Increased Susceptibility to Mechanical Stretch Drives the Persistence of Keloid Fibroblasts: An Investigation Using a Stretchable PDMS Platform
Abstract
:1. Introduction
2. Materials and Methods
2.1. Keloid Tissues and Human Dermal Fibroblasts
2.2. Cell Proliferation Assay
2.3. Gene Expression Profile
2.4. Quantitative Proteomics Analysis Using a TMT-Labelling Method
2.5. Western Blotting
2.6. Immunofluorescent Staining
2.7. Statistical Analysis
3. Results
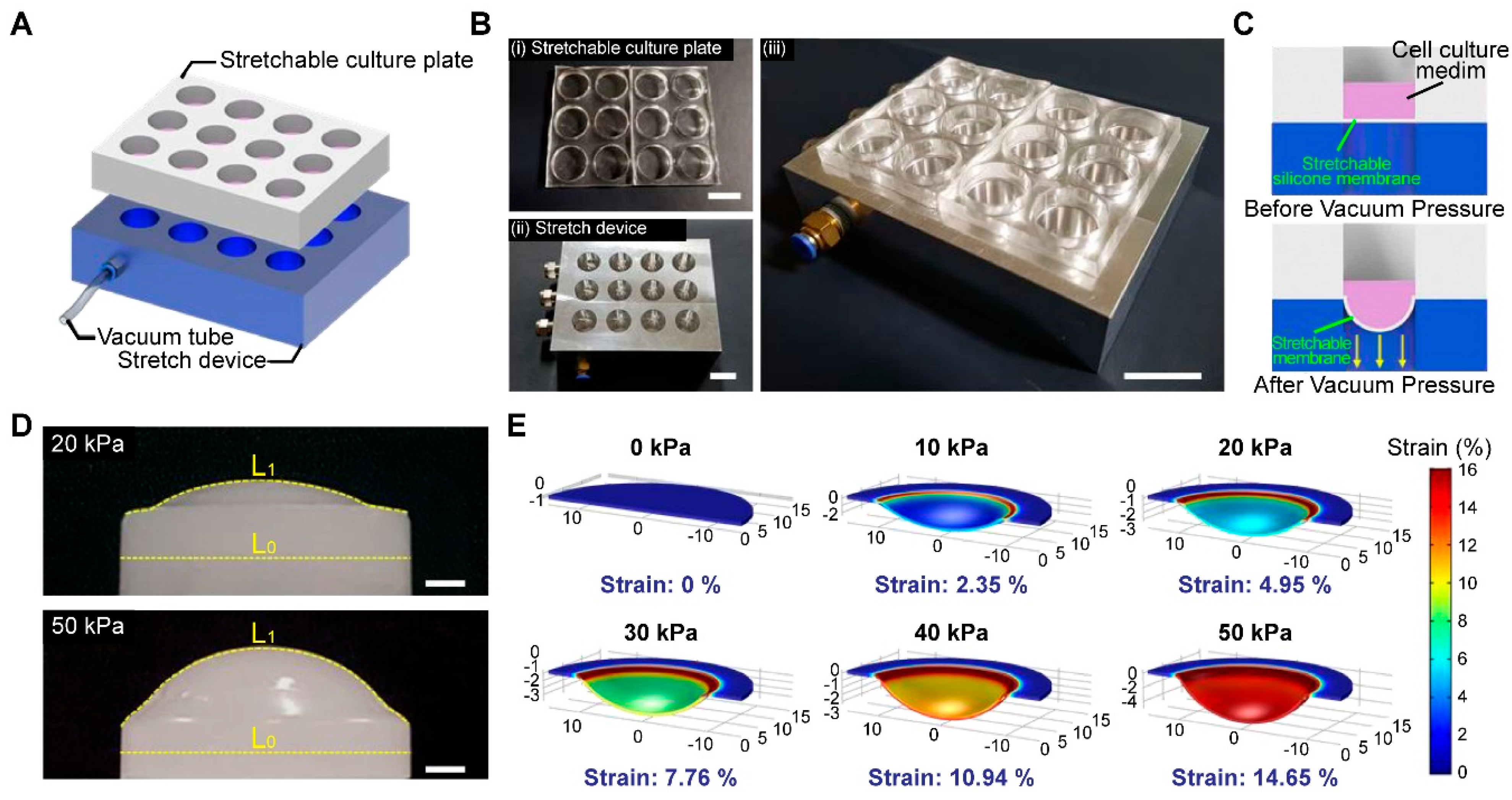
3.1. Fabrication of a High-Throughput, Mechanically Stretchable PDMS Cell Culture Platform
3.2. Human Dermal Fibroblasts (HDFs) and Keloid Fibroblasts (KFs) Showed Increased Proliferation Rates and Morphological Changes in Response to Mechanical Strain
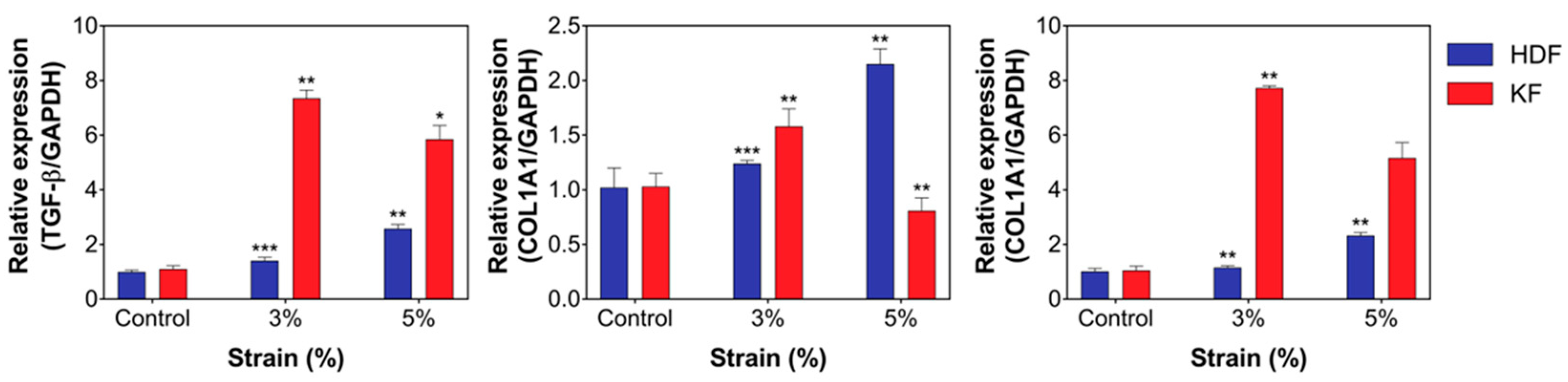
3.3. Mechanical Stretch Induced the Expression of Fibrotic Markers and ECM Components in HDFs and KFs
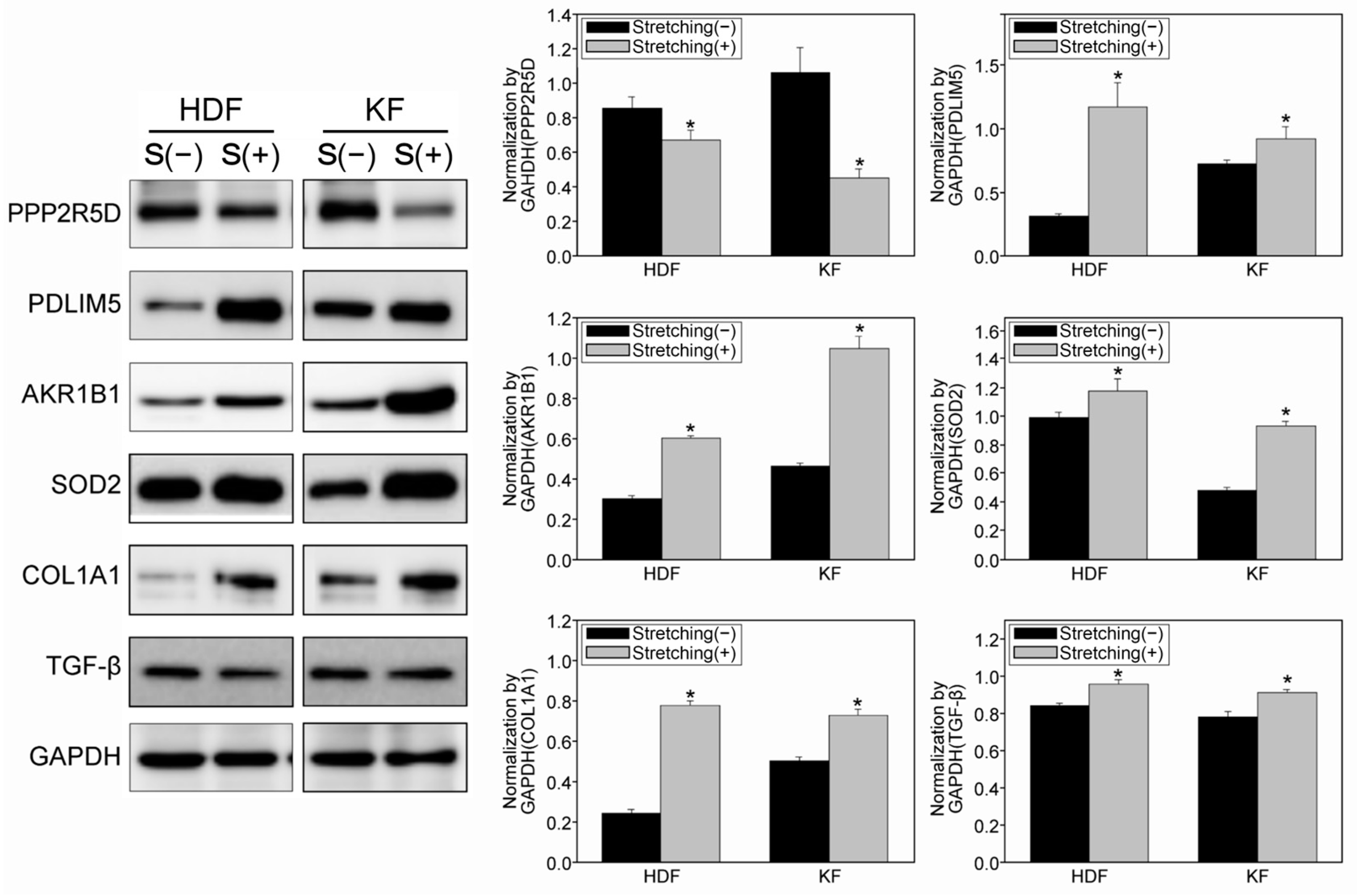
3.4. Quantitative Proteomic Analysis Using a Tandem Mass Tag (TMT)-Labelling Method Revealed Potential Target Proteins with Increased Mechanosensitivity in KFs
3.5. Immunohistochemical Staining of KFs Demonstrated an Increase and Change in the Cytoskeletal Composition Due to Cyclic Mechanical Stretch
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Jiang, D.; Liang, J.; Meltzer, E.B.; Gray, A.; Miura, R.; Wogensen, L.; Yamaguchi, Y.; Noble, P.W. Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. J. Exp. Med. 2011, 208, 1459–1471. [Google Scholar] [CrossRef] [PubMed]
- Rockey, D.C.; Bell, P.D.; Hill, J.A. Fibrosis—A Common Pathway to Organ Injury and Failure. N. Engl. J. Med. 2015, 373, 96. [Google Scholar] [CrossRef]
- Nanthakumar, C.B.; Hatley, R.J.D.; Lemma, S.; Gauldie, J.; Marshall, R.P.; Macdonald, S.J.F. Dissecting fibrosis: Therapeutic insights from the small-molecule toolbox. Nat. Rev. Drug Discov. 2015, 14, 693–720. [Google Scholar] [CrossRef]
- Ho, Y.Y.; Lagares, D.; Tager, A.M.; Kapoor, M. Fibrosis—A lethal component of systemic sclerosis. Nat. Rev. Rheumatol. 2014, 10, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Chiquet, M. Regulation of extracellular matrix gene expression by mechanical stress. Matrix Biol. 1999, 18, 417–426. [Google Scholar] [CrossRef]
