Antipsychotic Drugs Efficacy in Dextromethorphan-Induced Psychosis
Abstract
:1. Introduction
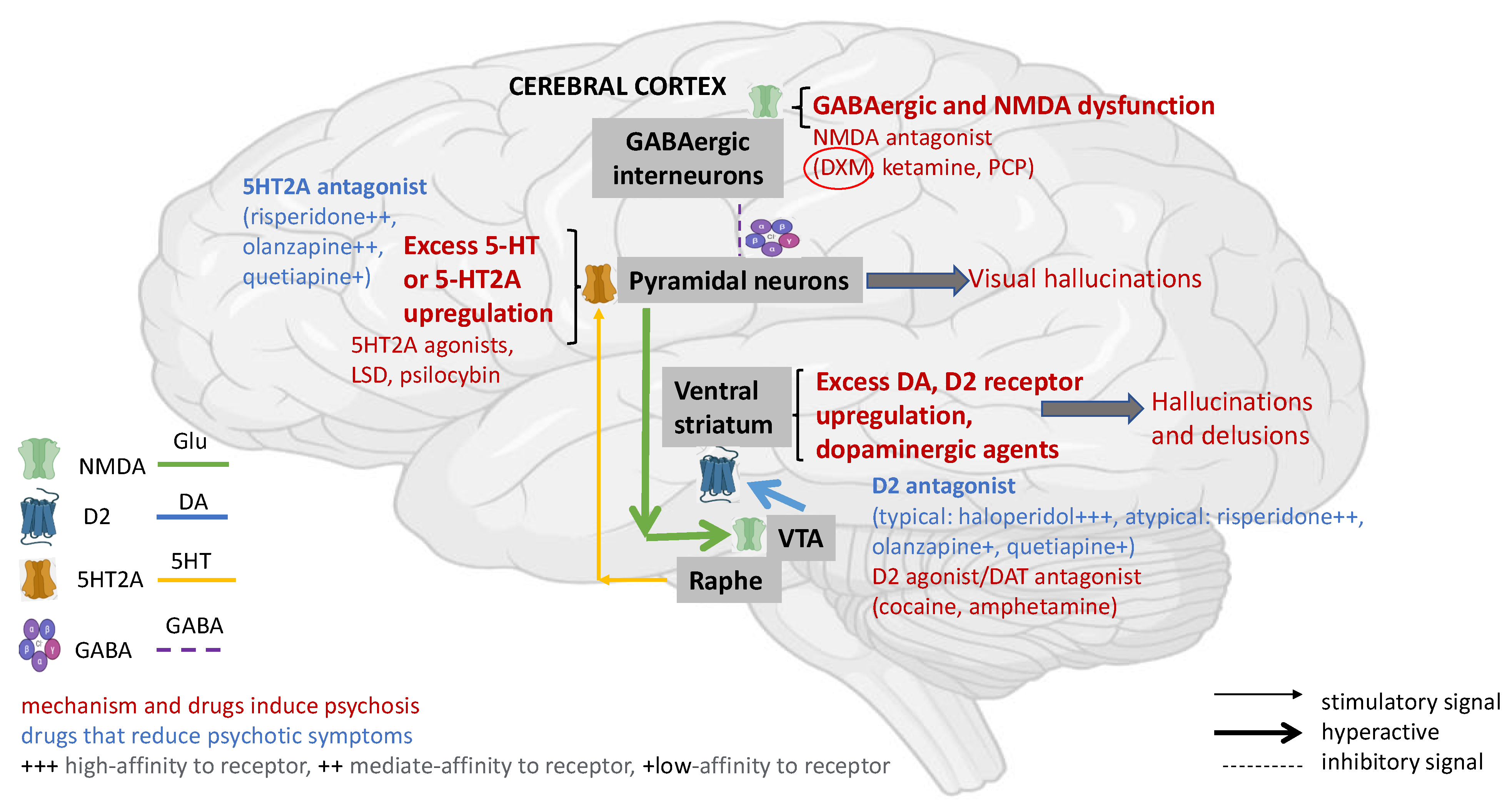
2. Pathophysiology of DXM Toxicity
3. Treatment of DXM Toxicity—Role of Antipsychotics
3.1. DXM Toxicity
3.2. Psychosis Induced by DXM Intoxication
4. Antipsychotics—General Overview
5. Antipsychotics in the Treatment of DXM-Induced Psychoses
| Receptor | Haloperidol | Risperidone | Olanzapine | Quetiapine |
|---|---|---|---|---|
| D2 | 0.1 | 0.5 | 4.0 | 30 |
| 5-HT2A | 50 | 0.2 | 2.5 | 250 |
 Data are Ki values in nM derived from functional antagonist R-SAT™ assays. Abbreviations: Ki (nM)—binding affinity data; D2—dopamine 2 receptor; 5-HT2A—serotonin 2A receptor. According to [72] with modification.
