Potential of Caffeic Acid and 10-Dehydrogingerdione as Lipid Regulators Relevant to Their Inhibitory Effect on miR-122 and ATP Citrate Lyase Activity in Diabetic Hyperlipidemic Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drugs and Chemicals
2.2. Animals
2.3. Experimental Design
2.4. Blood Sampling and Tissue Collection
2.5. Analytical Procedures
2.6. Quantitative Real Time PCR
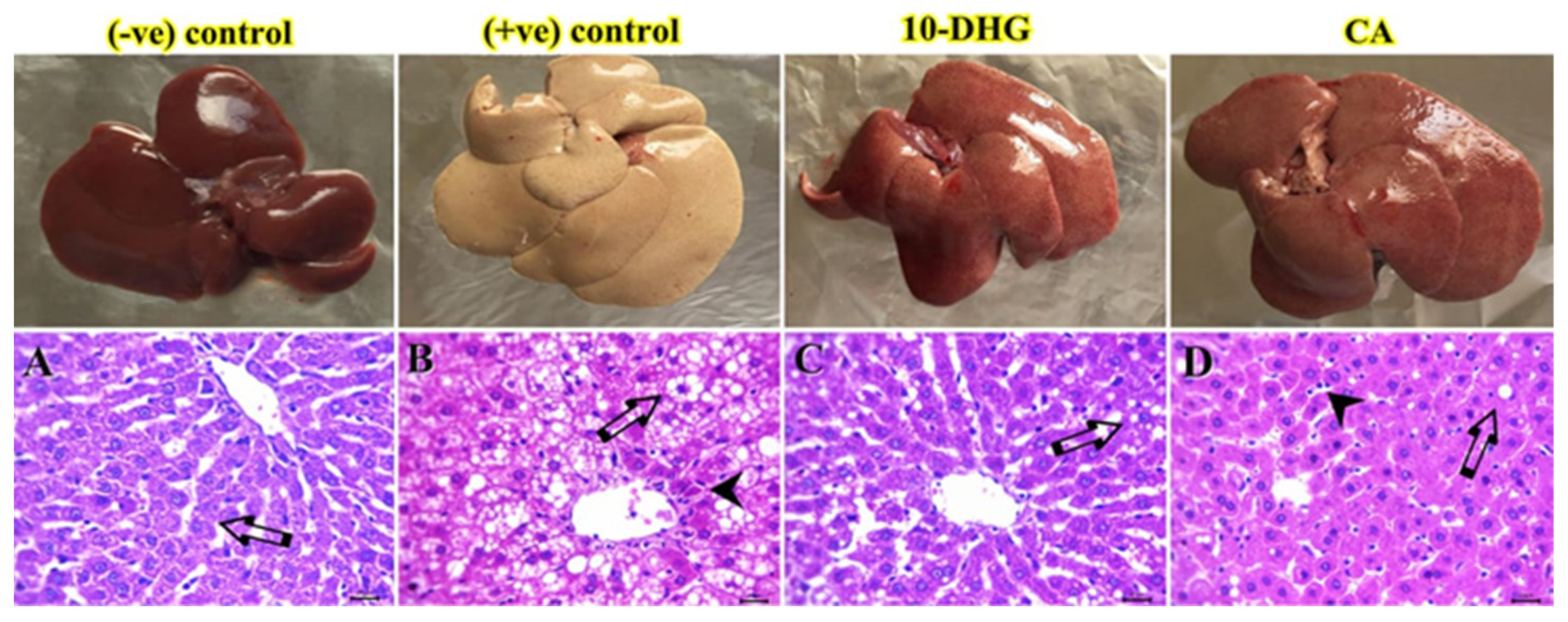
2.7. Histological Examination
2.8. Statistical Analysis
3. Results
3.1. Effect of Caffeic Acid and 10-Dehydrogengerdione on Body Weight and Liver Weight
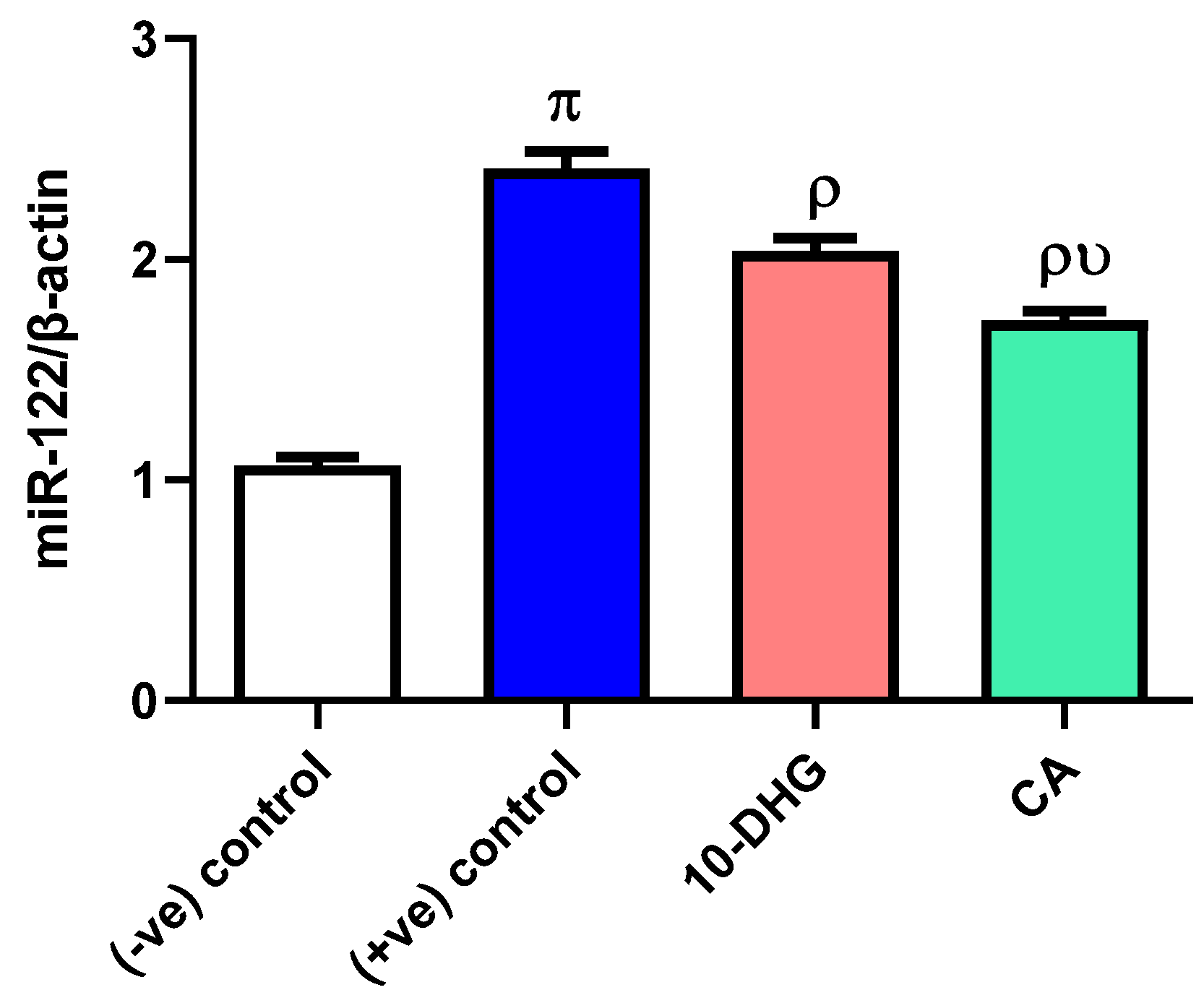
3.2. Effect of Caffeic Acid and 10-Dehydrogengerdione Treatment on miR-122 Expression
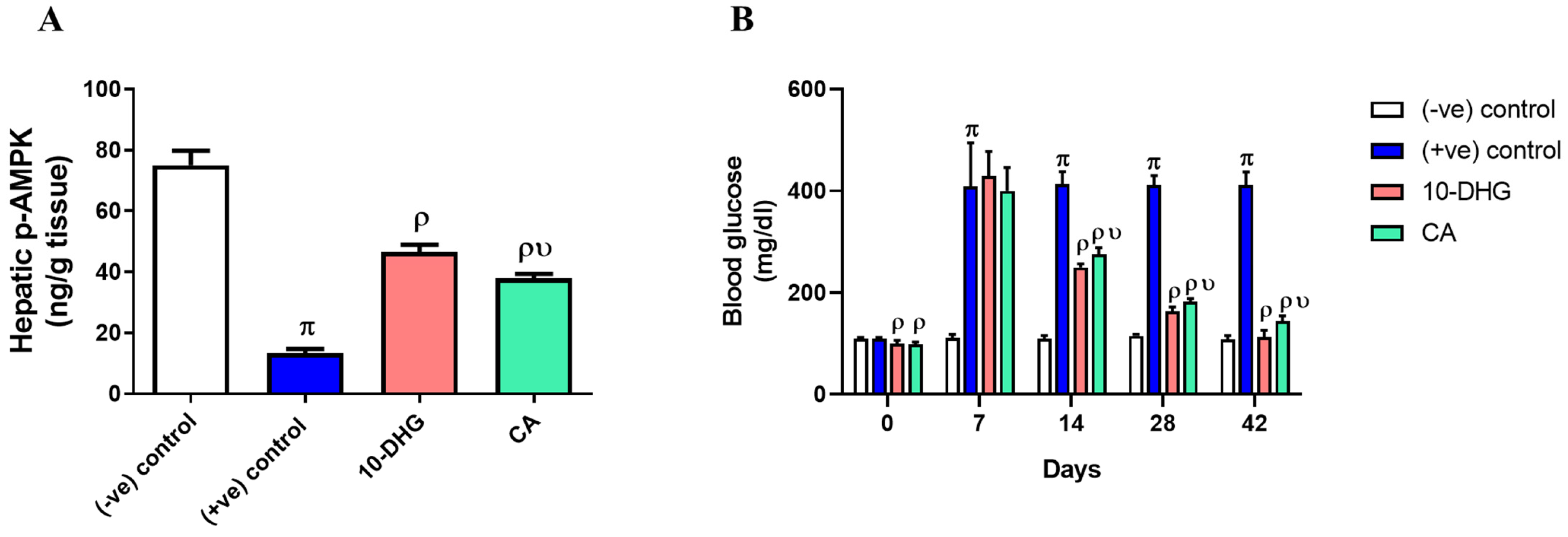
3.3. Effect of Caffeic Acid and 10-Dehydrogengerdione Treatment on Hepatic p-AMPK and Blood Glucose
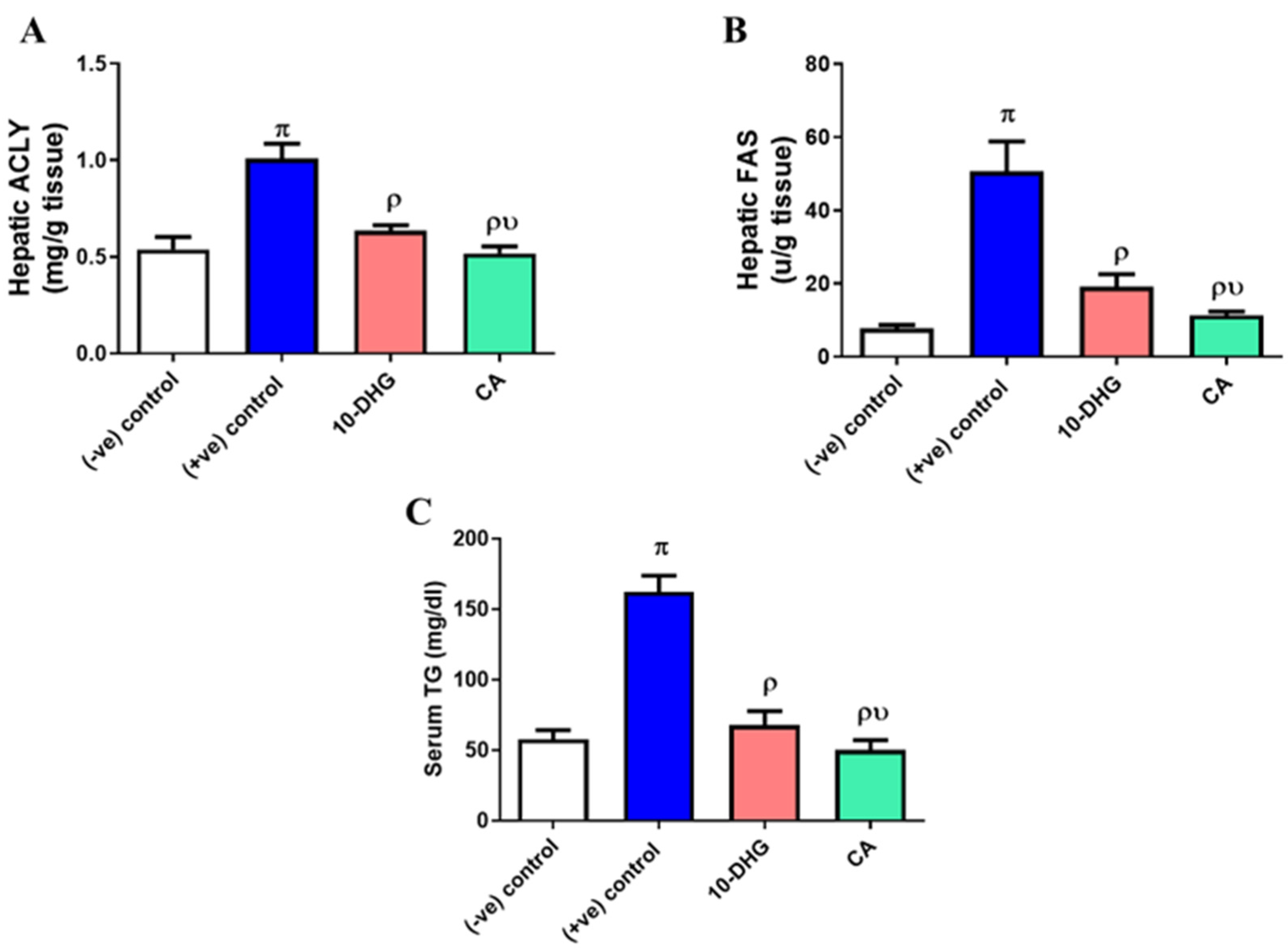
3.4. Effect of Caffeic Acid and 10-Dehydrogengerdione Treatment on Hepatic ACLY, FAS, and Serum Triglyceride
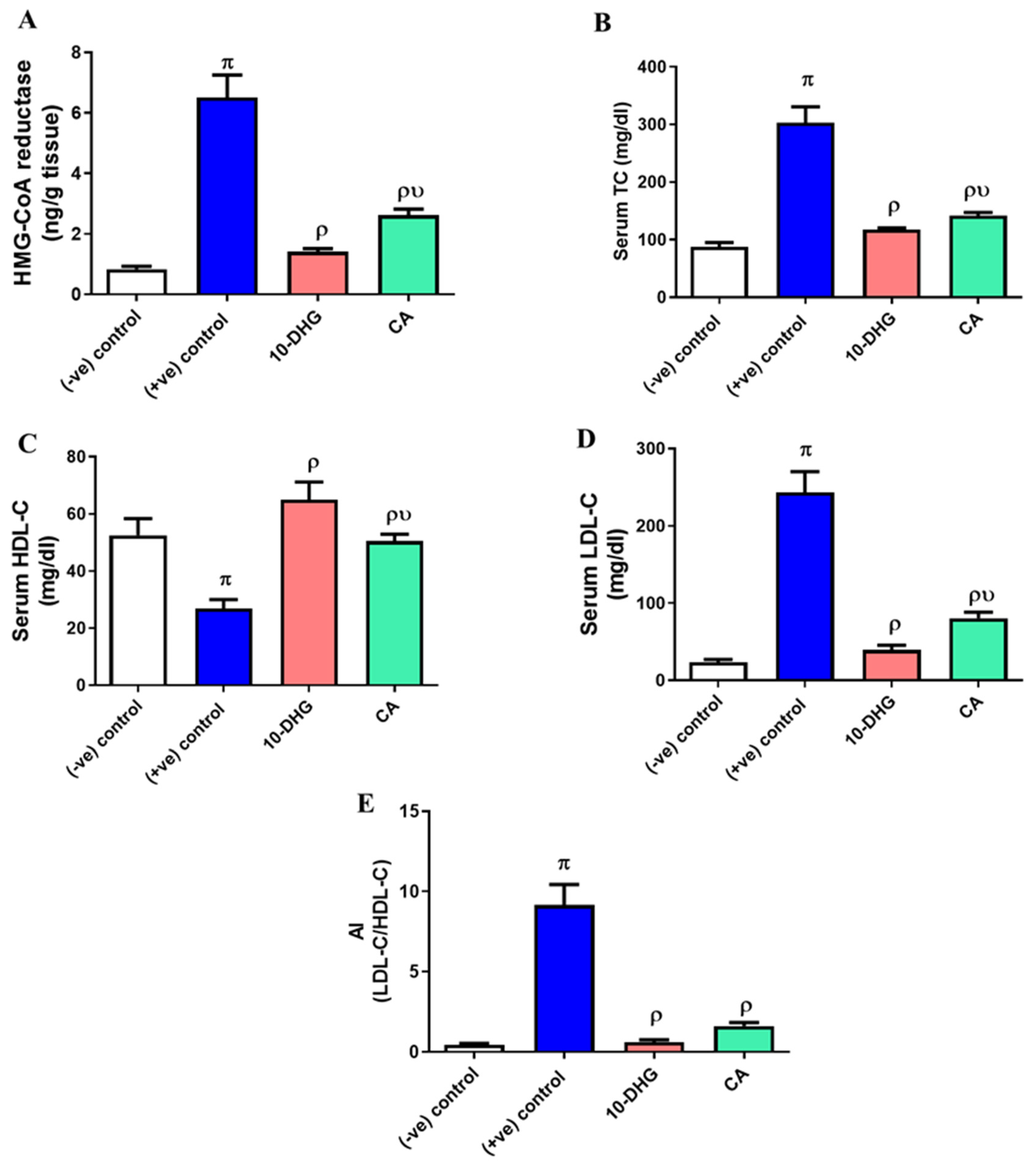
3.5. Effect of Caffeic Acid and 10-Dehydrogengerdione Treatment on Hepatic HMG-CoA Reductase Activity and Lipogram Pattern
3.6. Effect of Caffeic Acid and 10-Dehydrogengerdione Treatment on Hepatic HMG-CoA Reductase Activity and Lipogram Pattern
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Su, D.; Zhang, R.; Hou, F.; Chi, J.; Huang, F.; Yan, S.; Liu, L.; Deng, Y.; Wei, Z.; Zhang, M. Lychee pulp phenolics ameliorate hepatic lipid accumulation by reducing miR-33 and miR-122 expression in mice fed a high-fat diet. Food Funct. 