- Hinz, B. Tissue stiffness, latent TGF-beta1 activation, and mechanical signal transduction: Implications for the pathogenesis and treatment of fibrosis. Curr. Rheumatol. Rep. 2009, 11, 120–126. [Google Scholar] [CrossRef]
- Burd, A.; Huang, L. Hypertrophic response and keloid diathesis: Two very different forms of scar. Plast. Reconstr. Surg. 2005, 116, 150e–157e. [Google Scholar] [CrossRef] [PubMed]
- Gauglitz, G.G.; Korting, H.C.; Pavicic, T.; Ruzicka, T.; Jeschke, M.G. Hypertrophic scarring and keloids: Pathomechanisms and current and emerging treatment strategies. Mol. Med. 2011, 17, 113–125. [Google Scholar] [CrossRef]
- Shih, B.; Bayat, A. Genetics of keloid scarring. Arch. Dermatol. Res. 2010, 302, 319–339. [Google Scholar] [CrossRef]
- Jumper, N.; Hodgkinson, T.; Paus, R.; Bayat, A. Site-specific gene expression profiling as a novel strategy for unravelling keloid disease pathobiology. PLoS ONE 2017, 12, e0172955. [Google Scholar] [CrossRef]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in wound repair: Molecular and cellular mechanisms. J. Invest. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef]
- Dong, X.; Mao, S.; Wen, H. Upregulation of proinflammatory genes in skin lesions may be the cause of keloid formation (Review). Biomed. Rep. 2013, 1, 833–836. [Google Scholar] [CrossRef]
- Schäfer, M.; Werner, S. Cancer as an overhealing wound: An old hypothesis revisited. Nat. Rev. Mol. Cell Biol. 2008, 9, 628–638. [Google Scholar] [CrossRef]
- Al-Attar, A.; Mess, S.; Thomassen, J.M.; Kauffman, C.L.; Davison, S.P. Keloid pathogenesis and treatment. Plast. Reconstr. Surg. 2006, 117, 286–300. [Google Scholar] [CrossRef]
- Seifert, O.; Mrowietz, U. Keloid scarring: Bench and bedside. Arch. Dermatol. Res. 2009, 301, 259–272. [Google Scholar] [CrossRef]
- Shimazaki, M.; Nakamura, K.; Kii, I.; Kashima, T.; Amizuka, N.; Li, M.; Saito, M.; Fukuda, K.; Nishiyama, T.; Kitajima, S.; et al. Periostin is essential for cardiac healing after acute myocardial infarction. J. Exp. Med. 2008, 205, 295–303. [Google Scholar] [CrossRef]
