Resveratrol Effect on α-Lactalbumin Thermal Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Differential Scanning Calorimetry (DSC)
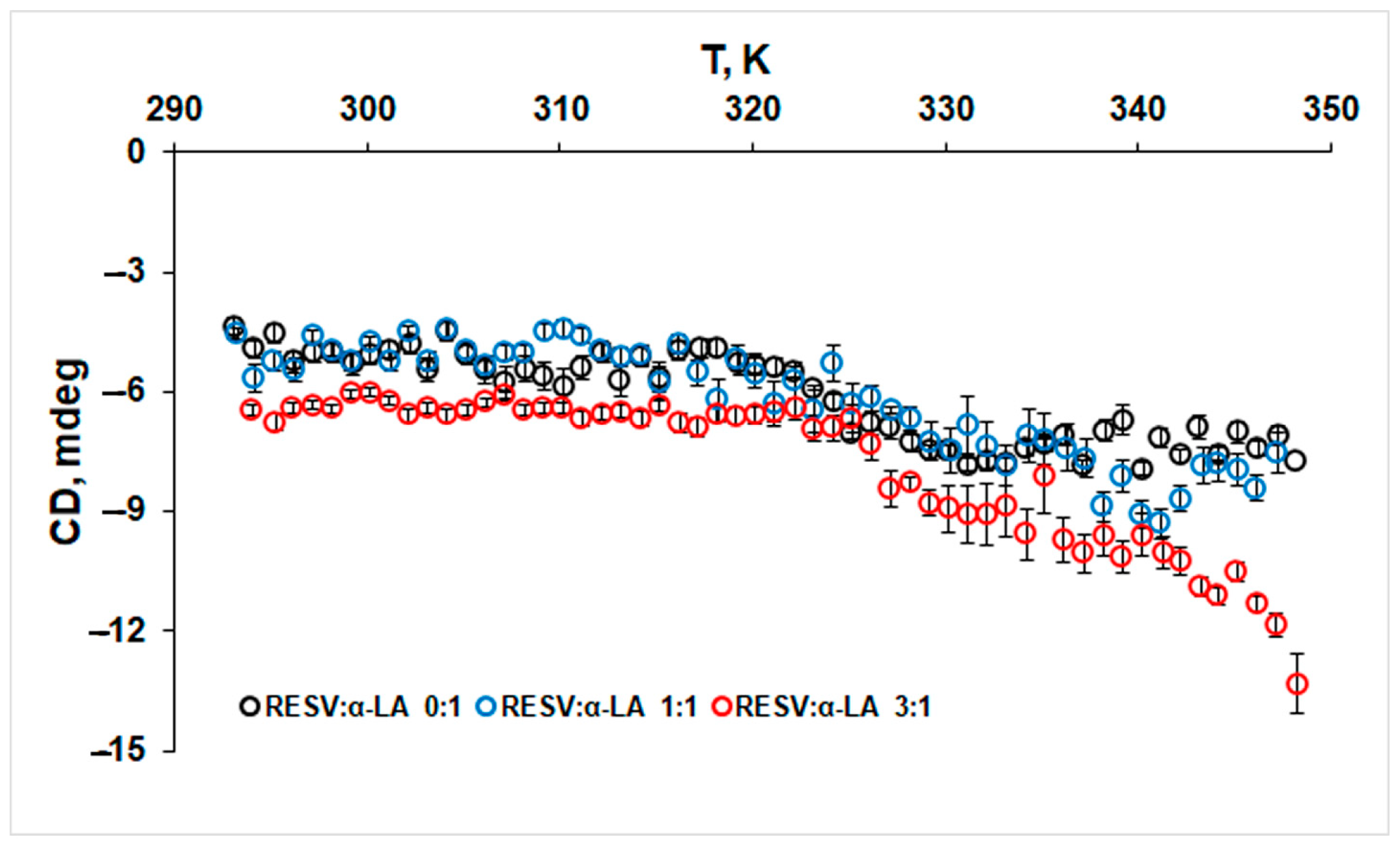
2.2.2. Circular Dichroism (CD)
2.2.3. Dynamic Light Scattering (DLS)
2.2.4. Molecular Docking
3. Results and Discussion
