Long-Term L-Glutamine Treatment Reduces Hemolysis without Ameliorating Hepatic Vaso-Occlusion and Liver Fibrosis in a Mouse Model of Sickle Cell Disease
Abstract
:1. Introduction
2. Methods
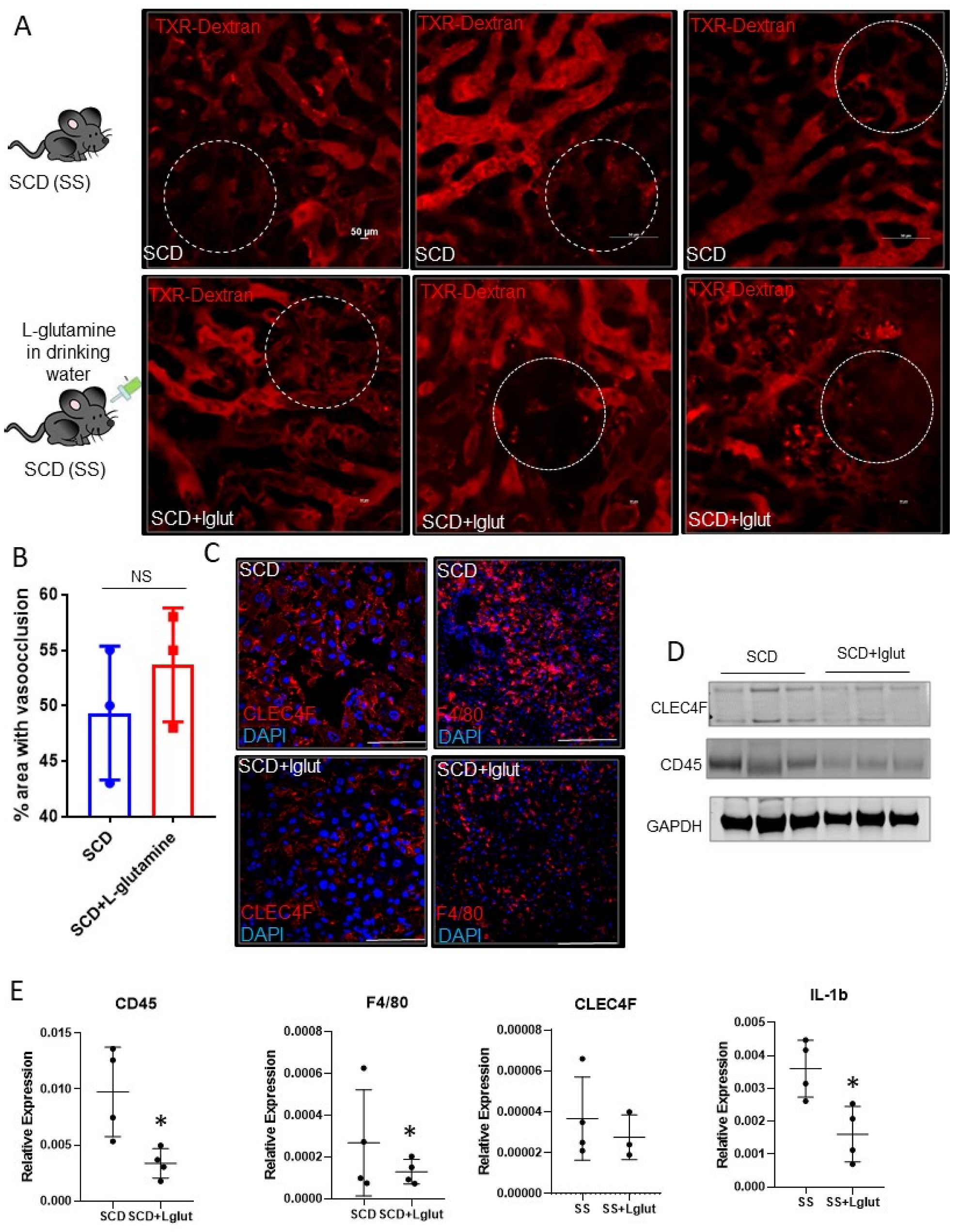
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar] [CrossRef] [PubMed]
- Ebert, E.C.; Nagar, M.; Hagspiel, K.D. Gastrointestinal and Hepatic Complications of Sickle Cell Disease. Clin. Gastroenterol. Hepatol. 2010, 8, 483–489. [Google Scholar] [CrossRef]
- Rees, D.C.; Williams, T.N.; Gladwin, M.T. Sickle-cell disease. Lancet 2010, 376, 2018–2031. [Google Scholar] [CrossRef] [PubMed]
- Sundd, P.; Gladwin, M.T.; Novelli, E.M. Pathophysiology of Sickle Cell Disease. Annu. Rev. Pathol. 2018, 14, 263–292. [Google Scholar] [CrossRef]
- Banerjee, S.; Owen, C.; Chopra, S. Sickle cell hepatopathy. Hepatology 2001, 33, 1021–1028. [Google Scholar] [CrossRef]
- Schubert, T.T. Hepatobiliary system in sickle cell disease. Gastroenterology 1986, 90, 2013–2021. [Google Scholar] [CrossRef] [PubMed]
- Vats, R.; Liu, S.; Zhu, J.; Mukhi, D.; Tutuncuoglu, E.; Cardenes, N.; Singh, S.; Brzoska, T.; Kosar, K.; Bamne, M.; et al. Impaired bile secretion promotes hepatobiliary injury in Sickle Cell Disease. Hepatology 2020, 72, 2165–2181. [Google Scholar] [CrossRef]
- Feld, J.J.; Kato, G.J.; Koh, C.; Shields, T.; Hildesheim, M.; Kleiner, D.E.; Taylor, J.G.; Sandler, N.G.; Douek, D.; Haynes-Williams, V.; et al. Liver injury is associated with mortality in sickle cell disease. Aliment. Pharmacol. Ther. 2015, 42, 912–921. [Google Scholar] [CrossRef]
- Koh, C.; Turner, T.; Zhao, X.; Minniti, C.P.; Feld, J.J.; Simpson, J.; Demino, M.; Conrey, A.K.; Jackson, M.J.; Seamon, C.; et al. Liver stiffness increases acutely during sickle cell vaso-occlusive crisis. Am. J. Hematol. 2013, 88, E250–E254. [Google Scholar] [CrossRef]
- Allali, S.; de Montalembert, M.; Brousse, V.; Heilbronner, C.; Taylor, M.; Brice, J.; Manzali, E.; Garcelon, N.; Lacaille, F. Hepatobiliary Complications in Children with Sickle Cell Disease: A Retrospective Review of Medical Records from 616 Patients. J. Clin. Med. 2019, 8, 1481. [Google Scholar] [CrossRef]