Data are Ki values in nM derived from functional antagonist R-SAT™ assays. Abbreviations: Ki (nM)—binding affinity data; D2—dopamine 2 receptor; 5-HT2A—serotonin 2A receptor. According to [72] with modification.6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FDA Approved Drug Products: Nuedexta Dextromethorphan Hydrobromide and Quinidine Sulfate Oral Capsules. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/021879s014lbl.pdf (accessed on 10 November 2022).
- Nguyen, L.; Thomas, K.L.; Lucke-Wold, B.P.; Cavendish, J.Z.; Crowe, M.S.; Matsumoto, R.R. Dextromethorphan: An update on its utility for neurological and neuropsychiatric disorders. Pharmacol. Ther. 2016, 159, 1–22. [Google Scholar] [CrossRef]
- Brown, G.R.; McLaughlin, K.; Vaughn, K. Identifying and treating patients with synthetic psychoactive drug intoxication. JAAPA 2018, 31, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Journey, J.D.; Agrawal, S.; Stern, E. Dextromethorphan Toxicity. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538502/ (accessed on 13 October 2022).
- Łukasik-Głębocka, M.; Sommerfeld, K. Acute dextromethorphan poisoning based on the records of the Department of Toxicology and Internal Diseases in Poznan. Przegl. Lek. 2009, 66, 853–856. [Google Scholar]
- Bryner, J.K.; Wang, U.K.; Hui, J.W.; Bedodo, M.; MacDougall, C.; Anderson, I.B. Dextromethorphan abuse in adolescence: An increasing trend: 1999–2004. Arch. Pediatr. Adolesc. Med. 2006, 160, 1217–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Non-Medical Use of Medicines: Health and Social Responses. Available online: https://www.emcdda.europa.eu/publications/mini-guides/non-medical-use-of-medicines-health-and-social-responses_sl (accessed on 10 October 2022).
- Morris, H.; Wallach, J. From PCP to MXE: A comprehensive review of the non-medical use of dissociative drugs. Drug Test Anal. 2014, 6, 614–632. [Google Scholar] [CrossRef]
- The Consumer Healthcare Products Association (CHPA). Preventing Dextromethorphan Abuse. Available online: https://www.chpa.org/about-consumer-healthcare/activities-initiatives/preventing-dextromethorphan-abuse (accessed on 21 November 2022).
- Traynor, K. Advisers vote against declaring dextromethorphan a controlled substance. Am. J. Health Syst. Pharm. 2010, 67, 1788, 1790, 1793. [Google Scholar] [CrossRef]
- Stanciu, C.N.; Penders, T.M.; Rouse, E.M. Recreational use of dextromethorphan, “Robotripping”—A brief review. Am. J. Addict. 2016, 25, 374–377. [Google Scholar] [CrossRef]
- Available online: https://neurogroove.info/trip/dxm-inaczej-niz-zwykle-czyli-30-dawka-optymalna (accessed on 20 October 2022).
- Price, L.H.; Lebel, J. Dextromethorphan-induced psychosis. Am. J. Psych. 2000, 157, 304. [Google Scholar] [CrossRef]
- Martinak, B.; Bolis, R.A.; Black, J.R.; Fargason, R.E.; Birur, B. Dextromethorphan in cough syrup: The Poor Man’s Psychosis. Psychopharmacol. Bull. 2017, 47, 59–63. [Google Scholar]
- Stahl, S.M. Stahl’s Essential Psychopharmacology: Neuroscientific Basis and Practical Applications, 4th ed.; Cambridge University Press: New York, NY, USA, 2013. [Google Scholar]
- Pascali, J.P.; Fais, P.; Vaiano, F.; Pigaiani, N.; D’Errico, S.; Furlanetto, S.; Palumbo, D.; Bertol, E. Internet pseudoscience: Testing opioid containing formulations with tampering potential. J. Pharm. Biomed. Anal. 2018, 153, 16–21. [Google Scholar] [CrossRef]
- Hendrickson, R.G. “DXemon juice:” analytical evaluation of an extraction process for the purification and freebasing of dextromethorphan from cold preparations. J. Med. Toxicol. 2008, 4, 309. [Google Scholar] [CrossRef] [Green Version]
- Drugs for cough. Med. Lett. Drugs Ther. 2018, 60, 206–208.