2017, 8, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Baselga-Escudero, L.; Pascual-Serrano, A.; Ribas-Latre, A.; Casanova, E.; Salvadó, M.J.; Arola, L.; Arola-Arnal, A.; Bladé, C. Long-term supplementation with a low dose of proanthocyanidins normalized liver miR-33a and miR-122 levels in high-fat diet-induced obese rats. Nutr. Res. 2015, 35, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Rottiers, V.; Näär, A.M. MicroRNAs in metabolism and metabolic disorders. Nat. Rev. Mol. Cell Biol. 2012, 13, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.H.; Wang, B.; Kota, J.; Yu, J.; Costinean, S.; Kutay, H.; Yu, L.; Bai, S.; La Perle, K.; Chivukula, R.R.; et al. Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liver. J. Clin. Investig. 2012, 122, 2871–2883. [Google Scholar] [CrossRef]
- Baselga-Escudero, L.; Bladé, C.; Ribas-Latre, A.; Casanova, E.; Salvadó, M.J.; Arola, L.; Arola-Arnal, A. Grape seed proanthocyanidins repress the hepatic lipid regulators miR-33 and miR-122 in rats. Mol. Nutr. Food Res. 2012, 56, 1636–1646. [Google Scholar] [CrossRef]
- Rotllan, N.; Fernández-Hernando, C. MicroRNA Regulation of Cholesterol Metabolism. Cholesterol 2012, 2012, 847849. [Google Scholar] [CrossRef]
- Rahayu Lestari, S.; Lukiati, B.; Nur Arifah, S.; Rofiqotun Nurul Alimah, A.; Gofur, A. Medicinal Uses of Single Garlic in Hyperlipidemia by Fatty Acid Synthase Enzyme Inhibitory: Molecular Docking. IOP Conf. Ser. Earth Environ. Sci. 2019, 276, 012008. [Google Scholar] [CrossRef]
- Pinkosky, S.L.; Groot, P.H.E.; Lalwani, N.D.; Steinberg, G.R. Targeting ATP-Citrate Lyase in Hyperlipidemia and Metabolic Disorders. Trends Mol. Med. 2017, 23, 1047–1063. [Google Scholar] [CrossRef]
- Xie, Z.; Zhang, M.; Song, Q.; Cheng, L.; Zhang, X.; Song, G.; Sun, X.; Gu, M.; Zhou, C.; Zhang, Y.; et al. Development of the novel ACLY inhibitor 326E as a promising treatment for hypercholesterolemia. Acta Pharm. Sin. B 2022, 13, 739–753. [Google Scholar] [CrossRef]