- Brittenham, G.M.; Cohen, A.R.; McLaren, C.E.; Martin, M.B.; Griffith, P.M.; Nienhuis, A.W.; Young, N.S.; Allen, C.J.; Farrell, D.E.; Harris, J.W. Hepatic iron stores and plasma ferritin concentration in patients with sickle cell anemia and thalassemia major. Am. J. Hematol. 1993, 42, 81–85. [Google Scholar] [CrossRef]
- Bandyopadhyay, R.; Bandyopadhyay, S.; Dutta, A. Sickle cell hepatopathy. Indian J. Pathol. Microbiol. 2008, 51, 284–285. [Google Scholar] [CrossRef]
- Hogen, R.; Kim, M.; Lee, Y.; Lo, M.; Kaur, N.; Kahn, J.; Chopra, S.; Qazi, Y.; Sedra, A.; Kim, J.; et al. Liver Transplantation in Patients with Sickle Cell Disease in the United States. J. Surg. Res. 2020, 255, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Suddle, A.R. Management of liver complications in sickle cell disease. Hematol. Am. Soc. Hematol. Educ. Program 2019, 2019, 345–350. [Google Scholar] [CrossRef]
- Mekeel, K.L.; Langham, M.R.; Gonzalez-Peralta, R.; Fujita, S.; Hemming, A.W. Liver transplantation in children with sickle-cell disease. Liver Transplant. 2007, 13, 505–508. [Google Scholar] [CrossRef]
- Morris, C.R.; Hamilton-Reeves, J.; Martindale, R.G.; Sarav, M.; Ochoa Gautier, J.B. Acquired Amino Acid Deficiencies: A Focus on Arginine and Glutamine. Nutr. Clin. Pract. 2017, 32, 30S–47S. [Google Scholar] [CrossRef]
- Niihara, Y.; Matsui, N.M.; Shen, Y.M.; Akiyama, D.A.; Johnson, C.S.; Sunga, M.A.; Magpayo, J.; Embury, S.H.; Kalra, V.K.; Ho Cho, S.; et al. L-glutamine therapy reduces endothelial adhesion of sickle red blood cells to human umbilical vein endothelial cells. BMC Hematol. 2005, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Niihara, Y.; Zerez, C.R.; Akiyama, D.S.; Tanaka, K.R. Oral L-glutamine therapy for sickle cell anemia: I. Subjective clinical improvement and favorable change in red cell NAD redox potential. Am. J. Hematol. 1998, 58, 117–121. [Google Scholar] [CrossRef]
- Niihara, Y.; Miller, S.T.; Kanter, J.; Lanzkron, S.; Smith, W.R.; Hsu, L.L.; Gordeuk, V.R.; Viswanathan, K.; Sarnaik, S.; Osunkwo, I.; et al. A Phase 3 Trial of l -Glutamine in Sickle Cell Disease. N. Engl. J. Med. 2018, 379, 226–235. [Google Scholar] [CrossRef]
- Cox, S.E.; Hart, E.; Kirkham, F.J.; Stotesbury, H. L-glutamine in sickle cell disease. Drugs Today 2020, 56, 257. [Google Scholar] [CrossRef]