- LePage, K.T.; Ishamel, J.E.; Low, C.M.; Traynelis, S.F.; Murray, T.F. Differential binding properties of [3H]dextrorphan and [3H]MK-801 in heterologously expressed NMDA receptors. Neuropharmacology 2005, 49, 1–16. [Google Scholar] [CrossRef]
- Stahl, S.M. Dextromethorphan/Bupropion: A novel oral NMDA (N-methyl-d-aspartate) receptor antagonist with multimodal activity. CNS Spectr. 2019, 24, 461–466. [Google Scholar] [CrossRef] [Green Version]
- Damaj, M.I.; Flood, P.; Ho, K.K.; May, E.L.; Martin, B.R. Effects of dextromethorphan and dextrorphan on nicotine and neuronal nicotinic receptors: In vitro and in vivo selectivity. J. Pharmacol. Exp. Ther. 2005, 312, 780–785. [Google Scholar] [CrossRef] [Green Version]
- Taylor, C.P.; Traynelis, S.F.; Siffert, J.; Pope, L.E.; Matsumoto, R.R. Pharmacology of dextromethorphan: Relevance to dextromethorphan/quinidine (Nuedexta(R)) clinical use. Pharmacol. Ther. 2016, 164, 170–182. [Google Scholar] [CrossRef] [Green Version]
- Baldo, B.A. Opioid analgesic drugs and serotonin toxicity (syndrome): Mechanisms, animal models, and links to clinical effects. Arch. Toxicol. 2018, 92, 2457–2473. [Google Scholar] [CrossRef]
- Bruggeman, C.; O’Day, C.S. Selective Serotonin Reuptake Inhibitor Toxicity. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Dy, P.; Arcega, V.; Ghali, W.; Wolfe, W. Serotonin syndrome caused by drug to drug interaction between escitalopram and dextromethorphan. BMJ Case Rep. 2017, 2017, bcr2017221486. [Google Scholar] [CrossRef]
- Forget, P.; le Polain de Waroux, B.; Wallemacq, P.; Gala, J.L. Life-threatening dextromethorphan intoxication associated with interaction with amitriptyline in a poor CYP2D6 metabolizer: A single case re-exposure study. J. Pain Symptom Manag. 2008, 36, 92–96. [Google Scholar] [CrossRef]
- Schwartz, A.R.; Pizon, A.F.; Brooks, D.E. Dextromethorphan-induced serotonin syndrome. Clin. Toxicol. 2008, 46, 771–773. [Google Scholar] [CrossRef]
- Boyer, E.W.; Shannon, M. The serotonin syndrome. N. Engl. J. Med. 2005, 352, 1112–1120. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, G.; Saleem, M.D.; Naim, H. How many deaths before we put cough syrups behind the counter? Perspect. Public Health 2014, 134, 309. [Google Scholar]
- Hill, M. Chemical toxicity. In Understanding Environmental Pollution; Cambridge University Press: Cambridge, MA, USA, 2010; pp. 57–88. [Google Scholar]
- Rammer, L.; Holmgren, P.; Sandler, H. Fatal intoxication by dextromethorphan: A report on two cases. Forensic Sci. Int. 1988, 37, 233–236. [Google Scholar] [CrossRef]
- Bem, J.L.; Peck, R. Dextromethorphan. An overview of safety issues. Drug Saf. 1992, 7, 190–199. [Google Scholar] [CrossRef]
- Logan, B.K.; Yeakel, J.K.; Goldfogel, G.; Frost, M.P.; Sandstrom, G.; Wickham, D.J. Dextromethorphan abuse leading to assault, suicide, or homicide. J. Forensic Sci. 2012, 57, 1388–1394. [Google Scholar] [CrossRef]
- Modi, D.; Bhalavat, R.; Patterson, J.C., 2nd. Suicidal and homicidal behaviors related to dextromethorphan abuse in a middle-aged woman. J. Addict. Med. 2013, 7, 143–144. [Google Scholar] [CrossRef]
- Stanciu, C.N.; Penders, T.M. Mania after misuse of dextromethorphan. J. Addict. Med. 2015, 9, 159–160. [Google Scholar] [CrossRef]