- Ando, J.; Yamamoto, K. Effects of shear stress and stretch on endothelial function. Antioxid. Redox Signal 2011, 15, 1389–1403. [Google Scholar] [CrossRef]
- Escudé, M.; Rigozzi, M.K.; Terentjev, E.M. How Cells Feel: Stochastic Model for a Molecular Mechanosensor. Biophys. J. 2014, 106, 124–133. [Google Scholar] [CrossRef]
- Huang, C.; Miyazaki, K.; Akaishi, S.; Watanabe, A.; Hyakusoku, H.; Ogawa, R. Biological effects of cellular stretch on human dermal fibroblasts. J. Plast. Reconstr. Aesthet. Surg. 2013, 66, e351–e361. [Google Scholar] [CrossRef]
- Kubow, K.E.; Vukmirovic, R.; Zhe, L.; Klotzsch, E.; Smith, M.L.; Gourdon, D.; Luna, S.; Vogel, V. Mechanical forces regulate the interactions of fibronectin and collagen I in extracellular matrix. Nat. Commun. 2015, 6, 8026. [Google Scholar] [CrossRef]
- Brown, J.J.; Bayat, A. Genetic susceptibility to raised dermal scarring. Br. J. Dermatol. 2009, 161, 8–18. [Google Scholar] [CrossRef]
- Huang, C.; Liu, L.; You, Z.; Wang, B.; Du, Y.; Ogawa, R. Keloid progression: A stiffness gap hypothesis. Int. Wound J. 2017, 14, 764–771. [Google Scholar] [CrossRef]
- Harn, H.I.; Ogawa, R.; Hsu, C.K.; Hughes, M.W.; Tang, M.J.; Chuong, C.M. The tension biology of wound healing. Exp. Dermatol. 2019, 28, 464–471. [Google Scholar] [CrossRef]
- Butler, P.D.; Longaker, M.T.; Yang, G.P. Current Progress in Keloid Research and Treatment. J. Am. Coll. Surg. 2008, 206, 731–741. [Google Scholar] [CrossRef]
- Naylor, M.C.; Brissett, A.E. Current concepts in the etiology and treatment of keloids. Facial Plast. Surg. 2012, 28, 504–512. [Google Scholar] [CrossRef]
- Ud-Din, S.; Bayat, A. Strategic management of keloid disease in ethnic skin: A structured approach supported by the emerging literature. Br. J. Dermatol. 2013, 169 (Suppl. 3), 71–81. [Google Scholar] [CrossRef]
- Arno, A.I.; Gauglitz, G.G.; Barret, J.P.; Jeschke, M.G. Up-to-date approach to manage keloids and hypertrophic scars: A useful guide. Burns 2014, 40, 1255–1266. [Google Scholar] [CrossRef]
- Bao, G.; Suresh, S. Cell and molecular mechanics of biological materials. Nat. Mater. 2003, 2, 715–725. [Google Scholar] [CrossRef]
