Hepatocellular Carcinoma in Mice Affects Neuronal Activity and Glia Cells in the Suprachiasmatic Nucleus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Experiments and Tissue Preparation
2.2. Immunohistochemistry
2.2.1. Chromogenic Reaction
2.2.2. Immunofluorescence
2.2.3. Antibody Specificity
2.3. Image Acquisition and Analysis
2.4. Statistical Analysis
3. Results
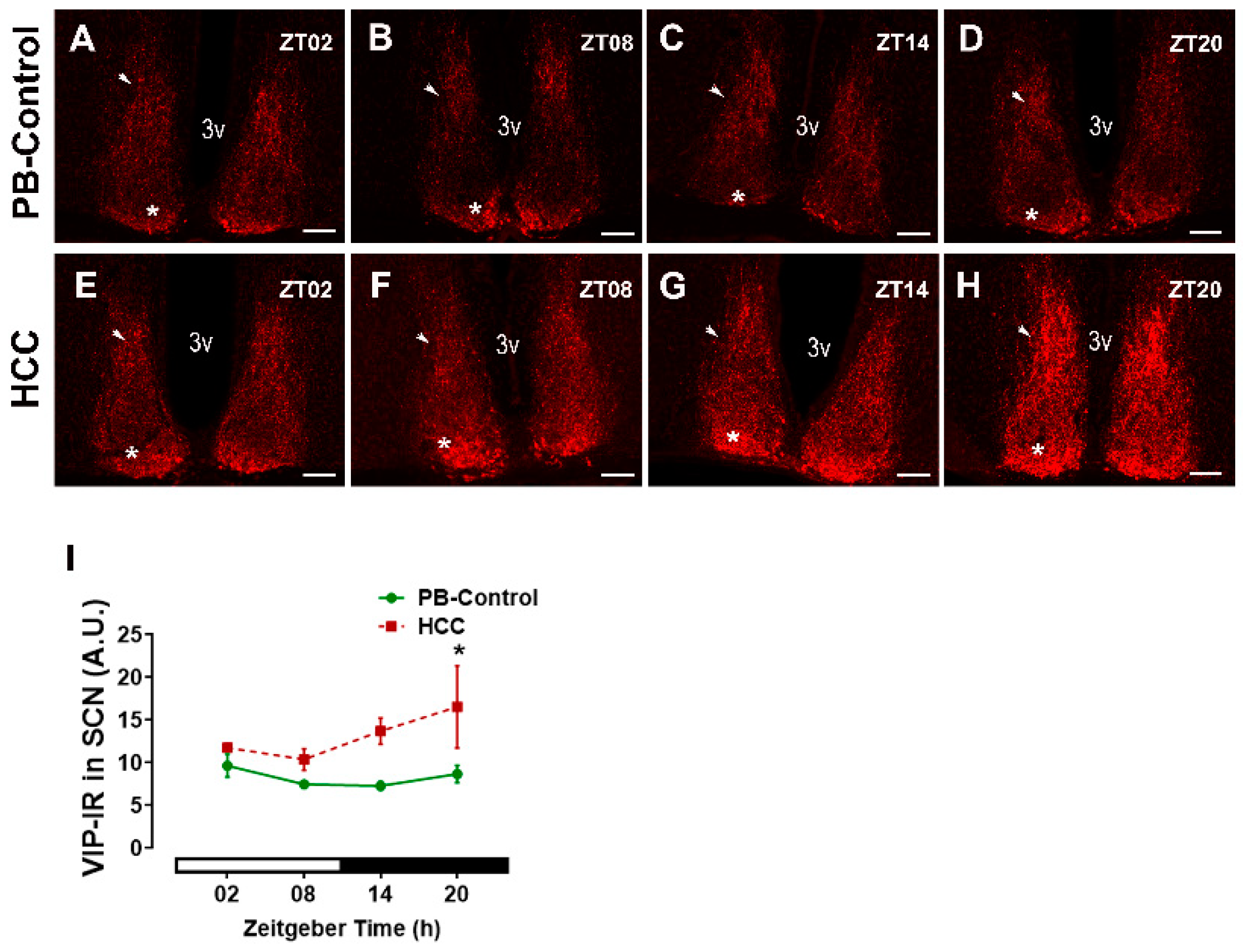
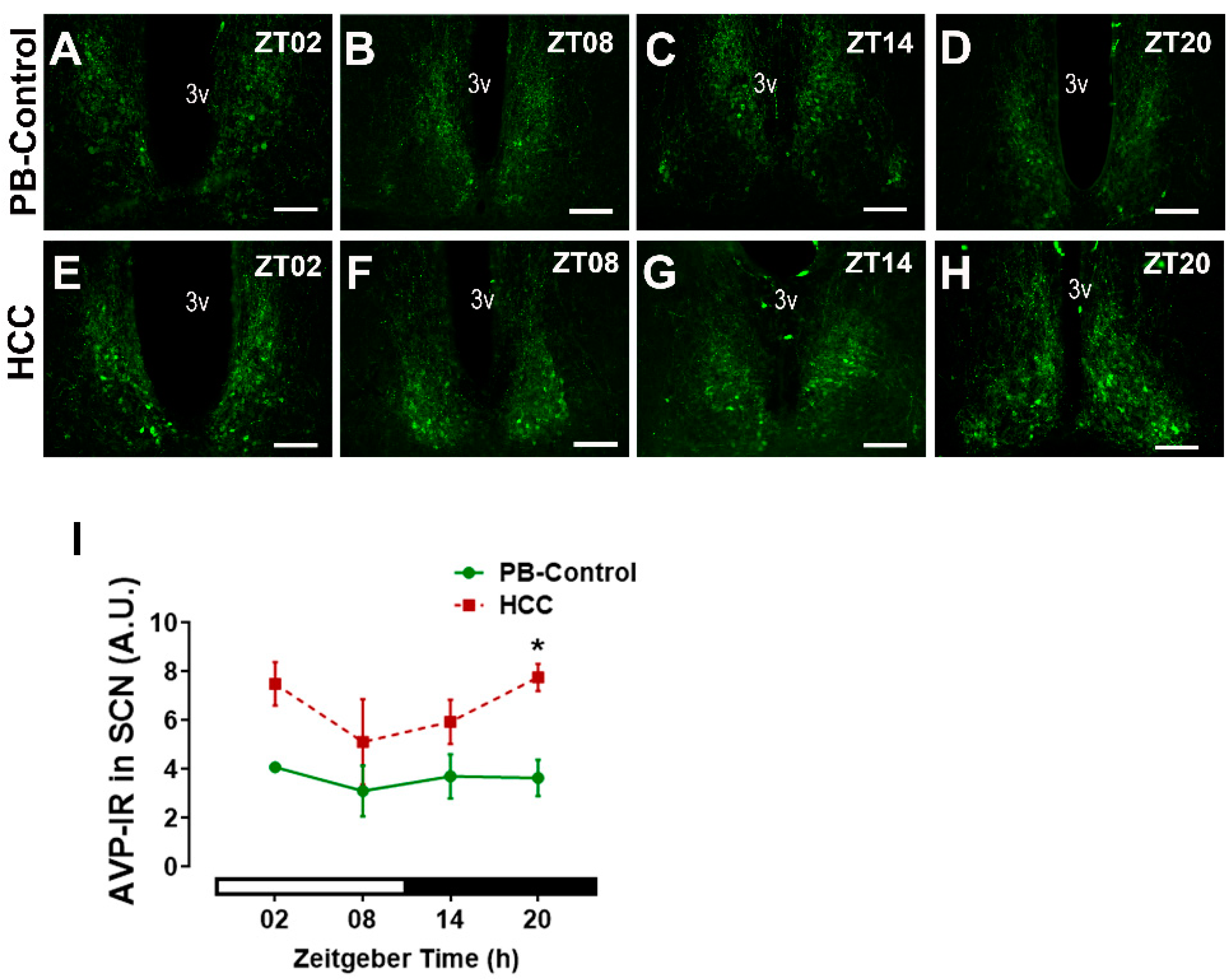
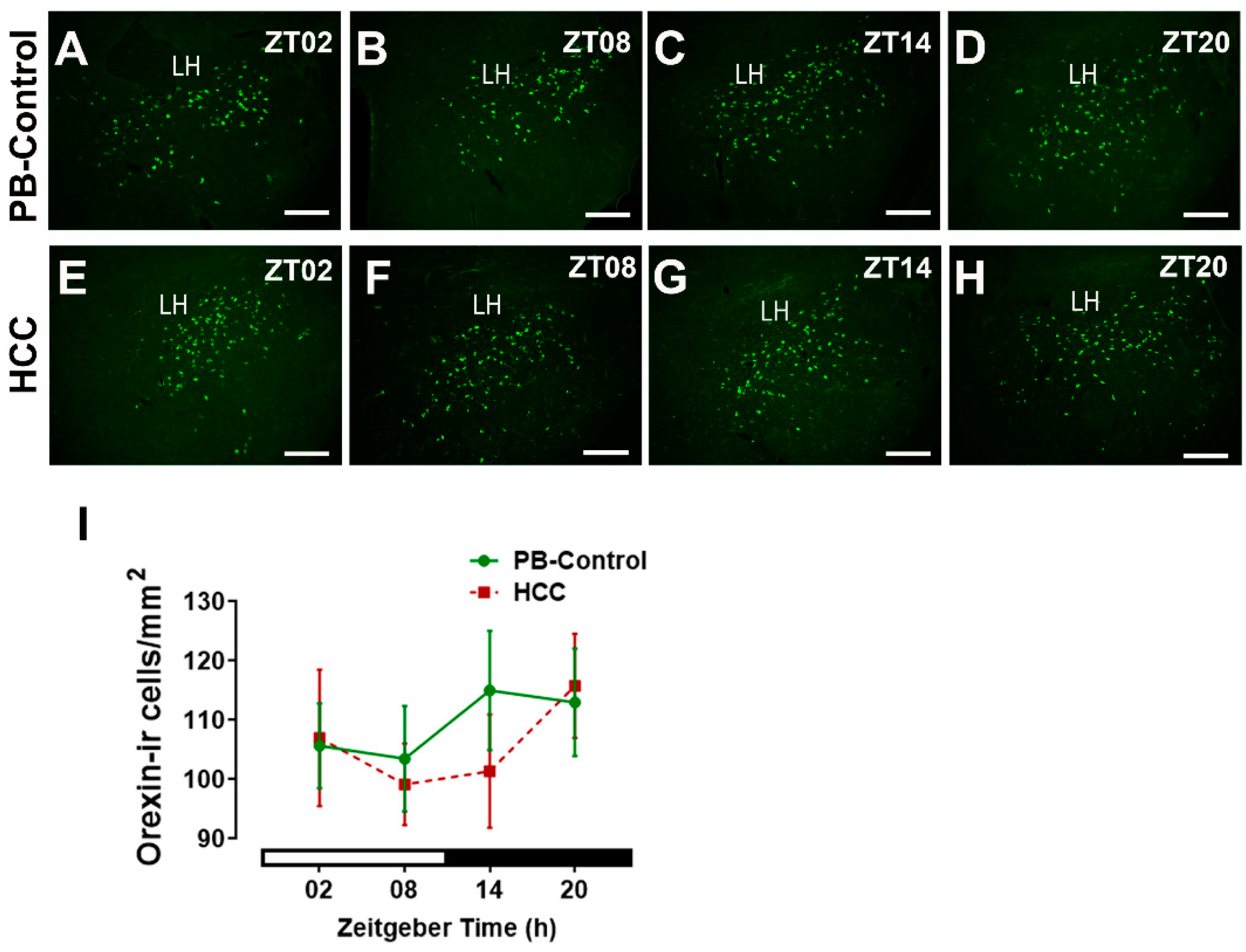
3.1. Effect of HCC on Neuropeptides in SCN and Lateral Hypothalamus
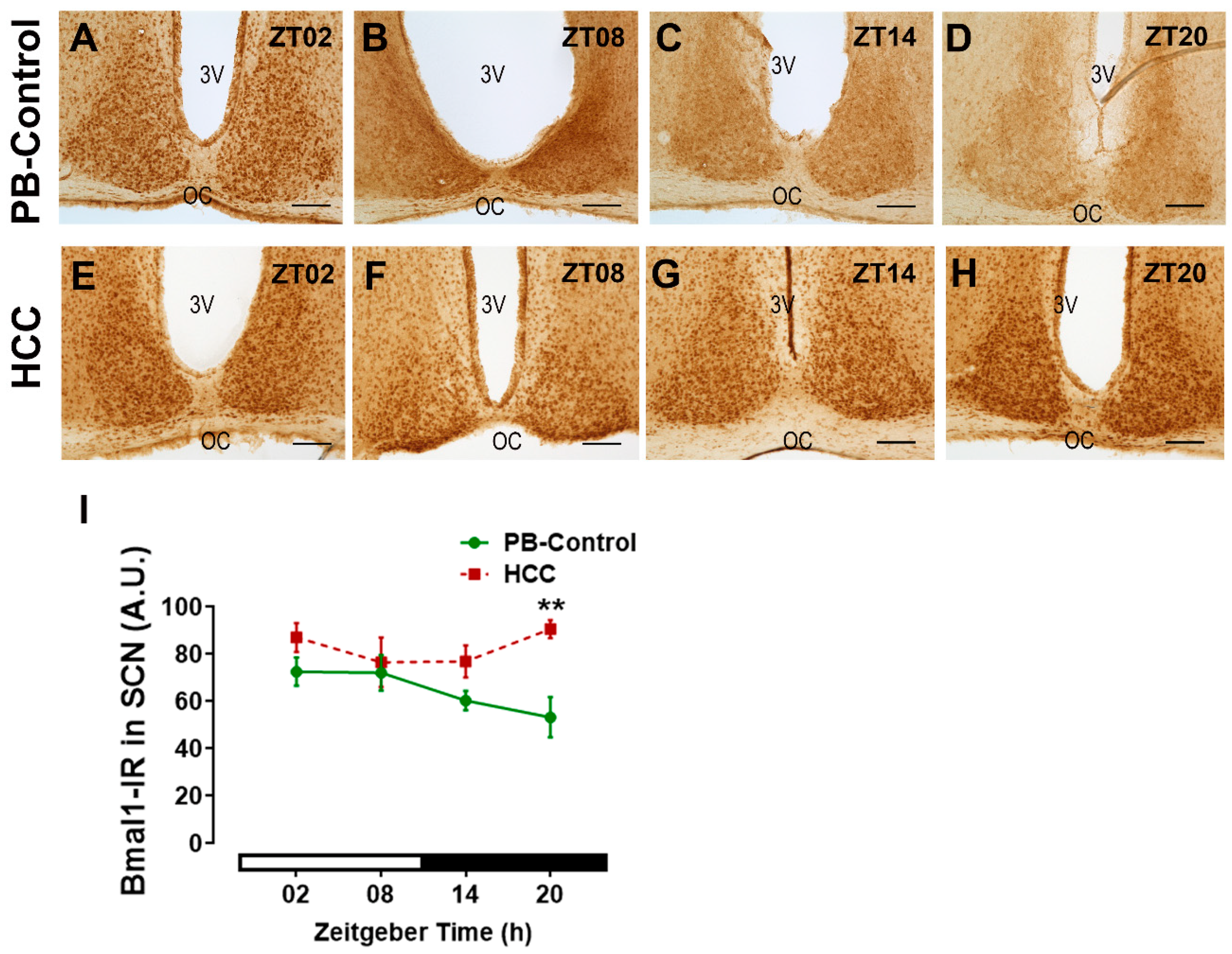
3.2. Effect of HCC on Bmal1-Immunoreactive Cells in SCN
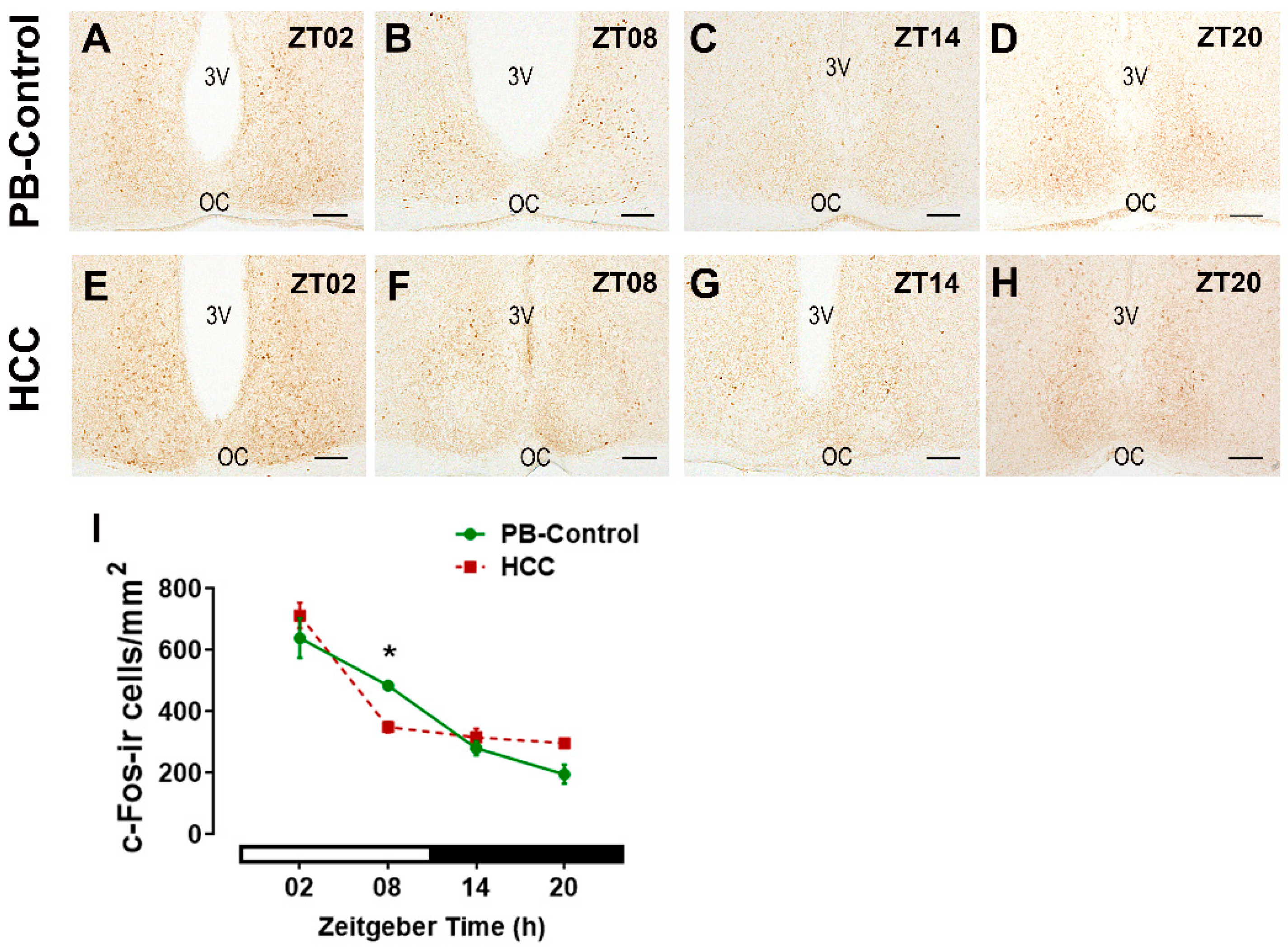
3.3. Effect of HCC on the Neuronal Activity Marker c-Fos in SCN
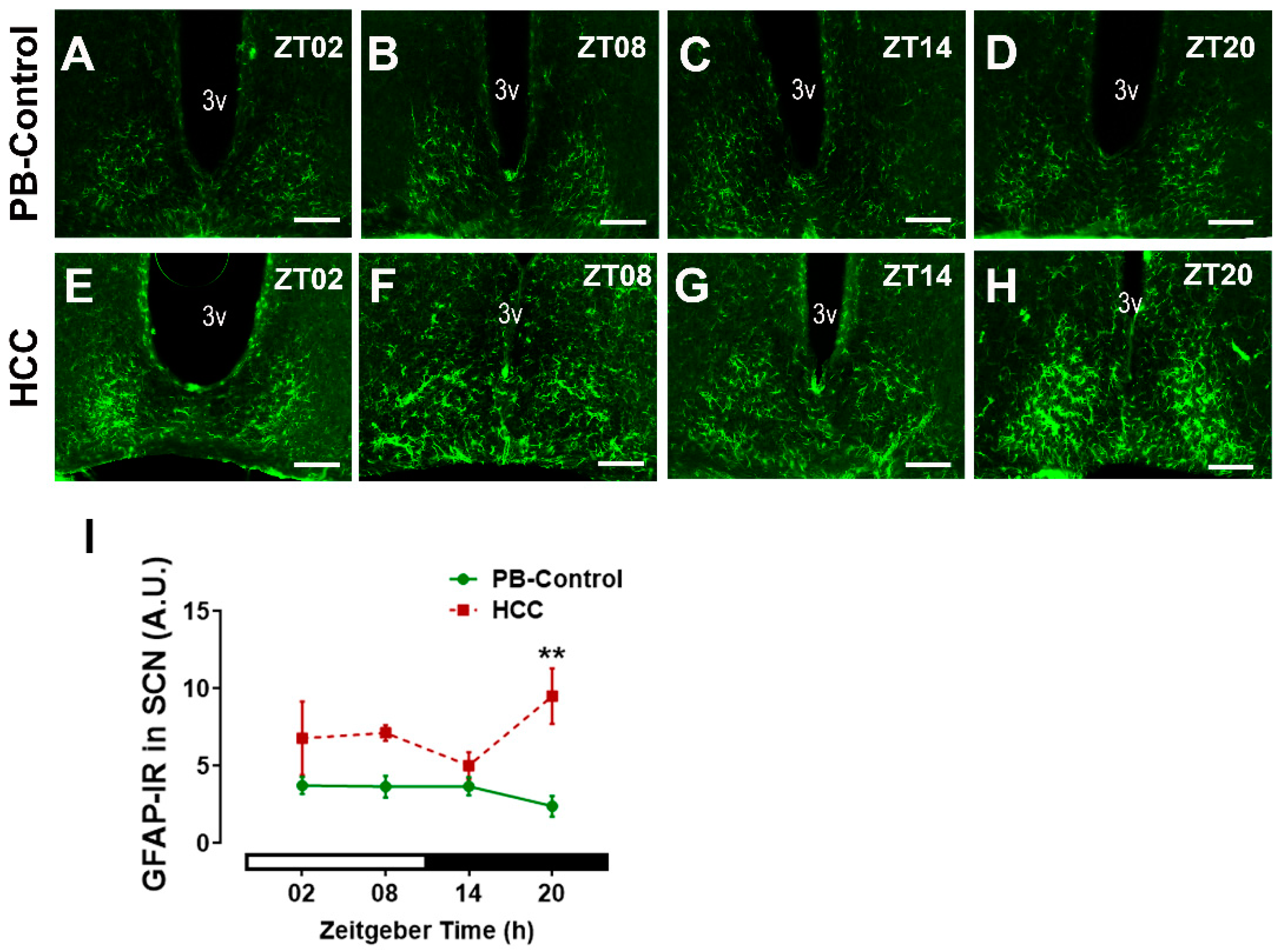
3.4. Effect of HCC on Astrocytic Marker GFAP in SCN
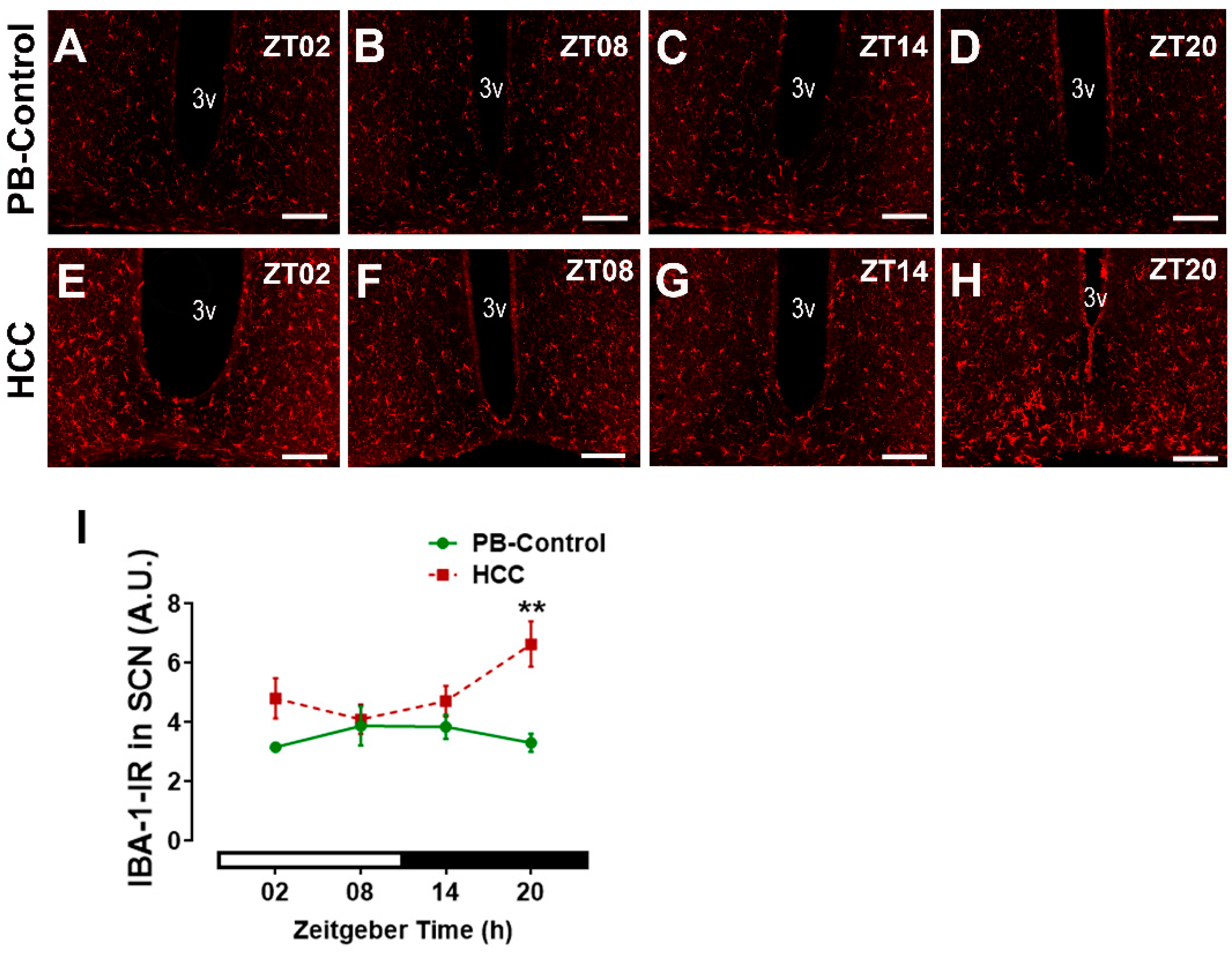
3.5. Effect of HCC on Microglial Marker IBA-1 in SCN
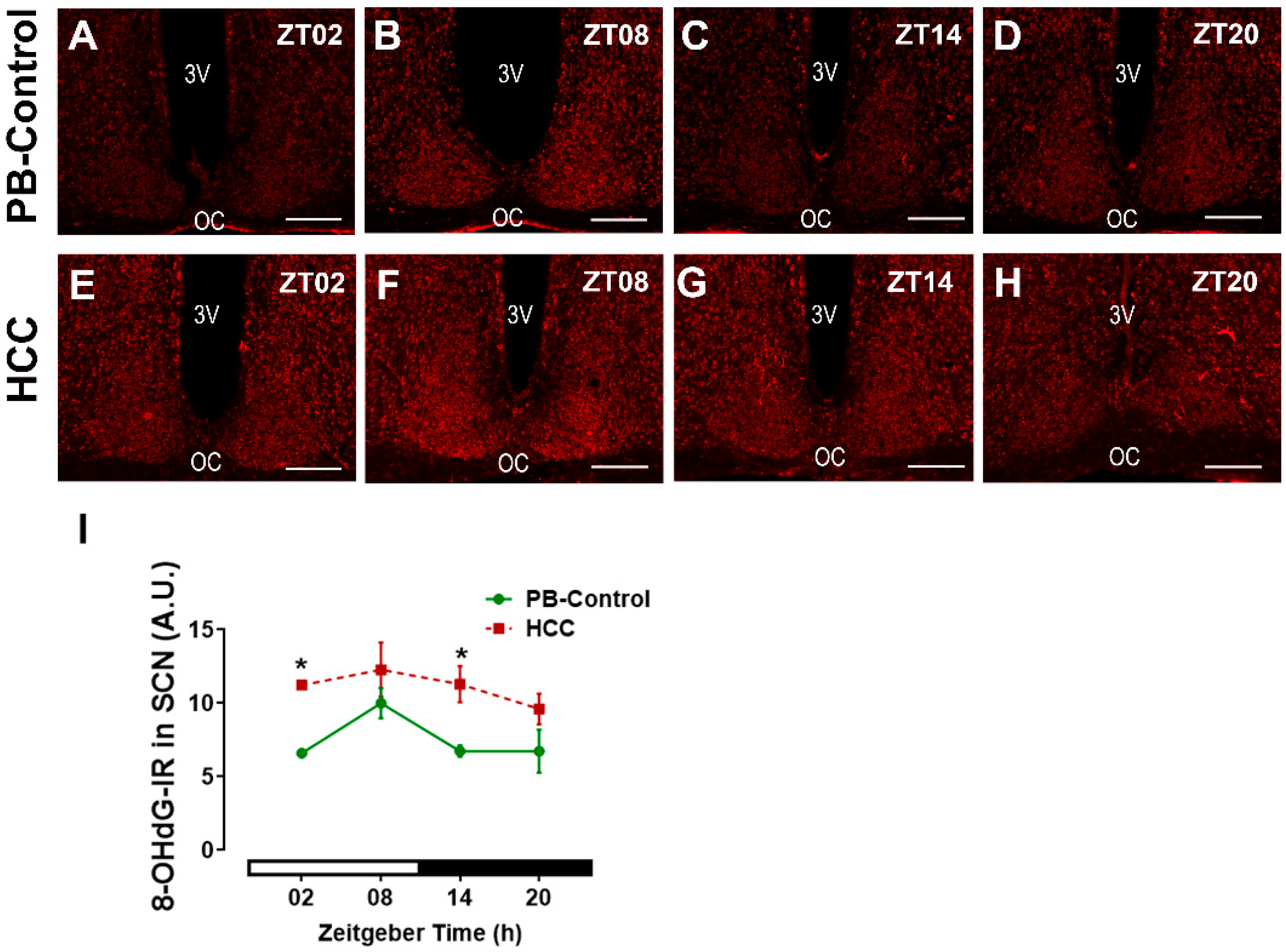
3.6. Effect of HCC on Oxidative Stress Marker 8-OHdG in SCN
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Swain, M.G.; Jones, D.E.J. Fatigue in chronic liver disease: New insights and therapeutic approaches. Liver Int. Off. J. Int. Assoc. Study Liver 2019, 39, 6–19. [Google Scholar] [CrossRef]
- Singal, A.G.; Kanwal, F.; Llovet, J.M. Global trends in hepatocellular carcinoma epidemiology: Implications for screening, prevention and therapy. Nat. Rev. Clin. Oncol. 2023, 20, 864–884. [Google Scholar] [CrossRef]
- Rao, A.; Rich, N.E.; Marrero, J.A.; Yopp, A.C.; Singal, A.G. Diagnostic and Therapeutic Delays in Patients With Hepatocellular Carcinoma. J. Natl. Compr. Cancer Netw. 2021, 19, 1063–1071. [Google Scholar] [CrossRef]
- Qiu, X.; Li, M.; Wu, L.; Xin, Y.; Mu, S.; Li, T.; Song, K. Severe Fatigue is an Important Factor in the Prognosis of Patients with Advanced Hepatocellular Carcinoma Treated with Sorafenib. Cancer Manag. Res. 2020, 12, 7983–7992. [Google Scholar] [CrossRef]
- Ee, C.; Kay, S.; Reynolds, A.; Lovato, N.; Lacey, J.; Koczwara, B. Lifestyle and integrative oncology interventions for cancer-related fatigue and sleep disturbances. Maturitas 2024, 187, 108056. [Google Scholar] [CrossRef]
- Sleight, A.G.; Crowder, S.L.; Skarbinski, J.; Coen, P.; Parker, N.H.; Hoogland, A.I.; Gonzalez, B.D.; Playdon, M.C.; Cole, S.; Ose, J.; et al. A New Approach to Understanding Cancer-Related Fatigue: Leveraging the 3P Model to Facilitate Risk Prediction and Clinical Care. Cancers 2022, 14, 1982. [Google Scholar] [CrossRef]
- Herzog, E.D.; Hermanstyne, T.; Smyllie, N.J.; Hastings, M.H. Regulating the Suprachiasmatic Nucleus (SCN) Circadian Clockwork: Interplay between Cell-Autonomous and Circuit-Level Mechanisms. Cold Spring Harb. Perspect. Biol. 2017, 9, a027706. [Google Scholar] [CrossRef]
- Silver, R.; Kriegsfeld, L.J. Circadian rhythms have broad implications for understanding brain and behavior. Eur. J. Neurosci. 2014, 39, 1866–1880. [Google Scholar] [CrossRef]
- Hastings, M.H.; Maywood, E.S.; Brancaccio, M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat. Rev. Neurosci. 2018, 19, 453–469. [Google Scholar] [CrossRef]