- Sadaf, A.; Quinn, C.T. L-glutamine for sickle cell disease: Knight or pawn? Exp. Biol. Med. 2020, 245, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.C.; Sun, C.W.; Ryan, T.M.; Pawlik, K.M.; Ren, J.; Townes, T.M. Correction of sickle cell disease by homologous recombination in embryonic stem cells. Blood 2006, 108, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xia, Y.; Zhu, G.; Yan, J.; Tan, C.; Deng, B.; Deng, J.; Yin, Y.; Ren, W. Glutamine supplementation improves intestinal cell proliferation and stem cell differentiation in weanling mice. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Pradhan-Sundd, T.; Vats, R.; Russell, J.M.; Singh, S.; Michael, A.A.; Molina, L.; Kakar, S.; Cornuet, P.; Poddar, M.; Watkins, S.C.; et al. Dysregulated bile transporters and impaired tight junctions during chronic liver injury in mice. Gastroenterology 2018, 155, 1218–1232. [Google Scholar] [CrossRef] [PubMed]
- Bennewitz, M.F.; Watkins, S.C.; Sundd, P. Quantitative intravital two-photon excitation microscopy reveals absence of pulmonary vaso-occlusion in unchallenged Sickle Cell Disease mice. IntraVital 2014, 3, e29748. [Google Scholar] [CrossRef] [PubMed]
- Pradhan-Sundd, T.; Zhou, L.; Vats, R.; Jiang, A.; Molina, L.; Singh, S.; Poddar, M.; Russell, J.; Stolz, D.B.; Oertel, M.; et al. Dual catenin loss in murine liver causes tight junctional deregulation and progressive intrahepatic cholestasis. Hepatology 2017, 67, 2320–2337. [Google Scholar] [CrossRef]
- Zhou, L.; Pradhan-Sundd, T.; Poddar, M.; Singh, S.; Kikuchi, A.; Stolz, D.B.; Shou, W.; Li, Z.; Nejak-Bowen, K.N.; Monga, S.P. Mice with hepatic loss of the desmosomal protein γ-catenin are prone to cholestatic injury and chemical carcinogenesis. Am. J. Pathol. 2015, 185, 3274–3289. [Google Scholar] [CrossRef]
- Vats, R.; Kaminski, T.W.; Ju, E.-M.; Brozska, T.; Tutuncuoglu, E.; Tejero, J.; Novelli, E.M.; Sundd, P.; Pradhan-Sundd, T. P-selectin deficiency promotes liver senescence in sickle cell disease mice. Blood 2021, 137, 2676–2680. [Google Scholar] [CrossRef]
- Sukhbaatar, N.; Weichhart, T. Iron regulation: Macrophages in control. Pharmaceuticals 2018, 11, 137. [Google Scholar] [CrossRef]