- Esau, C.; Davis, S.; Murray, S.F.; Yu, X.X.; Pandey, S.K.; Pear, M.; Watts, L.; Booten, S.L.; Graham, M.; McKay, R.; et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006, 3, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Ding, L.L.; Li, J.M.; Xu, B.L.; Yang, L.; Bi, K.S.; Wang, Z.T. ¹H-NMR and MS based metabolomics study of the intervention effect of curcumin on hyperlipidemia mice induced by high-fat diet. PLoS ONE 2015, 10, e0120950. [Google Scholar] [CrossRef]
- Chao, C.-T.; Yeh, H.-Y.; Tsai, Y.-T.; Chuang, P.-H.; Yuan, T.-H.; Huang, J.-W.; Chen, H.-W. Natural and non-natural antioxidative compounds: Potential candidates for treatment of vascular calcification. Cell Death Discov. 2019, 5, 145. [Google Scholar] [CrossRef]
- Elseweidy, M.M.; Mohamed, H.E.; Elrashidy, R.A.; Atteia, H.H.; Elnagar, G.M.; Ali, A.E.-M. Potential therapeutic roles of 10-dehydrogingerdione and/or pentoxifylline against calcium deposition in aortic tissues of high dietary cholesterol-fed rabbits. Mol. Cell. Biochem. 2019, 453, 131–142. [Google Scholar] [CrossRef]
- Silva, H.; Lopes, N.M.F. Cardiovascular Effects of Caffeic Acid and Its Derivatives: A Comprehensive Review. Front. Physiol. 2020, 11, 595516. [Google Scholar] [CrossRef] [PubMed]
- Ekeuku, S.O.; Pang, K.L.; Chin, K.Y. Effects of Caffeic Acid and Its Derivatives on Bone: A Systematic Review. Drug Des. Dev. Ther. 2021, 15, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.A.; Maalik, A.; Murtaza, G. Inhibitory mechanism against oxidative stress of caffeic acid. J. Food Drug Anal. 2016, 24, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Park, G.S.; Lee, S.Y.; Kim, J.Y.; Kim, Y.K. The conformation and CETP inhibitory activity of [10]-dehydrogingerdione