- Ono, D.; Honma, K.I.; Honma, S. Roles of Neuropeptides, VIP and AVP, in the Mammalian Central Circadian Clock. Front. Neurosci. 2021, 15, 650154. [Google Scholar]
- Morin, L.P. Neuroanatomy of the extended circadian rhythm system. Exp. Neurol. 2013, 243, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, M.; Korf, H.W.; von Gall, C. Chronotype and stability of spontaneous locomotor activity rhythm in BMAL1-deficient mice. Chronobiol. Int. 2015, 32, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, M.; Muller, C.M.; Mordel, J.; Meissl, H.; Ansari, N.; Deller, T.; Korf, H.W.; von Gall, C. The mammalian molecular clockwork controls rhythmic expression of its own input pathway components. J. Neurosci. 2009, 29, 6114–6123. [Google Scholar] [CrossRef]
- Brancaccio, M.; Edwards, M.D.; Patton, A.P.; Smyllie, N.J.; Chesham, J.E.; Maywood, E.S.; Hastings, M.H. Cell-autonomous clock of astrocytes drives circadian behavior in mammals. Science 2019, 363, 187–192. [Google Scholar] [CrossRef]
- Ali, A.A.H.; Stahr, A.; Ingenwerth, M.; Theis, M.; Steinhäuser, C.; von Gall, C. Connexin30 and Connexin43 show a time-of-day dependent expression in the mouse suprachiasmatic nucleus and modulate rhythmic locomotor activity in the context of chronodisruption. Cell Commun. Signal. 2019, 17, 61. [Google Scholar] [CrossRef]
- Mazuski, C.; Abel, J.H.; Chen, S.P.; Hermanstyne, T.O.; Jones, J.R.; Simon, T.; Doyle, F.J.; Herzog, E.D. Entrainment of Circadian Rhythms Depends on Firing Rates and Neuropeptide Release of VIP SCN Neurons. Neuron 2018, 99, 555–563.e555. [Google Scholar] [CrossRef]
- Todd, W.D.; Venner, A.; Anaclet, C.; Broadhurst, R.Y.; De Luca, R.; Bandaru, S.S.; Issokson, L.; Hablitz, L.M.; Cravetchi, O.; Arrigoni, E.; et al. Suprachiasmatic VIP neurons are required for normal circadian rhythmicity and comprised of molecularly distinct subpopulations. Nat. Commun. 2020, 11, 4410. [Google Scholar] [CrossRef]
- Jones, J.R.; Simon, T.; Lones, L.; Herzog, E.D. SCN VIP Neurons Are Essential for Normal Light-Mediated Resetting of the Circadian System. J. Neurosci. Off. J. Soc. Neurosci. 2018, 38, 7986–7995. [Google Scholar] [CrossRef] [PubMed]
- Reghunandanan, V. Vasopressin in circadian function of SCN. J. Biosci. 2020, 45, 140. [Google Scholar] [CrossRef]
- Gau, D.; Lemberger, T.; von Gall, C.; Kretz, O.; Le Minh, N.; Gass, P.; Schmid, W.; Schibler, U.; Korf, H.W.; Schutz, G. Phosphorylation of CREB Ser142 regulates light-induced phase shifts of the circadian clock. Neuron 2002, 34, 245–253. [Google Scholar] [CrossRef]
- Gall, C.v.; Duffield, G.E.; Hastings, M.H.; Kopp, M.D.A.; Dehghani, F.; Korf, H.-W.; Stehle, J.H. CREB in the Mouse SCN: A Molecular Interface Coding the Phase-Adjusting Stimuli Light, Glutamate, PACAP, and Melatonin for Clockwork Access. J. Neurosci. 1998, 18, 10389–10397. [Google Scholar] [CrossRef] [PubMed]
- von Gall, C. The Effects of Light and the Circadian System on Rhythmic Brain Function. Int. J. Mol. Sci. 2022, 23, 2778. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, Y.; Kiyohara, H.; Teratani, T.; Mikami, Y.; Kanai, T. Organ and brain crosstalk: The liver-brain axis in gastrointestinal, liver, and pancreatic diseases. Neuropharmacology 2022, 205, 108915. [Google Scholar] [CrossRef]