- Łukasik-Głębocka, M. Dekstrometorfan i benzydamina–nowe substancje odurzające. Serwis Inf. Narkom. 2008, 2, 16–19. [Google Scholar]
- Miller, S.C. Dextromethorphan psychosis, dependence and physical withdrawal. Addict. Biol. 2005, 10, 325–327. [Google Scholar] [CrossRef]
- Matin, N.; Kurani, A.; Kennedy, C.A.; Liu, Q.Y. Dextromethorphan-induced near-fatal suicide attempt in a slow metabolizer at cytochrome P450 2D6. Am. J. Geriatr. Pharmacother. 2007, 5, 162–165. [Google Scholar] [CrossRef]
- Romanelli, F.; Smith, K.M. Dextromethorphan abuse: Clinical effects and management. J. Am. Pharm. Assoc. 2009, 49, e20–e25. [Google Scholar] [CrossRef] [PubMed]
- Leighton, K.M. Paranoid psychosis after abuse of Actifed. BMJ 1982, 284, 789–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, G. Acute psychosis following intravenous abuse of pseudoephedrine: A case report. J. Pscyhopharm. 1996, 10, 324–325. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.K.; Goldfogel, G.; Hamilton, R.; Kuhlman, J. Five deaths resulting from abuse of dextromethorphan sold over the internet. J. Anal. Toxicol. 2009, 33, 99–103. [Google Scholar] [CrossRef] [Green Version]
- Burns, J.M.; Boyer, E.W. Antitussives and substance abuse. Subst. Abuse Rehabil. 2013, 4, 75–82. [Google Scholar]
- Nairn, S.J.; Díaz, J.E. Cold-syrup induced movement disorder. Pediatr. Emerg. Care 2001, 17, 191–192. [Google Scholar] [CrossRef] [PubMed]
- Soutullo, C.A.; Cottingham, E.M.; Keck, P.E. Psychosis associated with pseudoephedrine and dextromethorphan. J. Am. Acad. Child Adolesc. Psychiatry 1999, 38, 1471–1472. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.L.; Devanand, D.P.; Stahl, S.M. Dementia-related psychosis and the potential role for pimavanserin. CNS Spectr. 2022, 27, 7–15. [Google Scholar] [CrossRef]
- Whittemore, E.R.; Ilyin, V.I.; Woodward, R.M. Antagonism of N-methyl-D aspartate receptors by [sigma] site ligands: Potency, subtype-selectively and mechanism of inhibition. J. Pharmacol. Exp. Ther. 1997, 282, 32–38. [Google Scholar]
- Yamamoto, H.; Yamamoto, T.; Sagi, N.; Klenerova, V.; Goji, K.; Kawai, N.; Baba, A.; Takamori, E.; Moroji, T. Sigma ligands indirectly modulate the NMDA receptor-ion channel complex on intact neuronal cells via sigma 1 site. J. Neurosci. 1995, 15, 731–736. [Google Scholar] [CrossRef]
- Meoni, P.; Tortella, F.C.; Bowery, N.G. An autoradiographic study of dextromethorphan high-affinity binding sites in rat brain: Sodium-dependency and colocalization with paroxetine. Br. J. Pharmacol. 1997, 120, 1255–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okland, T.; Shirazi, M.; Rylander, M.; Holland, J. A case of aggressive psychosis in the setting of regular dextromethorphan abuse. Psychosomatics 2016, 57, 655–656. [Google Scholar] [CrossRef]
- Shen, W.W. A history of antipsychotic drug development. Compr. Psychiatry 1999, 40, 407–414. [Google Scholar] [CrossRef]