- Wipff, P.J.; Majd, H.; Acharya, C.; Buscemi, L.; Meister, J.J.; Hinz, B. The covalent attachment of adhesion molecules to silicone membranes for cell stretching applications. Biomaterials 2009, 30, 1781–1789. [Google Scholar] [CrossRef]
- Carpi, N.; Piel, M. Stretching micropatterned cells on a PDMS membrane. J. Vis. Exp. 2014, 83, e51193. [Google Scholar] [CrossRef]
- Cockerill, M.; Rigozzi, M.K.; Terentjev, E.M. Mechanosensitivity of the 2nd Kind: TGF-β Mechanism of Cell Sensing the Substrate Stiffness. PLoS ONE 2015, 10, e0139959. [Google Scholar] [CrossRef]
- Chen, K.D.; Li, Y.S.; Kim, M.; Li, S.; Yuan, S.; Chien, S.; Shyy, J.Y. Mechanotransduction in response to shear stress. Roles of receptor tyrosine kinases, integrins, and Shc. J. Biol. Chem. 1999, 274, 18393–18400. [Google Scholar] [CrossRef]
- Langevin, H.M.; Bouffard, N.A.; Badger, G.J.; Iatridis, J.C.; Howe, A.K. Dynamic fibroblast cytoskeletal response to subcutaneous tissue stretch ex vivo and in vivo. Am. J. Physiol. Cell Physiol. 2005, 288, C747–C756. [Google Scholar] [CrossRef]
- Schroer, A.K.; Shotwell, M.S.; Sidorov, V.Y.; Wikswo, J.P.; Merryman, W.D. I-Wire Heart-on-a-Chip II: Biomechanical analysis of contractile, three-dimensional cardiomyocyte tissue constructs. Acta Biomater. 2017, 48, 79–87. [Google Scholar] [CrossRef]
- Huang, J.W.; Pan, H.J.; Yao, W.Y.; Tsao, Y.W.; Liao, W.Y.; Wu, C.W.; Tung, Y.C.; Lee, C.H. Interaction between lung cancer cell and myofibroblast influenced by cyclic tensile strain. Lab. Chip 2013, 13, 1114–1120. [Google Scholar] [CrossRef]
- Öhlund, D.; Elyada, E.; Tuveson, D. Fibroblast heterogeneity in the cancer wound. J. Exp. Med. 2014, 211, 1503–1523. [Google Scholar] [CrossRef]
- Hinz, B.; Phan, S.H.; Thannickal, V.J.; Galli, A.; Bochaton-Piallat, M.L.; Gabbiani, G. The myofibroblast: One function, multiple origins. Am. J. Pathol. 2007, 170, 1807–1816. [Google Scholar] [CrossRef]
- Hinz, B. Formation and function of the myofibroblast during tissue repair. J. Investig. Dermatol. 2007, 127, 526–537. [Google Scholar] [CrossRef]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef]
- Hinz, B.; Gabbiani, G. Cell-matrix and cell-cell contacts of myofibroblasts: Role in connective tissue remodeling. Thromb. Haemost. 2003, 90, 993–1002. [Google Scholar] [CrossRef]