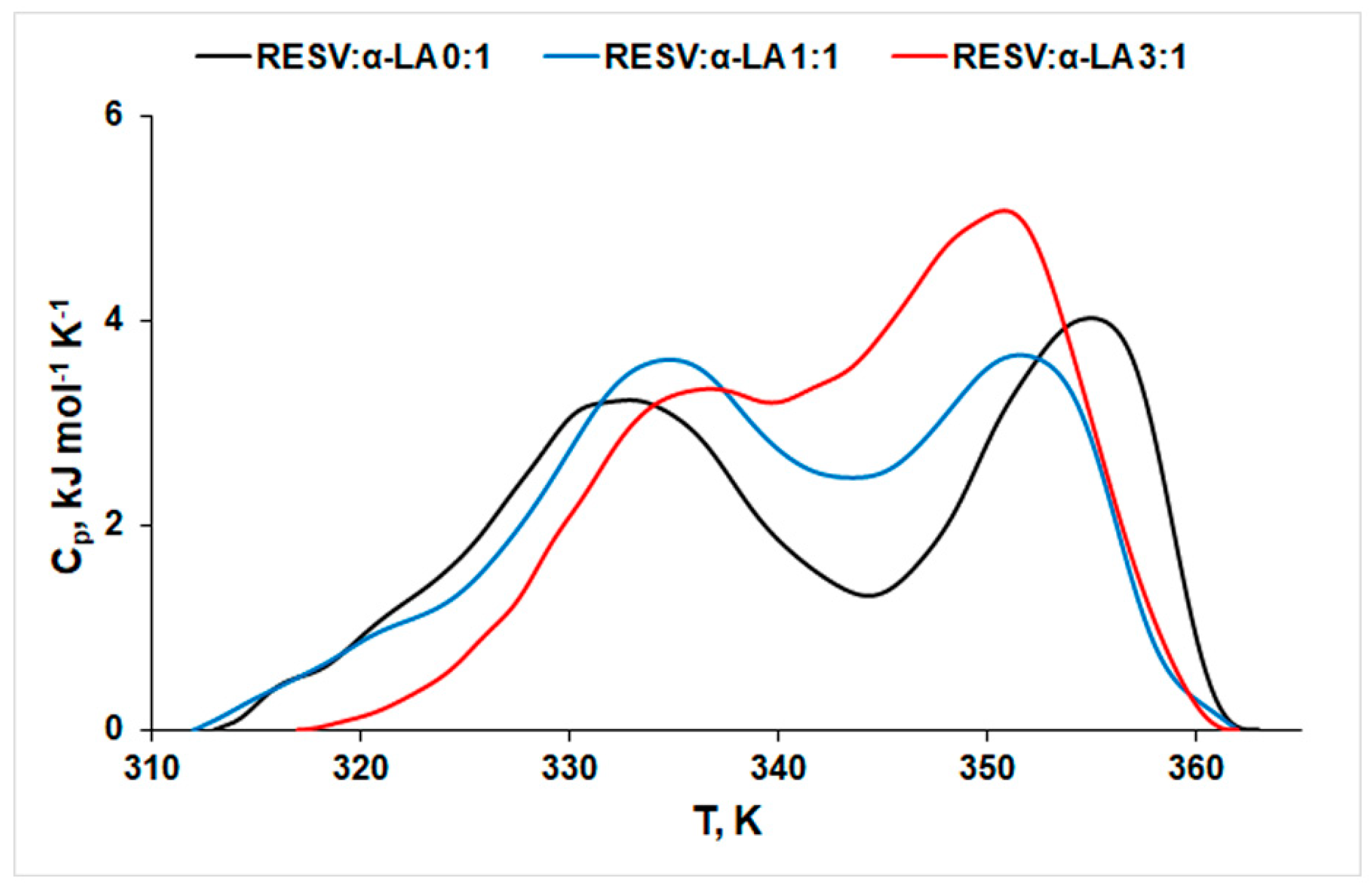
3.1. Thermal Stability of Protein in the Absence and Presence of RESV
- (1)
- On one side, the peak corresponding to the first (lower temperature, T1) process shifts to higher values, indicating a stabilizing effect of RESV. The same effect on protein structure was reported for doxorubicin [49].