isolated from Zingiber officinale. Arch. Pharm. Res. 2011, 34, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.K.; Gupta, P.S.; Singh, S.K. Antidiabetic, anti-hyperlipidemic and antioxidant activities of Bauhinia variegata flower extract. Biocatal. Agric. Biotechnol. 2019, 19, 101142. [Google Scholar] [CrossRef]
- Jaishree, V.; Narsimha, S. Swertiamarin and quercetin combination ameliorates hyperglycemia, hyperlipidemia and oxidative stress in streptozotocin-induced type 2 diabetes mellitus in wistar rats. Biomed. Pharmacother. Biomed. Pharmacother. 2020, 130, 110561. [Google Scholar] [CrossRef]
- Elseweidy, M.M.; Elnagar, G.M.; Elsawy, M.M.; Ali, A.A.; Zein, N. Losartan and azelastine either alone or in combination as modulators for endothelial dysfunction and platelets activation in diabetic hyperlipidemic rats. J. Pharm. Pharmacol. 2020, 72, 1812–1821. [Google Scholar] [CrossRef]
- Sethi, A.; Parmar, H.S.; Kumar, A. The effect of aspirin on atherogenic diet-induced diabetes mellitus. Basic Clin. Pharmacol. Toxicol. 2011, 108, 371–377. [Google Scholar] [CrossRef]
- Elseweidy, M.M.; Elnagar, G.M.; Elsawy, M.M.; Zein, N. Azelastine a potent antihistamine agent, as hypolipidemic and modulator for aortic calcification in diabetic hyperlipidemic rats model. Arch. Physiol. Biochem. 2022, 128, 1611–1618. [Google Scholar] [CrossRef]
- Wang, Y.; Kaur, G.; Kumar, M.; Kushwah, A.S.; Kabra, A.; Kainth, R. Caffeic Acid Prevents Vascular Oxidative Stress and Atherosclerosis against Atherosclerogenic Diet in Rats. Evid.-Based Complement. Altern. Med. 2022, 2022, 8913926. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Sharma, H.; Pathan, R.A.; Kumar, V.; Javed, S.; Bhandari, U. Anti-apoptotic potential of rosuvastatin pretreatment in murine model of cardiomyopathy. Int. J. Cardiol. 