- Desmoulière, A.; Chaponnier, C.; Gabbiani, G. Tissue repair, contraction, and the myofibroblast. Wound Repair. Regen. 2005, 13, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Huang, X.; Hecker, L.; Kurundkar, D.; Kurundkar, A.; Liu, H.; Jin, T.H.; Desai, L.; Bernard, K.; Thannickal, V.J. Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J. Clin. Investig. 2013, 123, 1096–1108. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef]
- Chapman, K.E.; Sinclair, S.E.; Zhuang, D.; Hassid, A.; Desai, L.P.; Waters, C.M. Cyclic mechanical strain increases reactive oxygen species production in pulmonary epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2005, 289, L834–L841. [Google Scholar] [CrossRef]
- Rana, M.K.; Srivastava, J.; Yang, M.; Chen, C.S.; Barber, D.L. Hypoxia increases the abundance but not the assembly of extracellular fibronectin during epithelial cell transdifferentiation. J. Cell Sci. 2015, 128, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Guan, H.; Liu, J.Q.; Zheng, Z.; Zhou, Q.; Zhang, J.; Su, L.L.; Hu, D.H. Hypoxia drives the transition of human dermal fibroblasts to a myofibroblast-like phenotype via the TGF-β1/Smad3 pathway. Int. J. Mol. Med. 2017, 39, 153–159. [Google Scholar] [CrossRef]
- Laffin, B.; Petrash, M. Expression of the Aldo-Ketoreductases AKR1B1 and AKR1B10 in Human Cancers. Front. Pharmacol. 2012, 3, 104. [Google Scholar] [CrossRef]
- Gao, Y.H.; Li, C.X.; Shen, S.M.; Li, H.; Chen, G.Q.; Wei, Q.; Wang, L.S. Hypoxia-inducible factor 1α mediates the down-regulation of superoxide dismutase 2 in von Hippel-Lindau deficient renal clear cell carcinoma. Biochem. Biophys. Res. Commun. 2013, 435, 46–51. [Google Scholar] [CrossRef]
- Rahaman, S.O.; Grove, L.M.; Paruchuri, S.; Southern, B.D.; Abraham, S.; Niese, K.A.; Scheraga, R.G.; Ghosh, S.; Thodeti, C.K.; Zhang, D.X.; et al. TRPV4 mediates myofibroblast differentiation and pulmonary fibrosis in mice. J. Clin. Investig. 2014, 124, 5225–5238. [Google Scholar] [CrossRef]
- Yang, X.R.; Lin, M.J.; Sham, J.S. Physiological functions of transient receptor potential channels in pulmonary arterial smooth muscle cells. Adv. Exp. Med. Biol. 2010, 661, 109–122. [Google Scholar] [CrossRef]