- Hunt, N.J.; Wahl, D.; Westwood, L.J.; Lockwood, G.P.; Le Couteur, D.G.; Cogger, V.C. Targeting the liver in dementia and cognitive impairment: Dietary macronutrients and diabetic therapeutics. Adv. Drug Deliv. Rev. 2022, 190, 114537. [Google Scholar] [CrossRef]
- Görg, B.; Qvartskhava, N.; Bidmon, H.J.; Palomero-Gallagher, N.; Kircheis, G.; Zilles, K.; Häussinger, D. Oxidative stress markers in the brain of patients with cirrhosis and hepatic encephalopathy. Hepatology 2010, 52, 256–265. [Google Scholar] [CrossRef]
- Haussinger, D.; Dhiman, R.K.; Felipo, V.; Gorg, B.; Jalan, R.; Kircheis, G.; Merli, M.; Montagnese, S.; Romero-Gomez, M.; Schnitzler, A.; et al. Hepatic encephalopathy. Nat. Rev. Dis. Primers 2022, 8, 43. [Google Scholar] [CrossRef]
- Haussinger, D.; Butz, M.; Schnitzler, A.; Gorg, B. Pathomechanisms in hepatic encephalopathy. Biol. Chem. 2021, 402, 1087–1102. [Google Scholar] [CrossRef] [PubMed]
- Schulien, I.; Hasselblatt, P. Diethylnitrosamine-induced liver tumorigenesis in mice. Methods Cell Biol. 2021, 163, 137–152. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Huang, J.; Xu, J.; Chen, F.; Zhu, Z.; Gao, L.; Qin, J.; Liu, B.; Liang, C. Sleep Disturbance Affects Immune Factors in Clinical Liver Cancer Patients. Curr. Oncol. 2022, 29, 7943–7952. [Google Scholar] [CrossRef]
- Huang, T.W.; Cheung, D.S.T.; Xu, X.; Loh, E.W.; Lai, J.H.; Su, W.W.; Wu, S.S.; Lin, C.C. Relationship Between Diurnal Cortisol Profile and Sleep Quality in Patients With Hepatocellular Carcinoma. Biol. Res. Nurs. 2020, 22, 139–147. [Google Scholar] [CrossRef]
- Qu, M.; Zhang, G.; Qu, H.; Vu, A.; Wu, R.; Tsukamoto, H.; Jia, Z.; Huang, W.; Lenz, H.J.; Rich, J.N.; et al. Circadian regulator BMAL1::CLOCK promotes cell proliferation in hepatocellular carcinoma by controlling apoptosis and cell cycle. Proc. Natl. Acad. Sci. USA 2023, 120, e2214829120. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Shen, X.; Fasae, M.B.; Zhi, F.; Chai, L.; Ou, Y.; Feng, H.; Liu, S.; Liu, Y.; Yang, S. The Expression and Function of Circadian Rhythm Genes in Hepatocellular Carcinoma. Oxidative Med. Cell. Longev. 2021, 2021, 4044606. [Google Scholar] [CrossRef]
- Lin, Y.M.; Chang, J.H.; Yeh, K.T.; Yang, M.Y.; Liu, T.C.; Lin, S.F.; Su, W.W.; Chang, J.G. Disturbance of circadian gene expression in hepatocellular carcinoma. Mol. Carcinog. 2008, 47, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.L.; Yu, C.; Jiang, J.X.; Liu, L.P.; Fang, X.; Wu, C. Hepatitis B virus X protein disrupts the balance of the expression of circadian rhythm genes in hepatocellular carcinoma. Oncol. Lett. 2014, 8, 2715–2720. [Google Scholar] [CrossRef]
- Hassan, S.A.; Ali, A.A.H.; Sohn, D.; Flögel, U.; Jänicke, R.U.; Korf, H.W.; von Gall, C. Does timing matter in radiotherapy of hepatocellular carcinoma? An experimental study in mice. Cancer Med. 2021, 10, 7712–7725. [Google Scholar] [CrossRef]