- Kane, J.; Honigfeld, G.; Singer, J.; Meltzer, H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch. Gen. Psychiatry 1988, 45, 789–796. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, J.P.; Lieberman, J.A.; Stroup, T.S.; Davis, S.M.; Meltzer, H.Y.; Rosenheck, R.A.; Swartz, M.S.; Perkins, D.O.; Keefe, R.S.E.; Davis, C.E.; et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am. J. Psychiatry 2006, 163, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.W.; Barnes, T.R.; Davies, L.; Murray, R.M.; Dunn, G.; Hayhurst, K.P.; Markwick, A.; Lloyd, H.; Jones, P.B. Randomized controlled trial of effect of prescription of clozapine versus other second-generation antipsychotic drugs in resistant schizophrenia. Schizophr. Bull. 2006, 32, 715–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chokhawala, K.; Stevens, L. Antipsychotic Medications. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Nucifora, F.C., Jr.; Mihaljevic, M.; Lee, B.J.; Sawa, A. Clozapine as a model for antipsychotic development. Neurotherapeutics 2017, 14, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Burry, L.; Mehta, S.; Perreault, M.M.; Luxenberg, J.S.; Siddiqi, N.; Hutton, B.; Fergusson, D.A.; Bell, C.; Rose, L. Antipsychotics for treatment of delirium in hospitalised non-ICU patients. Cochrane Database Syst. Rev. 2018, 6, CD005594. [Google Scholar] [CrossRef] [Green Version]
- Severance, E.G.; Dickerson, F.B.; Yolken, R.H. Autoimmune phenotypes in schizophrenia reveal novel treatment targets. Pharmacol. Ther. 2018, 189, 184–198. [Google Scholar] [CrossRef]
- Desai, N.; Patel, P.B.; Shah, S.; Patel, T.K.; Shah, S.N.; Vatsala, E. Prevalence and pattern of antipsychotic induced movement disorders in a tertiary care teaching hospital in India-a cross-sectional study. Int. J. Psychiatry Clin. Pract. 2018, 22, 101–108. [Google Scholar] [CrossRef]
- Khanna, P.; Suo, T.; Komossa, K.; Ma, H.; Rummel-Kluge, C.; El-Sayeh, H.G.; Leucht, S.; Xia, J. Aripiprazole versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst. Rev. 2014, 1, CD006569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lieberman, J.A.; Stroup, T.S.; McEvoy, J.P.; Swartz, M.S.; Rosenheck, R.A.; Perkins, D.O.; Keefe, R.S.; Davis, S.M.; Davis, C.E.; Lebowitz, B.D.; et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N. Engl. J. Med. 2005, 353, 1209–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amaladoss, A.; O’Brien, S. Cough syrup psychosis. CJEM 2011, 13, 53–56. [Google Scholar] [CrossRef] [Green Version]
- Beasley, C.M., Jr.; Tollefson, G.; Tran, P.; Satterlee, W.; Sanger, T.; Hamilton, S. Olanzapine versus placebo and haloperidol: Acute phase results of the North American Double-Blind Olanzapine Trial. Neuropsychopharmacology 1996, 14, 111–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dellva, M.A.; Tran, P.; Tollefson, G.D.; Wentley, A.L.; Beasley, C.M., Jr. Standard olanzapine versus placebo and ineffective-dose olanzapine in the maintenance treatment of schizophrenia. Psychiatr. Serv. 1997, 48, 1571–1577. [Google Scholar] [PubMed] [Green Version]
- Cáceda, R.; D’Orio, B. A young man’s “trip” to heaven and hell. Curr. Psychiatry 2008, 7, 72–88. [Google Scholar]
- Shoja Shafti, S.; Gilanipoor, M. A Comparative study between olanzapine and risperidone in the management of schizophrenia. Schizophr. Res. Treatment. 2014, 2014, 307202. [Google Scholar] [CrossRef] [Green Version]
- Indivior. PERSERIS (Risperidone) for Extended-Release Injectable Suspension, for Subcutaneous Use. Prescr Inf. July 2018. Available online: http://www.indivior.com/wp-content/uploads/2018/07/FDA-Labelrevised.pdf (accessed on 27 November 2022).