2011, 150, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Gibson-Corley, K.N.; Olivier, A.K.; Meyerholz, D.K. Principles for valid histopathologic scoring in research. Vet. Pathol. 2013, 50, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef]
- Shizu, R.; Shindo, S.; Yoshida, T.; Numazawa, S. MicroRNA-122 down-regulation is involved in phenobarbital-mediated activation of the constitutive androstane receptor. PLoS ONE 2012, 7, e41291. [Google Scholar] [CrossRef]
- Kwon, I.G.; Ha, T.K.; Ryu, S.-W.; Ha, E. Roux-en-Y gastric bypass stimulates hypothalamic miR-122 and inhibits cardiac and hepatic miR-122 expressions. J. Surg. Res. 2015, 199, 371–377. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.Y.; Wei, X.C.; Wang, Q.; Yang, J.Y.; Hou, H.; Du, Z.W.; Wu, X.A. Effects of dibutyl phthalate on lipid metabolism in liver and hepatocytes based on PPARα/SREBP-1c/FAS/GPAT/AMPK signal pathway. Food Chem. Toxicol. 2021, 149, 112029. [Google Scholar] [CrossRef]
- Liu, S.; Wu, Z.; Guo, S.; Meng, X.; Chang, X. Polyphenol-rich extract from wild Lonicera caerulea berry reduces cholesterol accumulation by mediating the expression of hepatic miR-33 and miR-122, HMGCR, and CYP7A1 in rats. J. Funct. Foods 2018, 40, 648–658. [Google Scholar] [CrossRef]
- Baselga-Escudero, L.; Arola-Arnal, A.; Pascual-Serrano, A.; Ribas-Latre, A.; Casanova, E.; Salvadó, M.J.; Arola, L.; Blade, C. Chronic administration of proanthocyanidins or docosahexaenoic acid reverses the increase of miR-33a and miR-122 in dyslipidemic obese rats. PLoS ONE 2013, 8, e69817. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, J.; Li, Y.; Wan, Y.; Lin, J.; Shen, A.; Xu, W.; Li, H.; Zhang, Y.; Xu, J.; et al. Artemisia capillaris formula inhibits hepatic steatosis via an miR-122-induced decrease in fatty acid synthase expression in vivo and in vitro. Mol. Med. Rep. 2016, 13, 4751–4758. [Google Scholar] [CrossRef]