- Yassine, M.; Hassan, S.A.; Sommer, S.; Yücel, L.A.; Bellert, H.; Hallenberger, J.; Sohn, D.; Korf, H.W.; von Gall, C.; Ali, A.A.H. Radiotherapy of the Hepatocellular Carcinoma in Mice Has a Time-of-Day-Dependent Impact on the Mouse Hippocampus. Cells 2022, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.A.; Ali, A.A.H.; Yassine, M.; Sohn, D.; Pfeffer, M.; Jänicke, R.U.; Korf, H.W.; von Gall, C. Relationship between locomotor activity rhythm and corticosterone levels during HCC development, progression, and treatment in a mouse model. J. Pineal Res. 2021, 70, e12724. [Google Scholar] [CrossRef]
- Garofalo, S.; Cocozza, G.; Mormino, A.; Bernardini, G.; Russo, E.; Ielpo, D.; Andolina, D.; Ventura, R.; Martinello, K.; Renzi, M.; et al. Natural killer cells and innate lymphoid cells 1 tune anxiety-like behavior and memory in mice via interferon-γ and acetylcholine. Nat. Commun. 2023, 14, 3103. [Google Scholar] [CrossRef]
- Suzuki, S.; Handa, R.J. Estrogen receptor-beta, but not estrogen receptor-alpha, is expressed in prolactin neurons of the female rat paraventricular and supraoptic nuclei: Comparison with other neuropeptides. J. Comp. Neurol. 2005, 484, 28–42. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, J.; Zhang, P.P.; Chen, L.; Tang, L.L.; Yang, X.J.; He, Q.M.; Wen, X.; Sun, Y.; Liu, N.; et al. ARNTL hypermethylation promotes tumorigenesis and inhibits cisplatin sensitivity by activating CDK5 transcription in nasopharyngeal carcinoma. J. Exp. Clin. Cancer Res. 2019, 38, 11. [Google Scholar] [CrossRef]
- Imai, Y.; Ibata, I.; Ito, D.; Ohsawa, K.; Kohsaka, S. A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem. Biophys. Res. Commun. 1996, 224, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Pegram, C.N.; Eng, L.F.; Wikstrand, C.J.; McComb, R.D.; Lee, Y.L.; Bigner, D.D. Monoclonal antibodies reactive with epitopes restricted to glial fibrillary acidic proteins of several species. Neurochem. Pathol. 1985, 3, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, C.; Malaguti, M.; Rizzo, B.; Barbalace, M.C.; Fabbri, D.; Hrelia, S. Neuroprotective effect of sulforaphane against methylglyoxal cytotoxicity. Chem. Res. Toxicol. 2015, 28, 1234–1245. [Google Scholar] [CrossRef]
- Moreno-Paublete, R.; Canlon, B.; Cederroth, C.R. Differential Neural Responses Underlying the Inhibition of the Startle Response by Pre-Pulses or Gaps in Mice. Front. Cell. Neurosci. 2017, 11, 19. [Google Scholar] [CrossRef]
- Öztürk, M.; Ingenwerth, M.; Sager, M.; von Gall, C.; Ali, A.A.H. Does a Red House Affect Rhythms in Mice with a Corrupted Circadian System? Int. J. Mol. Sci. 2021, 22, 2288. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.B.J.; Paxinos, G. Paxinos and Franklin’s The Mouse Brain in Stereotaxic Coordinates, 4th ed.; Academic Press: Amsterdam, The Netherland, 2013. [Google Scholar]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef]
- Lier, J.; Streit, W.J.; Bechmann, I. Beyond Activation: Characterizing Microglial Functional Phenotypes. Cells 2021, 10, 2236. [Google Scholar] [CrossRef]
- Mogavero, M.P.; DelRosso, L.M.; Fanfulla, F.; Bruni, O.; Ferri, R. Sleep disorders and cancer: State of the art and future perspectives. Sleep. Med. Rev. 2021, 56, 101409. [Google Scholar] [CrossRef]
- Verma, D.; Hashim, O.; Jayapalan, J.; Subramanian, P. Effect of melatonin on antioxidant status and circadian activity rhythm during hepatocarcinogenesis in mice. J. Cancer Res. Ther. 2014, 10, 1040–1044. [Google Scholar]