- (2)
- On the other side, the temperature values corresponding to the second peak (T2) decrease with increasing concentration of RESV, pointing to a destabilization effect of both bound and free forms of the ligand on the protein structure. One may notice that RESV action on this second process is more pronounced than its action on the first process (|ΔT1| = 2.6 K, |ΔT2| = 4.2 K). Higher values of ∆Hcal obtained in the presence of RESV indicate a lower exposure of the hydrophobic regions of the native protein to the solvent molecules, during the unfolding process in the presence of RESV [19]. The positive values of ΔS sign for rearrangements within the protein molecule in the unfolding process.
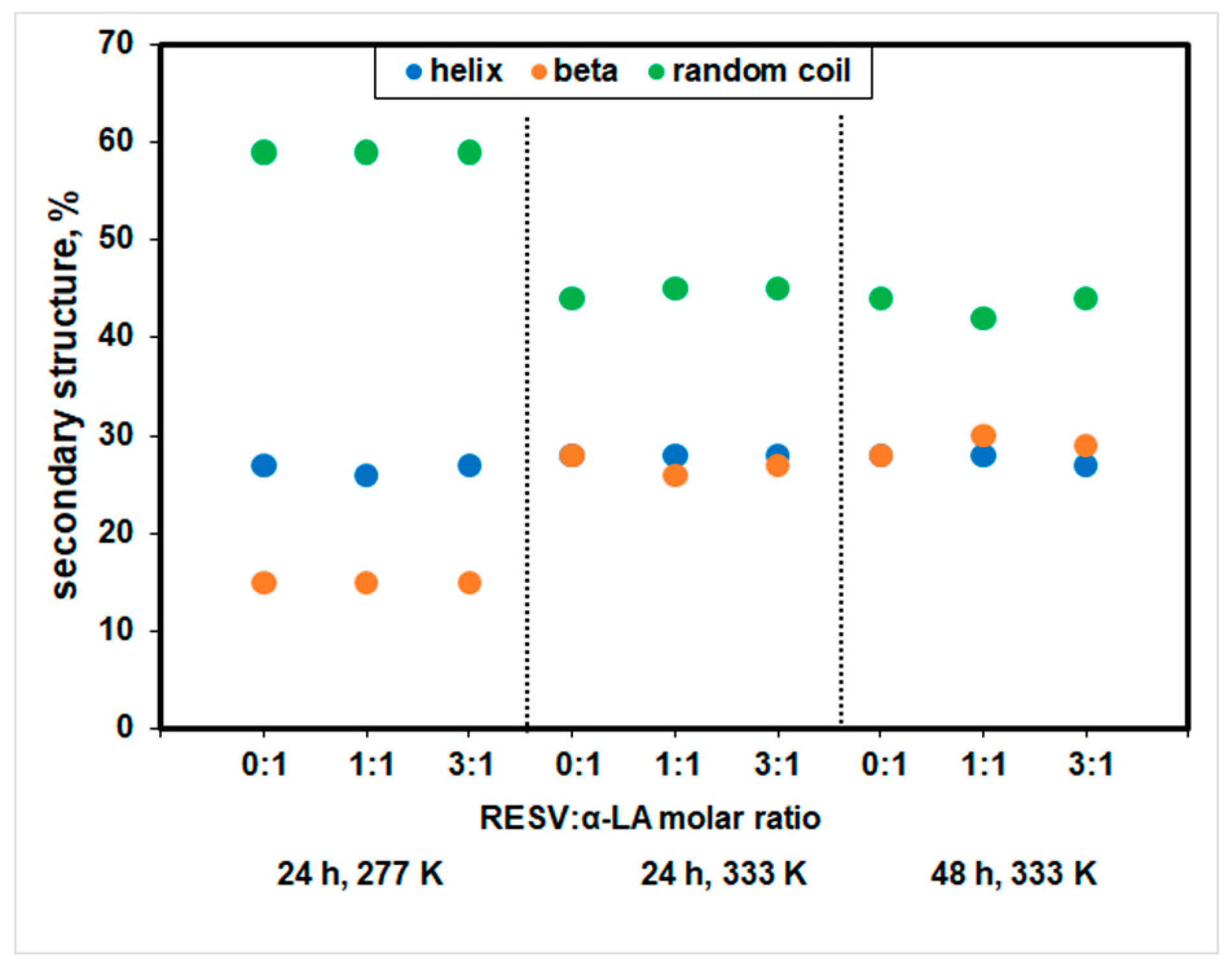
3.2. Changes in the Secondary Structure of Protein
3.3. Dynamic Light Scattering
3.4. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brew, K. α-Lactalbumin. In Advanced Dairy Chemistry: Volume 1A: Proteins: Basic Aspects, 4th ed.; McSweeney, P.L.H., Fox, P.F., Eds.; Springer: New York, NY, USA, 2013; pp. 261–273. [Google Scholar] [CrossRef]
- Gołębiowski, A.; Pomastowski, P.; Rafińska, K.; Zuvela, P.; Wong, M.W.; Pryshchepa, O.; Madajski, P.; Buszewski, B. Functionalization of Alpha-Lactalbumin by Zinc Ions. ACS Omega 2022, 7, 38459–38474. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.R.; Brew, K. Calcium regulates folding and disulfide-bond formation in α-lactalbumin. Biochem. Biophys. Res. Commun. 1989, 163, 1390–1396. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Zhang, M.; Jiang, L.; Jing, H.; Ren, F.Z. Influence of pH on the structure and oleic acid binding ability of bovine α-lactalbumin. Protein J. 2012, 31, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Zhang, M.; Ge, K.S.; Xing, H.Z.; Ren, F.Z. α-Lactalbumin-oleic acid complex kills tumor cells by inducing excess energy metabolism but inhibiting mRNA expression of the related enzymes. J. Dairy Sci. 2018, 101, 4853–4863. [Google Scholar] [CrossRef]
- Layman, D.K.; Lönnerdal, B.; Fernstrom, J.D. Applications for α-lactalbumin in human nutrition. Nutr. Rev. 2018, 76, 444–460. [Google Scholar] [CrossRef]
- Wu, W.; Hu, X.; Zeng, Z.; Wu, D.; Li, H.; Li, H. Characterization of the binding properties of sorafenib to C-MYC G-quadruplexes: Evidence for screaning potential ligands. J. Phys. Chem. B 2023, 127, 874–883. [Google Scholar] [CrossRef]
- Pellegrini, A.; Thomas, U.; Bramaz, N.; Hunziker, P.; von Fellenberg, R. Isolation and identification of three bactericidal domains in the bovine α-lactalbumin molecule. Biochim. Biophys. Acta 1999, 1426, 439–448. [Google Scholar] [CrossRef]
- Shi, R.; Liu, Y.; Ma, Y.; Li, J.; Zhang, W.; Jiang, Z.; Hou, J. Insight into binding behavior, structure, and foam properties of α-lactalbumin/glycyrrhizic acid complex in an acidic environment. Food Hydrocoll. 2022, 125, 107411. [Google Scholar] [CrossRef]