- Yang, X.R.; Lin, A.H.; Hughes, J.M.; Flavahan, N.A.; Cao, Y.N.; Liedtke, W.; Sham, J.S. Upregulation of osmo-mechanosensitive TRPV4 channel facilitates chronic hypoxia-induced myogenic tone and pulmonary hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 302, L555–L568. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, P.K.; Jaggi, A.S. TRPV4 channels: Physiological and pathological role in cardiovascular system. Basic. Res. Cardiol. 2015, 110, 54. [Google Scholar] [CrossRef] [PubMed]
- Kornreich, M.; Avinery, R.; Malka-Gibor, E.; Laser-Azogui, A.; Beck, R. Order and disorder in intermediate filament proteins. FEBS Lett. 2015, 589, 2464–2476. [Google Scholar] [CrossRef]
- Parrish, A.R. The cytoskeleton as a novel target for treatment of renal fibrosis. Pharmacol. Ther. 2016, 166, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zahalak, G.I.; McConnaughey, W.B.; Elson, E.L. Determination of cellular mechanical properties by cell poking, with an application to leukocytes. J. Biomech. Eng. 1990, 112, 283–294. [Google Scholar] [CrossRef]
- Suresh, S. Biomechanics and biophysics of cancer cells. Acta Biomater. 2007, 3, 413–438. [Google Scholar] [CrossRef]
- Han, B.; Bai, X.H.; Lodyga, M.; Xu, J.; Yang, B.B.; Keshavjee, S.; Post, M.; Liu, M. Conversion of mechanical force into biochemical signaling. J. Biol. Chem. 2004, 279, 54793–54801. [Google Scholar] [CrossRef]
- Kilkenny, D.M.; Rocheleau, J.V.; Price, J.; Reich, M.B.; Miller, G.G. c-Src regulation of fibroblast growth factor-induced proliferation in murine embryonic fibroblasts. J. Biol. Chem. 2003, 278, 17448–17454. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Ma, L.; Zhou, X.; Ponnusamy, M.; Tang, J.; Zhuang, M.A.; Tolbert, E.; Bayliss, G.; Bai, J.; Zhuang, S. Src inhibition blocks renal interstitial fibroblast activation and ameliorates renal fibrosis. Kidney Int. 2016, 89, 68–81. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, Y. Therapy for kidney fibrosis: Is the Src kinase a potential target? Kidney Int. 2016, 89, 12–14. [Google Scholar] [CrossRef]