- Moody, T.W. Peptide receptors as cancer drug targets. Ann. N. Y. Acad. Sci. 2019, 1455, 141–148. [Google Scholar] [CrossRef]
- Amidi, A.; Wu, L.M. Circadian disruption and cancer- and treatment-related symptoms. Front. Oncol. 2022, 12, 1009064. [Google Scholar] [CrossRef]
- Costa, R.; Mangini, C.; Domenie, E.D.; Zarantonello, L.; Montagnese, S. Circadian rhythms and the liver. Liver Int. 2023, 43, 534–545. [Google Scholar] [CrossRef]
- Fattovich, G.; Stroffolini, T.; Zagni, I.; Donato, F. Hepatocellular carcinoma in cirrhosis: Incidence and risk factors. Gastroenterology 2004, 127, S35–S50. [Google Scholar] [CrossRef]
- Hamnett, R.; Chesham, J.E.; Maywood, E.S.; Hastings, M.H. The Cell-Autonomous Clock of VIP Receptor VPAC2 Cells Regulates Period and Coherence of Circadian Behavior. J. Neurosci. Off. J. Soc. Neurosci. 2021, 41, 502–512. [Google Scholar] [CrossRef]
- Kahan, A.; Mahe, K.; Dutta, S.; Kassraian, P.; Wang, A.; Gradinaru, V. Immediate responses to ambient light in vivo reveal distinct subpopulations of suprachiasmatic VIP neurons. iScience 2023, 26, 107865. [Google Scholar] [CrossRef]
- Collins, B.; Pierre-Ferrer, S.; Muheim, C.; Lukacsovich, D.; Cai, Y.; Spinnler, A.; Herrera, C.G.; Wen, S.; Winterer, J.; Belle, M.D.C.; et al. Circadian VIPergic Neurons of the Suprachiasmatic Nuclei Sculpt the Sleep-Wake Cycle. Neuron 2020, 108, 486–499.e5. [Google Scholar] [CrossRef]
- Kalsbeek, A.; Buijs, R.M. Organization of the neuroendocrine and autonomic hypothalamic paraventricular nucleus. Handb. Clin. Neurol. 2021, 180, 45–63. [Google Scholar] [CrossRef]
- Takahashi, Y.; Okamura, H.; Yanaihara, N.; Hamada, S.; Fujita, S.; Ibata, Y. Vasoactive intestinal peptide immunoreactive neurons in the rat suprachiasmatic nucleus demonstrate diurnal variation. Brain Res. 1989, 497, 374–377. [Google Scholar] [CrossRef]
- Bult, A.; van der Zee, E.A.; Compaan, J.C.; Lynch, C.B. Differences in the number of arginine-vasopressin-immunoreactive neurons exist in the suprachiasmatic nuclei of house mice selected for differences in nest-building behavior. Brain Res. 1992, 578, 335–338. [Google Scholar] [CrossRef]
- Hofman, M.A. The human circadian clock and aging. Chronobiol. Int. 2000, 17, 245–259. [Google Scholar] [CrossRef]
- Hara, M.; Takeba, Y.; Iiri, T.; Ohta, Y.; Ootaki, M.; Watanabe, M.; Watanabe, D.; Koizumi, S.; Otsubo, T.; Matsumoto, N. Vasoactive intestinal peptide increases apoptosis of hepatocellular carcinoma by inhibiting the cAMP/Bcl-xL pathway. Cancer Sci. 2018, 110, 235–244. [Google Scholar] [CrossRef]
- Ravindranathan, S.; Passang, T.; Li, J.-M.; Wang, S.; Dhamsania, R.; Ware, M.B.; Zaidi, M.Y.; Zhu, J.; Cardenas, M.; Liu, Y.; et al. Targeting vasoactive intestinal peptide-mediated signaling enhances response to immune checkpoint therapy in pancreatic ductal adenocarcinoma. Nat. Commun. 2022, 13, 6418. [Google Scholar] [CrossRef]
- Reubi, J.C.; Läderach, U.; Waser, B.; Gebbers, J.-O.; Robberecht, P.; Laissue, J.A. Vasoactive Intestinal Peptide/Pituitary Adenylate Cyclase-activating Peptide Receptor Subtypes in Human Tumors and Their Tissues of Origin1. Cancer Res. 2000, 60, 3105–3112. [Google Scholar]