- Friesen, J.A.; Rodwell, V.W. The 3-hydroxy-3-methylglutaryl coenzyme-A (HMG-CoA) reductases. Genome Biol. 2004, 5, 248. [Google Scholar] [CrossRef] [PubMed]
- Krützfeldt, J.; Rajewsky, N.; Braich, R.; Rajeev, K.G.; Tuschl, T.; Manoharan, M.; Stoffel, M. Silencing of microRNAs in vivo with ‘antagomirs’. Nature 2005, 438, 685–689. [Google Scholar] [CrossRef] [PubMed]
- de Alencar Silva, A.; Pereira-de-Morais, L.; Rodrigues da Silva, R.E.; de Menezes Dantas, D.; Brito Milfont, C.G.; Gomes, M.F.; Araújo, I.M.; Kerntopf, M.R.; Alencar de Menezes, I.R.; Barbosa, R. Pharmacological screening of the phenolic compound caffeic acid using rat aorta, uterus and ileum smooth muscle. Chem. Biol. Interact. 2020, 332, 109269. [Google Scholar] [CrossRef] [PubMed]
- Joven, J.; Espinel, E.; Rull, A.; Aragonès, G.; Rodríguez-Gallego, E.; Camps, J.; Micol, V.; Herranz-López, M.; Menéndez, J.A.; Borrás, I.; et al. Plant-derived polyphenols regulate expression of miRNA paralogs miR-103/107 and miR-122 and prevent diet-induced fatty liver disease in hyperlipidemic mice. Biochim. Biophys. Acta (BBA) Gen. Subj. 2012, 1820, 894–899. [Google Scholar] [CrossRef]
- Liao, C.C.; Ou, T.T.; Wu, C.H.; Wang, C.J. Prevention of diet-induced hyperlipidemia and obesity by caffeic acid in C57BL/6 mice through regulation of hepatic lipogenesis gene expression. J. Agric. Food Chem. 2013, 61, 11082–11088. [Google Scholar] [CrossRef]
- Tyszka-Czochara, M.; Bukowska-Strakova, K.; Kocemba-Pilarczyk, K.A.; Majka, M. Caffeic Acid Targets AMPK Signaling and Regulates Tricarboxylic Acid Cycle Anaplerosis while Metformin Downregulates HIF-1α-Induced Glycolytic Enzymes in Human Cervical Squamous Cell Carcinoma Lines. Nutrients 2018, 10, 841. [Google Scholar] [CrossRef]