- Li, M.; Zhou, D.; Wu, D.; Hu, X.; Hu, J.; Geng, F.; Cheng, L. Comparative analysis of the interaction between alpha-lactalbumin and two edible azo colorants equipped with different sulfonyl group numbers. Food Chem. 2023, 416, 135826. [Google Scholar] [CrossRef]
- Al-Hanish, A.; Stanic-Vucinic, D.; Mihailovic, J.; Prodic, I.; Minic, S.; Stojadinovic, M.; Radibratovic, M.; Milcic, M.; Cirkovic Velickovic, T. Noncovalent interactions of bovine α-lactalbumin with green tea polyphenol, epigalocatechin-3-gallate. Food Hydrocoll. 2016, 61, 241–250. [Google Scholar] [CrossRef]
- Delavari, B.; Saboury, A.A.; Atri, M.S.; Ghasemi, A.; Bigdeli, B.; Khammari, A.; Maghami, P.; Moosavi-Movahedi, A.A.; Haertlé, T.; Goliaei, B. Alpha-lactalbumin: A new carrier for vitamin D3 food enrichment. Food Hydrocoll. 2015, 45, 124–131. [Google Scholar] [CrossRef]
- Mohammadi, F.; Moeeni, M. Analysis of binding interaction of genistein and kaempferol with bovine α-lactalbumin. J. Funct. Foods 2015, 12, 458–467. [Google Scholar] [CrossRef]
- Morris, A.M.; Watzky, M.A.; Finke, R.G. Protein aggregation kinetics, mechanism, and curve-fitting: A review of the literature. Biochim. Biophys. Acta Proteins Proteom. 2009, 1794, 375–397. [Google Scholar] [CrossRef]
- Ikeguchi, M.; Kuwajima, K.; Mitani, M.; Sugai, S. Evidence for identity between the equilibrium unfolding intermediate and a transient folding intermediate: A comparative study of the folding reactions of alpha-lactalbumin and lysozyme. Biochemistry 1986, 25, 6965–6972. [Google Scholar] [CrossRef]
- Wijesinha-Bettoni, R.; Dobson, C.M.; Redfield, C. Comparison of the denaturant-induced unfolding of the bovine and human α-lactalbumin molten globules. J. Mol. Biol. 2001, 312, 261–273. [Google Scholar] [CrossRef]
- Litwińczuk, A.; Ryu, S.R.; Nafie, L.A.; Lee, J.W.; Kim, H.I.; Jung, Y.M.; Czarnik-Matusewicz, B. The transition from the native to the acid-state characterized by multi-spectroscopy approach: Study for the holo-form of bovine α-lactalbumin. Biochim. Biophys. Acta 2014, 1844, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Yüksel, Z.; Avcı, E.; Erdem, Y.K. Characterization of binding interactions between green tea flavanoids and milk proteins. Food Chem. 2010, 121, 450–456. [Google Scholar] [CrossRef]
- Precupas, A.; Popa, V.T. Impact of Sinapic Acid on Bovine Serum Albumin Thermal Stability. Int. J. Mol. Sci. 2024, 25, 936. [Google Scholar] [CrossRef] [PubMed]
- Precupas, A.; Sandu, R.; Neculae, A.V.F.; Neacsu, A.; Popa, V.T. Calorimetric, spectroscopic and computational investigation of morin binding effect on bovine serum albumin stability. J. Mol. Liq. 2021, 333, 115953. [Google Scholar] [CrossRef]
