. 2021, 109, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Ramnath, R.D.; Ng, S.W.; Guglielmotti, A.; Bhatia, M. Role of MCP-1 in endotoxemia and sepsis. Int. Immunopharmacol. 2008, 8, 810–818. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Nicholson, D.W. Apoptosis and caspases regulate death and inflammation in sepsis. Nat. Rev. Immunol. 2006, 6, 813–822. [Google Scholar] [CrossRef]
- Taneja, R.; Parodo, J.; Song, H.J.; Kapus, A.; Rotstein, O.D.; Marshall, J.C. Delayed neutrophil apoptosis in sepsis is associated with maintenance of mitochondrial transmembrane potential and reduced caspase-9 activity. Crit. Care Med. 2004, 32, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.F.; Cao, K.; Jiang, J.P.; Guan, W.X.; Du, J.F. Neutrophil dysregulation during sepsis: An overview and update. J. Cell. Mol. Med. 2017, 21, 1687–1697. [Google Scholar] [CrossRef] [Green Version]
- Treacher, D.F.; Sabbato, M.; Brown, K.A.; Gant, V. The effects of leucodepletion in patients who develop the systemic inflammatory response syndrome following cardiopulmonary bypass. Perfusion 2001, 16 (Suppl. S1), 67–73. [Google Scholar] [CrossRef]
- Zhou, M.Y.; Lo, S.K.; Bergenfeldt, M.; Tiruppathi, C.; Jaffe, A.; Xu, N.; Malik, A.B. In vivo expression of neutrophil inhibitory factor via gene transfer prevents lipopolysaccharide-induced lung neutrophil infiltration and injury by a β2 integrin-dependent mechanism. J. Clin. Investig. 1998, 101, 2427–2437. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Li, L. Reduced oxidative tissue damage during endotoxemia in IRAK-1 deficient mice. Mol. Immunol. 2012, 50, 244–252. [Google Scholar] [CrossRef] [Green Version]
- Guerrero-Fonseca, I.M.; García-Ponce, A.; Vadillo, E.; Lartey, N.L.; Vargas-Robles, H.; Chánez-Paredes, S.; Castellanos-Martínez, R.; Nava, P.; Betanzos, A.; Neumann, B.M.; et al. HS1 deficiency protects against sepsis by attenuating neutrophil-inflicted lung damage. Eur. J. Cell Biol. 2022, 101, 151214. [Google Scholar] [CrossRef] [PubMed]
- Chiswick, E.L.; Mella, J.R.; Bernardo, J.; Remick, D.G. Acute-Phase Deaths from Murine Polymicrobial Sepsis Are Characterized by Innate Immune Suppression Rather Than Exhaustion. J. Immunol. 2015, 195, 3793–3802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotchkiss, R.S.; Strasser, A.; McDunn, J.E.; Swanson, P.E. Mechanisms of disease: Cell death. N. Engl. J. Med. 2009, 361, 1570–1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lartey, N.L.; Vargas-Robles, H.; Guerrero-Fonseca, I.M.; García-Ponce, A.; Salinas-Lara, C.; Rottner, K.; Schnoor, M. The Actin-Binding Protein Cortactin Promotes Sepsis Severity by Supporting Excessive Neutrophil Infiltration into the Lung. Biomedicines 2022, 10, 1019. https://doi.org/10.3390/biomedicines10051019
Lartey NL, Vargas-Robles H, Guerrero-Fonseca IM, García-Ponce A, Salinas-Lara C, Rottner K, Schnoor M. The Actin-Binding Protein Cortactin Promotes Sepsis Severity by Supporting Excessive Neutrophil Infiltration into the Lung. Biomedicines. 2022; 10(5):1019. https://doi.org/10.3390/biomedicines10051019
Chicago/Turabian StyleLartey, Nathaniel L., Hilda Vargas-Robles, Idaira M. Guerrero-Fonseca, Alexander García-Ponce, Citlaltepetl Salinas-Lara, Klemens Rottner, and Michael Schnoor. 2022. "The Actin-Binding Protein Cortactin Promotes Sepsis Severity by Supporting Excessive Neutrophil Infiltration into the Lung" Biomedicines 10, no. 5: 1019. https://doi.org/10.3390/biomedicines10051019