- Winn, N.C.; Volk, K.M.; Hasty, A.H. Regulation of tissue iron homeostasis: The macrophage “ferrostat.”. JCI Insight 2020, 5, e132964. [Google Scholar] [CrossRef]
- Slusarczyk, P.; Mleczko-Sanecka, K. The multiple facets of iron recycling. Genes 2021, 12, 1364. [Google Scholar] [CrossRef] [PubMed]
- Schroit, A.J.; Tanaka, Y.; Madsen, J.; Fidler, I.J. The recognition of red blood cells by macrophages: Role of phosphatidylserine and possible implications of membrane phospholipid asymmetry. Biol Cell 1984, 51, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Willekens, F.L.A.; Werre, J.M.; Kruijt, J.K.; Roerdinkholder-Stoelwinder, B.; Groenen-Döpp, Y.A.; van den Bos, A.G.; Bosman, G.J.; van Berkel, T.J. Liver Kupffer cells rapidly remove red blood cell-derived vesicles from the circulation by scavenger receptors. Blood 2005, 105, 2141–2145. [Google Scholar] [CrossRef] [PubMed]
- Pace, B.S.; Starlard-Davenport, A.; Kutlar, A. Sickle cell disease: Progress towards combination drug therapy. Br. J. Haematol. 2021, 194, 240–251. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katoch, O.; Ungalara, R.; Kaminski, T.; Li, Z.; Dubey, R.K.; Burholt, I.; Gudapati, S.; Pradhan-Sundd, T. Long-Term L-Glutamine Treatment Reduces Hemolysis without Ameliorating Hepatic Vaso-Occlusion and Liver Fibrosis in a Mouse Model of Sickle Cell Disease. Biomedicines 2023, 11, 2412. https://doi.org/10.3390/biomedicines11092412
Katoch O, Ungalara R, Kaminski T, Li Z, Dubey RK, Burholt I, Gudapati S, Pradhan-Sundd T. Long-Term L-Glutamine Treatment Reduces Hemolysis without Ameliorating Hepatic Vaso-Occlusion and Liver Fibrosis in a Mouse Model of Sickle Cell Disease. Biomedicines. 2023; 11(9):2412. https://doi.org/10.3390/biomedicines11092412
Chicago/Turabian StyleKatoch, Omika, Ramakrishna Ungalara, Tomasz Kaminski, Ziming Li, Rikesh K. Dubey, Isabella Burholt, Shweta Gudapati, and Tirthadipa Pradhan-Sundd. 2023. "Long-Term L-Glutamine Treatment Reduces Hemolysis without Ameliorating Hepatic Vaso-Occlusion and Liver Fibrosis in a Mouse Model of Sickle Cell Disease" Biomedicines 11, no. 9: 2412. https://doi.org/10.3390/biomedicines11092412