- Moody, T.W.; Nuche-Berenguer, B.; Jensen, R.T. Vasoactive intestinal peptide/pituitary adenylate cyclase activating polypeptide, and their receptors and cancer. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 38–47. [Google Scholar] [CrossRef]
- Su, Y.; van der Spek, R.; Foppen, E.; Kwakkel, J.; Fliers, E.; Kalsbeek, A. Effects of adrenalectomy on daily gene expression rhythms in the rat suprachiasmatic and paraventricular hypothalamic nuclei and in white adipose tissue. Chronobiol. Int. 2015, 32, 211–224. [Google Scholar] [CrossRef]
- Oesch, L.T.; Adamantidis, A.R. Sleep and Metabolism: Implication of Lateral Hypothalamic Neurons. In The Orexin System. Basic Science and Role in Sleep Pathology; Steiner, M.A., Yanagisawa, M., Clozel, M., Eds.; S.Karger AG: Basel, Switzerland, 2021; Volume 45. [Google Scholar]
- Aston-Jones, G.; Smith, R.J.; Sartor, G.C.; Moorman, D.E.; Massi, L.; Tahsili-Fahadan, P.; Richardson, K.A. Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction. Brain Res. 2010, 1314, 74–90. [Google Scholar] [CrossRef]
- Loh, D.H.; Dragich, J.M.; Kudo, T.; Schroeder, A.M.; Nakamura, T.J.; Waschek, J.A.; Block, G.D.; Colwell, C.S. Effects of vasoactive intestinal peptide genotype on circadian gene expression in the suprachiasmatic nucleus and peripheral organs. J. Biol. Rhythm. 2011, 26, 200–209. [Google Scholar] [CrossRef]
- Brancaccio, M.; Maywood, E.S.; Chesham, J.E.; Loudon, A.S.; Hastings, M.H. A Gq-Ca2+ axis controls circuit-level encoding of circadian time in the suprachiasmatic nucleus. Neuron 2013, 78, 714–728. [Google Scholar] [CrossRef]
- Vosko, A.; van Diepen, H.C.; Kuljis, D.; Chiu, A.M.; Heyer, D.; Terra, H.; Carpenter, E.; Michel, S.; Meijer, J.H.; Colwell, C.S. Role of vasoactive intestinal peptide in the light input to the circadian system. Eur. J. Neurosci. 2015, 42, 1839–1848. [Google Scholar] [CrossRef]
- An, S.; Harang, R.; Meeker, K.; Granados-Fuentes, D.; Tsai, C.A.; Mazuski, C.; Kim, J.; Doyle, F.J., 3rd; Petzold, L.R.; Herzog, E.D. A neuropeptide speeds circadian entrainment by reducing intercellular synchrony. Proc. Natl. Acad. Sci. USA 2013, 110, E4355–E4361. [Google Scholar] [CrossRef]
- Bunger, M.K.; Wilsbacher, L.D.; Moran, S.M.; Clendenin, C.; Radcliffe, L.A.; Hogenesch, J.B.; Simon, M.C.; Takahashi, J.S.; Bradfield, C.A. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 2000, 103, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- von Gall, C.; Noton, E.; Lee, C.; Weaver, D.R. Light does not degrade the constitutively expressed BMAL1 protein in the mouse suprachiasmatic nucleus. Eur. J. Neurosci. 2003, 18, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Hou, Y.; Wang, Y.; Yuan, L.; Cheng, L.; Zhang, T. Circadian misalignment impairs oligodendrocyte myelination via Bmal1 overexpression leading to anxiety and depression-like behaviors. J. Pineal Res. 2024, 76, e12935. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Ikeda, M. Bmal1 is an essential regulator for circadian cytosolic Ca2+ rhythms in suprachiasmatic nucleus neurons. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 12029–12038. [Google Scholar] [CrossRef] [PubMed]