- Precupas, A.; Popa, V.T. Insight into Protein–Ligand Interaction: The Effect on Protein Aggregation. In Advances in Chemistry Research. Volume 85; Taylor, J.C., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2024; pp. 289–340. [Google Scholar]
- Prigent, S.V.E.; Gruppen, H.; Visser, A.J.W.G.; Van Koningsveld, G.A.H.D.; Alfons, G.J.V. Effects of non-covalent interactions with 5-o-caffeoylquinic acid (CGA) on the heat denaturation and solubility of globular proteins. J. Agric. Food Chem. 2003, 51, 5088–5095. [Google Scholar] [CrossRef]
- Kanakis, C.D.; Hasni, I.; Bourassa, P.; Hamdani, S.; Tarantilis, P.A.; Tajmir-Riahi, H.A. Milk β-lactoglobulin complexes with tea polyphenols. Food Chem. 2011, 127, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Fatehi, Y.; Sahraei, A.; Mohammadi, F. Myricetin and morin hydrate inhibit amyloid fibril formation of bovine α-lactalbumin (BLA). Int. J. Biol. Macromol. 2024, 254, 127908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yu, D.; Sun, J.; Guo, H.; Ding, Q.; Liu, R.; Ren, F. Interaction of milk whey protein with common phenolic acids. J. Mol. Struct. 2014, 1058, 228–233. [Google Scholar] [CrossRef]
- Precupas, A.; Leonties, A.R.; Neacsu, A.; Angelescu, D.G.; Popa, V.T. Bovine hemoglobin thermal stability in the presence of naringenin: Calorimetric, spectroscopic and molecular modeling studies. J. Mol. Liq. 2022, 361, 119617. [Google Scholar] [CrossRef]
- Precupas, A.; Leonties, A.R.; Neacsu, A.; Sandu, R.; Popa, V.T. Gallic acid influence on bovine serum albumin thermal stability. New J. Chem. 2019, 43, 3891–3898. [Google Scholar] [CrossRef]
- Precupas, A.; Sandu, R.; Leonties, A.; Anghel, D.; Popa, V.T. Complex interaction of caffeic acid with bovine serum albumin: Calorimetric, spectroscopic and molecular docking evidence. New J. Chem. 2017, 41, 15003–15015. [Google Scholar] [CrossRef]
- Precupas, A.; Popa, V.T. Microcalorimetric investigation of ligand binding to BSA and its influence on the protein stability. In Bovine Serum Albumin: Properties and Applications; Masuelli, M.A., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2020; pp. 157–197. [Google Scholar]
- Le Bourvellec, C.; Renard, C.M.G.C. Interactions between polyphenols and macromolecules: Quantification methods and mechanisms. Crit. Rev. Food. Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef]
- Ioniţă, S.; Lincu, D.; Mitran, R.A.; Ziko, L.; Sedky, N.K.; Deaconu, M.; Brezoiu, A.M.; Matei, C.; Berger, D. Resveratrol Encapsulation and Release from Pristine and Functionalized Mesoporous Silica Carriers. Pharmaceutics 2022, 14, 203. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Tajmir-Riahi, H.A.; Subirade, M. Interaction of beta-lactoglobulin with resveratrol and its biological implications. Biomacromolecules 2008, 9, 50–56. [Google Scholar] [CrossRef]