- Xu, W.; Luo, Q.; Wen, X.; Xiao, M.; Mei, Q. Antioxidant and anti-diabetic effects of caffeic acid in a rat model of diabetes. Trop. J. Pharm. Res. 2020, 19, 1227–1232. [Google Scholar] [CrossRef]
- Gao, H.; Guan, T.; Li, C.; Zuo, G.; Yamahara, J.; Wang, J.; Li, Y. Treatment with ginger ameliorates fructose-induced Fatty liver and hypertriglyceridemia in rats: Modulation of the hepatic carbohydrate response element-binding protein-mediated pathway. Evid.-Based Complement. Altern. Med. Ecam 2012, 2012, 570948. [Google Scholar] [CrossRef]
- Roufogalis, B.D. Zingiber officinale (Ginger): A Future Outlook on Its Potential in Prevention and Treatment of Diabetes and Prediabetic States. New J. Sci. 2014, 2014, 674684. [Google Scholar] [CrossRef]
- Al Hroob, A.M.; Abukhalil, M.H.; Alghonmeen, R.D.; Mahmoud, A.M. Ginger alleviates hyperglycemia-induced oxidative stress, inflammation and apoptosis and protects rats against diabetic nephropathy. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 106, 381–389. [Google Scholar] [CrossRef]
- Alshathly, M.R. Efficacy of Ginger (Zingiber officinale) in Ameliorating Streptozotocin-Induced Diabetic Liver Injury in Rats: Histological and Biochemical Studies. J. Microsc. Ultrastruct. 2019, 7, 91–101. [Google Scholar] [CrossRef] [PubMed]
| Gene | Sequence |
|---|---|
| miR-122 | F: 5′-GGGGTGGAGTGTGACAATG-3 |
| R: 5′-CAGTGCGTGTCGTGGAGT-3′ | |
| β-Actin | F: 5′-GCCGGGACCTGACTGACTAC-3 |
| R: 5′-TTCTCCTTAATGTCACGCACGAT-3 |
| Parameters | Initial Body Weight | Final Body Weight | Liver Weight | Liver Weight/Final Body Weight Ratio (%) | |