- Moore, N.A.; Tye, N.C.; Axton, M.S.; Risius, F.C. The behavioral pharmacology of olanzapine, a novel “atypical” antipsychotic agent. J. Pharmacol. Exp. Ther. 1992, 262, 545–551. [Google Scholar]
- Corena-McLeod, M. Comparative pharmacology of risperidone and paliperidone. Drugs R D 2015, 15, 163–174. [Google Scholar] [CrossRef]
- Siu, A.; Drachtman, R. Dextromethorphan: A review of N-methyl-D-aspartate receptor antagonist in the management of pain. CNS Drug Rev. 2007, 13, 96–106. [Google Scholar] [CrossRef]
- Zawertailo, L.A.; Tyndale, R.F.; Busto, U.; Sellers, E.M. Effect of metabolic blockade on the psychoactive effects of dextromethorphan. Hum. Psychopharmacol. Clin. Exp. 2010, 25, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Hacksell, U.; Burstein, E.S.; McFarland, K.; Mills, R.G.; Williams, H. On the discovery and development of pimavanserin: A novel drug candidate for Parkinson’s psychosis. Neurochem. Res. 2014, 39, 2008–2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, J.G.; Kane, K.; Flockhart, D.A. Potent inhibition of CYP2D6 by haloperidol metabolites: Stereoselective inhibition by reduced haloperidol. Br. J. Clin. Pharmacol. 2001, 51, 45–52. [Google Scholar] [CrossRef]
- Hildebrand, M.; Seifert, W.; Reichenberger, A. Determination of dextromethorphan metabolizer phenotype in healthy volunteers. Eur. J. Clin. Pharmacol. 1989, 36, 315–318. [Google Scholar] [CrossRef]
- Zawertailo, L.A.; Kaplan, H.L.; Busto, U.E.; Tyndale, R.F.; Sellers, E.M. Psychotropic effects of dextromethorphan are altered by the CYP2D6 polymorphism: A pilot study. J. Clin. Psychopharmacol. 1998, 18, 332–337. [Google Scholar] [CrossRef]
- Logan, B.K. Combined dextromethorphan and chlorpheniramine intoxication in impaired drivers. J. Forensic. Sci. 2009, 54, 1176–1180. [Google Scholar] [CrossRef] [PubMed]


| DXM-Induced Intoxication Level | Dose [mg/kg b.w] | Effects |
|---|---|---|
| I plateau | 1.5–2.5 |
|
| II plateau | 2.5–7.5 |
|
| III plateau | 7.5–15.0 |
|
| IV plateau | >15 |
|
| Antipsychotics | Mechanism of Action (Target Receptors) * | |
|---|---|---|
| First-Generation Class (Typical, Conventional) | Haloperidol | Primarily via D2 antagonism |
| Droperidol | ||
| Fluphenazine | ||
| Chlorpromazine | ||
| Thioridazine | ||
| Thiothixene | ||
| Loxapine | ||
| Molindone | ||
| Trifluoperazine | ||
| Perphenazine | ||
| Second-Generation Class (Atypical) | Olanzapine | Primarily via D2 (weak), D4, and 5HT2A antagonism; also, through α-adrenergic, muscarinic, and histamine receptors |
| Risperidone | ||
| Ziprasidone | ||
| Clozapine | ||
| Quetiapine | ||
| Aripiprazole | ||
| Asenapine | ||
| Zotepine | ||
| Sulpiride | ||
| Paliperidone | ||
| Iloperidone | ||
| Lurasidone | ||
| Levosulpiride | ||
| Amisulpride | ||
| Perazine | ||
| Sertindole | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaremba, M.; Serafin, P.; Kleczkowska, P. Antipsychotic Drugs Efficacy in Dextromethorphan-Induced Psychosis. Biomedicines 2023, 11, 123. https://doi.org/10.3390/biomedicines11010123
Zaremba M, Serafin P, Kleczkowska P. Antipsychotic Drugs Efficacy in Dextromethorphan-Induced Psychosis. Biomedicines. 2023; 11(1):123. https://doi.org/10.3390/biomedicines11010123
Chicago/Turabian StyleZaremba, Malgorzata, Pawel Serafin, and Patrycja Kleczkowska. 2023. "Antipsychotic Drugs Efficacy in Dextromethorphan-Induced Psychosis" Biomedicines 11, no. 1: 123. https://doi.org/10.3390/biomedicines11010123