- Barca-Mayo, O.; Pons-Espinal, M.; Follert, P.; Armirotti, A.; Berdondini, L.; De Pietri Tonelli, D. Astrocyte deletion of Bmal1 alters daily locomotor activity and cognitive functions via GABA signalling. Nat. Commun. 2017, 8, 14336. [Google Scholar] [CrossRef]
- Patton, A.P.; Smyllie, N.J.; Chesham, J.E.; Hastings, M.H. Astrocytes Sustain Circadian Oscillation and Bidirectionally Determine Circadian Period, But Do Not Regulate Circadian Phase in the Suprachiasmatic Nucleus. J. Neurosci. 2022, 42, 5522–5537. [Google Scholar] [CrossRef]
- Ali, A.A.H.; Schwarz-Herzke, B.; Rollenhagen, A.; Anstötz, M.; Holub, M.; Lübke, J.; Rose, C.R.; Schnittler, H.J.; von Gall, C. Bmal1-deficiency affects glial synaptic coverage of the hippocampal mossy fiber synapse and the actin cytoskeleton in astrocytes. Glia 2020, 68, 947–962. [Google Scholar] [CrossRef]
- Lawrence, J.M.; Schardien, K.; Wigdahl, B.; Nonnemacher, M.R. Roles of neuropathology-associated reactive astrocytes: A systematic review. Acta Neuropathol. Commun. 2023, 11, 42. [Google Scholar] [CrossRef]
- Matsui, F.; Yamaguchi, S.T.; Kobayashi, R.; Ito, S.; Nagashima, S.; Zhou, Z.; Norimoto, H. Ablation of microglia does not alter circadian rhythm of locomotor activity. Mol. Brain 2023, 16, 34. [Google Scholar] [CrossRef]
- Honzlová, P.; Semenovykh, K.; Sumová, A. The Circadian Clock of Polarized Microglia and Its Interaction with Mouse Brain Oscillators. Cell. Mol. Neurobiol. 2023, 43, 1319–1333. [Google Scholar] [CrossRef]
- Ito, D.; Imai, Y.; Ohsawa, K.; Nakajima, K.; Fukuuchi, Y.; Kohsaka, S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res. Mol. Brain Res. 1998, 57, 1–9. [Google Scholar] [CrossRef]
- Lawther, A.J.; Phillips, A.J.K.; Chung, N.C.; Chang, A.; Ziegler, A.I.; Debs, S.; Sloan, E.K.; Walker, A.K. Disrupting circadian rhythms promotes cancer-induced inflammation in mice. Brain Behav. Immun. Health 2022, 21, 100428. [Google Scholar] [CrossRef]
- Gonzalo-Gobernado, R.; Reimers, D.; Herranz, A.S.; Díaz-Gil, J.J.; Osuna, C.; Asensio, M.J.; Baena, S.; Rodríguez-Serrano, M.; Bazán, E. Mobilization of neural stem cells and generation of new neurons in 6-OHDA-lesioned rats by intracerebroventricular infusion of liver growth factor. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2009, 57, 491–502. [Google Scholar] [CrossRef]
- Pekny, M.; Pekna, M. Astrocyte reactivity and reactive astrogliosis: Costs and benefits. Physiol. Rev. 2014, 94, 1077–1098. [Google Scholar] [CrossRef]
- Jayakumar, A.R.; Norenberg, M.D. Endothelial-astrocytic interactions in acute liver failure. Metab. Brain Dis. 2013, 28, 183–186. [Google Scholar] [CrossRef]
- Tilg, H.; Diehl, A.M. Cytokines in alcoholic and nonalcoholic steatohepatitis. N. Engl. J. Med. 2000, 343, 1467–1476. [Google Scholar] [CrossRef]
- Li, H.; Tofigh, A.M.; Amirfakhraei, A.; Chen, X.; Tajik, M.; Xu, D.; Motevalli, S. Modulation of astrocyte activity and improvement of oxidative stress through blockage of NO/NMDAR pathway improve posttraumatic stress disorder (PTSD)-like behavior induced by social isolation stress. Brain Behav. 2022, 12, e2620. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, C.; Huang, J.; Tang, X.; Liu, C.; Huang, K.; Xu, J.; Guo, G.; Tong, A.; Zhou, L. The role of astrocytes in oxidative stress of central nervous system: A mixed blessing. Cell Prolif. 2020, 53, e12781. [Google Scholar] [CrossRef]