- McCubrey, J.A.; Steelman, L.S.; Chappell, W.H.; Abrams, S.L.; Wong, E.W.; Chang, F.; Lehmann, B.; Terrian, D.M.; Milella, M.; Tafuri, A.; et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim. Biophys. Acta 2007, 1773, 1263–1284. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, K.; Ruan, M.; Yang, J.; Gao, Z. Gallic acid inhibits fibroblast growth and migration in keloids through the AKT/ERK signaling pathway. Acta Biochim. Biophys. Sin. (Shanghai) 2018, 50, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, J.C.; Lee, M.H.; Yang, C.E.; Lee, J.H.; Lee, W.J. High-Mobility Group Box 1 Mediates Fibroblast Activity via RAGE-MAPK and NF-κB Signaling in Keloid Scar Formation. Int. J. Mol. Sci. 2017, 19, 76. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Schätti, O.; Grad, S.; Goldhahn, J.; Salzmann, G.; Li, Z.; Alini, M.; Stoddart, M.J. A combination of shear and dynamic compression leads to mechanically induced chondrogenesis of human mesenchymal stem cells. Eur. Cell Mater. 2011, 22, 214–225. [Google Scholar] [CrossRef]
- Huang, C.; Akaishi, S.; Ogawa, R. Mechanosignaling pathways in cutaneous scarring. Arch. Dermatol. Res. 2012, 304, 589–597. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Won, C.; Ham, S.; Han, H.; Shin, S.; Jang, J.; Lee, S.; Kwon, C.; Cho, S.; Park, H.; et al. Increased Susceptibility to Mechanical Stretch Drives the Persistence of Keloid Fibroblasts: An Investigation Using a Stretchable PDMS Platform. Biomedicines 2024, 12, 2169. https://doi.org/10.3390/biomedicines12102169
Kim J, Won C, Ham S, Han H, Shin S, Jang J, Lee S, Kwon C, Cho S, Park H, et al. Increased Susceptibility to Mechanical Stretch Drives the Persistence of Keloid Fibroblasts: An Investigation Using a Stretchable PDMS Platform. Biomedicines. 2024; 12(10):2169. https://doi.org/10.3390/biomedicines12102169
Chicago/Turabian StyleKim, Jihee, Chihyeong Won, Seoyoon Ham, Heetak Han, Sungsik Shin, Jieun Jang, Sanghyeon Lee, Chaebeen Kwon, Sungjoon Cho, Hyeonjoo Park, and et al. 2024. "Increased Susceptibility to Mechanical Stretch Drives the Persistence of Keloid Fibroblasts: An Investigation Using a Stretchable PDMS Platform" Biomedicines 12, no. 10: 2169. https://doi.org/10.3390/biomedicines12102169