- Mohammadi, F.; Moeeni, M. Study on the interactions of trans-resveratrol and curcumin with bovine α-lactalbumin by spectroscopic analysis and molecular docking. Mater. Sci. Eng. C 2015, 50, 358–366. [Google Scholar] [CrossRef]
- Precupas, A.; Gheorghe, D.; Botea-Petcu, A.; Leonties, A.R.; Sandu, R.; Popa, V.T.; Mariussen, E.; El Yamani, N.; Rundén-Pran, E.; Dumit, V.; et al. Thermodynamic parameters at bio/nano interface and nanomaterial toxicity: A case study on BSA interaction with ZnO, SiO2 and TiO2. Chem. Res. Toxicol. 2020, 33, 2054–2071. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, D.; Teodorescu, F.; Díez-Villares, S.; Sandu, R.; Neacsu, A.; Neacsu, D.A.; Serban, A.; Botea-Petcu, A.; Popa, V.T.; Garcia-Fernandez, J.; et al. PEGylation effects on the interaction of sphingomyelin nanoemulsions with serum albumin: A thermodynamic investigation. Macromol. Mater. Eng. 2023, 308, 2200622. [Google Scholar] [CrossRef]
- Precupas, A.; Sandu, R.; Popa, V.T. Quercetin influence on thermal denaturation of bovine serum albumin. J. Phys. Chem. B 2016, 120, 9362–9375. [Google Scholar] [CrossRef]
- Akkerman, M.; Rauh, V.M.; Christensen, M.; Johansen, L.B.; Hammershøj, M.; Larsen, L.B. Effect of heating strategies on whey protein denaturation-Revisited by liquid chromatography quadrupole time-of-flight mass spectrometry. J. Dairy Sci. 2016, 99, 152–166. [Google Scholar] [CrossRef]
- Whitmore, L.; Wallace, B.A. Protein secondary structure analyses from circular dichroism spectroscopy: Methods and Reference Databases. Biopolymers 2008, 89, 392–400. [Google Scholar] [CrossRef]
- Andrade, M.A.; Chacón, P.; Merelo, J.J.; Morán, F. Evaluation of secondary structure of proteins from UV circular dichroism using an unsupervised learning neural network. Prot. Eng. 1993, 6, 383–390. [Google Scholar] [CrossRef]
- Chrysina, E.D.; Brew, K.; Acharya, K.R. Crystal structures of apo- and holo-bovine alpha-lactalbumin at 2. 2-A resolution reveal an effect of calcium on inter-lobe interactions. J. Biol. Chem. 2000, 275, 37021–37029. [Google Scholar] [CrossRef]
- RSCB Protein Data Bank. Available online: https://www.rcsb.org/pdb (accessed on 30 April 2024).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Varlan, A.; Hillebrand, M. Exploring the capabilities of TDDFT calculations to explain the induced chirality upon a binding process: A simple case, 3-carboxycoumarin. J. Mol. Struct. 2013, 1036, 341–349. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 16, 2785–2791. [Google Scholar] [CrossRef]
- Dassault Systemes BIOVIA. Discovery Studio Modeling Environment, Release 2019, San Diego, Dassault Systemes. Available online: https://discover.3ds.com/discovery-studio-visualizer-download (accessed on 30 April 2024).