|---|---|---|---|---|---|
| Groups | |||||
| (-ve) control | 146.2 ± 2.64 | 337.2 ± 6.76 | 8.29 ± 0.53 | 2.56 ± 0.21 | |
| (+ve) control | 151 ± 6.60 | 174.8 ± 7.33 π | 16.10 ± 0.44 π | 9.22 ± 0.49 π | |
| 10-DHG | 149.3 ± 5.05 | 292.2 ± 37.79 ρ | 11.32 ± 1.72 ρ | 3.9 ± 0.69 ρ | |
| CA | 147.4 ± 4.23 | 288 ± 16.19 ρ | 11.17 ± 0.25 ρ | 3.86 ± 0.25 ρ | |
| Organ | Main Lesions | (-ve) Control | (+ve) Control | 10-DHG | CA |
|---|---|---|---|---|---|
| Liver | Acute cell swelling | 0 | 2 | 0 | 1 |
| Lipidosis and foamy cells | 0 | 3 | 1 | 1 | |
| Necrotic and apoptotic cells | 0 | 2 | 0 | 1 | |
| Lymphocytes infiltrates | 0 | 2 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elseweidy, M.M.; Elawady, A.S.; Sobh, M.S.; Alqhtani, A.H.; Al-Gabri, N.A.; Elnagar, G.M. Potential of Caffeic Acid and 10-Dehydrogingerdione as Lipid Regulators Relevant to Their Inhibitory Effect on miR-122 and ATP Citrate Lyase Activity in Diabetic Hyperlipidemic Rats. Biomedicines 2023, 11, 726. https://doi.org/10.3390/biomedicines11030726
Elseweidy MM, Elawady AS, Sobh MS, Alqhtani AH, Al-Gabri NA, Elnagar GM. Potential of Caffeic Acid and 10-Dehydrogingerdione as Lipid Regulators Relevant to Their Inhibitory Effect on miR-122 and ATP Citrate Lyase Activity in Diabetic Hyperlipidemic Rats. Biomedicines. 2023; 11(3):726. https://doi.org/10.3390/biomedicines11030726
Chicago/Turabian StyleElseweidy, Mohamed M., Alaa S. Elawady, Mohammed S. Sobh, Abdulmohsen H. Alqhtani, Naif A. Al-Gabri, and Gehad M. Elnagar. 2023. "Potential of Caffeic Acid and 10-Dehydrogingerdione as Lipid Regulators Relevant to Their Inhibitory Effect on miR-122 and ATP Citrate Lyase Activity in Diabetic Hyperlipidemic Rats" Biomedicines 11, no. 3: 726. https://doi.org/10.3390/biomedicines11030726