- Owusu Apenten, R.K. Thermodynamic parameters for 3-state thermal denaturation of human and bovine α-lactalbumin. Thermochim. Acta 1995, 262, 1–12. [Google Scholar] [CrossRef]
- Gill, P.; Moghadam, T.T.; Ranjbar, B. Differential scanning calorimetry techniques: Applications in biology and nanoscience. JBT 2010, 21, 167–193. [Google Scholar] [PubMed]
- Delavari, B.; Mamashli, F.; Bigdeli, B.; Poursoleiman, A.; Karami, L.; Zolmajd-Haghighi, Z.; Ghasemi, A.; Samaei-Daryan, S.; Hosseini, M.; Haertlé, T.; et al. A biophysical study on the mechanism of interactions of DOX or PTX with α-lactalbumin as a delivery carrier. Sci. Rep. 2018, 8, 17345. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, N.J. Applications of circular dichroism in protein and peptide analysis. Trends Analyt Chem. 1999, 18, 236–244. [Google Scholar] [CrossRef]
- Spöttel, J.; Brockelt, J.; Falke, S.; Rohn, S. Characterization of conjugates between α-Lactalbumin and Benzyl Isothiocyanate—Effects on Molecular Structure and Proteolytic Stability. Molecules 2021, 26, 6247. [Google Scholar] [CrossRef]
- Romano, A.; Lajterer, C.; Shpigelman, A.; Lesmes, U. Bovine alpha-lactalbumin assemblies with capsaicin: Formation, interactions, loading and physiochemical characterization. Food Chem. 2021, 352, 129306. [Google Scholar] [CrossRef]
- Vanderheeren, G.; Hanssens, I. Thermal Unfolding of Bovine α-Lactalbumin. Comparison of circular dichroism with hydrophobicity measurements. J. Biol. Chem. 1994, 269, 7090–7094. [Google Scholar] [CrossRef]
- Katouzian, I.; Jafari, S.M.; Maghsoudlou, Y.; Karami, L.; Eikani, M.H. Experimental and molecular docking study of the binding interactions between bovine α-lactalbumin and oleuropein. Food Hydrocoll. 2020, 105, 105859. [Google Scholar] [CrossRef]
- Diao, M.; Liang, Y.; Zhao, J.; Zhang, J.; Zhang, T. Complexation of ellagic acid with α-lactalbumin and its antioxidant property. Food Chem. 2022, 372, 131307. [Google Scholar] [CrossRef]



| Ligand–Protein System | RESV Concentration, M | α-LA Concentration, M |
|---|---|---|
| RESV:α-LA 0:1 | 0 | 1.43 × 10−4 |
| RESV:α-LA 1:1 | 1.43 × 10−4 | 1.43 × 10−4 |
| RESV:α-LA 3:1 | 4.28 × 10−4 | 1.43 × 10−4 |
| Thermodynamic Parameters | RESV:α-LA 0:1 | RESV:α-LA 1:1 | RESV:α-LA 3:1 |
|---|---|---|---|
| T1, K | 333.14 ± 0.07 | 334.05 ± 0.09 | 335.74 ± 1.32 |
| T2, K | 355.20 ± 0.05 | 352.04 ± 0.16 | 350.99 ± 0.42 |
| ∆H1, kJ mol−1 | 54.69 ± 0.07 | 43.54 ± 0.22 | 58.18 ± 3.17 |
| ∆H2, kJ mol−1 | 45.37 ± 0.06 | 57.86 ± 0.22 | 47.75 ± 2.55 |
| ∆Hcal, kJ mol−1 | 100.33 | 101.40 | 105.93 |
| ∆S, kJ mol−1 K−1 | 0.283 | 0.289 | 0.312 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Precupas, A.; Gheorghe, D.; Leonties, A.R.; Popa, V.T. Resveratrol Effect on α-Lactalbumin Thermal Stability. Biomedicines 2024, 12, 2176. https://doi.org/10.3390/biomedicines12102176
Precupas A, Gheorghe D, Leonties AR, Popa VT. Resveratrol Effect on α-Lactalbumin Thermal Stability. Biomedicines. 2024; 12(10):2176. https://doi.org/10.3390/biomedicines12102176
Chicago/Turabian StylePrecupas, Aurica, Daniela Gheorghe, Anca Ruxandra Leonties, and Vlad Tudor Popa. 2024. "Resveratrol Effect on α-Lactalbumin Thermal Stability" Biomedicines 12, no. 10: 2176. https://doi.org/10.3390/biomedicines12102176