- Iizumi, T.; Takahashi, S.; Mashima, K.; Minami, K.; Izawa, Y.; Abe, T.; Hishiki, T.; Suematsu, M.; Kajimura, M.; Suzuki, N. A possible role of microglia-derived nitric oxide by lipopolysaccharide in activation of astroglial pentose-phosphate pathway via the Keap1/Nrf2 system. J. Neuroinflamm. 2016, 13, 99. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-hydroxy-2′-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 120–139. [Google Scholar] [CrossRef]
- Simicic, D.; Cudalbu, C.; Pierzchala, K. Overview of oxidative stress findings in hepatic encephalopathy: From cellular and ammonium-based animal models to human data. Anal. Biochem. 2022, 654, 114795. [Google Scholar] [CrossRef] [PubMed]
- Rich, T.; Innominato, P.F.; Boerner, J.; Mormont, M.C.; Iacobelli, S.; Baron, B.; Jasmin, C.; Lévi, F. Elevated Serum Cytokines Correlated with Altered Behavior, Serum Cortisol Rhythm, and Dampened 24-Hour Rest-Activity Patterns in Patients with Metastatic Colorectal Cancer. Clin. Cancer Res. 2005, 11, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Crews, F.T.; Sarkar, D.K.; Qin, L.; Zou, J.; Boyadjieva, N.; Vetreno, R.P. Neuroimmune Function and the Consequences of Alcohol Exposure. Alcohol. Res. 2015, 37, 331–341, 344–351. [Google Scholar] [PubMed]
- Padilla, J.; Osman, N.M.; Bissig-Choisat, B.; Grimm, S.L.; Qin, X.; Major, A.M.; Yang, L.; Lopez-Terrada, D.; Coarfa, C.; Li, F.; et al. Circadian dysfunction induces NAFLD-related human liver cancer in a mouse model. J. Hepatol. 2024, 80, 282–292. [Google Scholar] [CrossRef]
- Kotsiliti, E. Understanding HCC sex disparities. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 147. [Google Scholar] [CrossRef]
- Walton, J.C.; Bumgarner, J.R.; Nelson, R.J. Sex Differences in Circadian Rhythms. Cold Spring Harb. Perspect. Biol. 2022, 14, a039107. [Google Scholar] [CrossRef]
| Antibody | Manufacturer | Concentration |
|---|---|---|
| Anti-VIP (rabbit, monoclonal, cat #4245) | BMA Biomedicals (Augst, Switzerland) | 1:4000 |
| Anti-AVP (rabbit, polyclonal, cat # AHP372) | BIO-RAD (Berkeley, CA, USA) | 1:1000 |
| Anti-Bmal1 (rabbit, monoclonal, cat #14268-1-AP) | Proteintech (Rosemont, IL, USA) | 1:500 |
| Anti-orexin (rabbit, monoclonal, cat # 16743) | Cells Signaling Technology (Danvers, MA, USA) | 1:2000 |
| Anti-IBA1 (rabbit, polyclonal, cat # 019-19741) | WAKO (Osaka, Japan) | 1:2000 |
| Anti-GFAP (mouse, monoclonal, cat # 556330) | BD Biosciences (Eysins, Switzerland) | 1:500 |
| Anti-8-OHDG (mouse, monoclonal, cat #AM03160PU-N) | ORIGENE (Herford, Germany) | 1:1000 |
| Anti-c-Fos (rabbit, monoclonal, cat #4384) | Cells Signaling Technology (Danvers, MA, USA) | 1:1000 |
| Antibody | Manufacturer | Concentration |
|---|---|---|
| Anti-rabbit IgG Biotin (goat, cat # BA-1000) | Vector Laboratories (Burlingame, CA, USA) | 1:500 |
| Anti-rabbit IgG Alexa Fluor 488 (goat; cat # A-11036) | Molecular Probes (Eugene, OR, USA) | 1:500 |
| Anti-mouse IgG Alexa Fluor 568 (goat; cat # A-11031) | Molecular Probes (Eugene, OR, USA) | 1:500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yassine, M.; Hassan, S.A.; Yücel, L.A.; Purath, F.F.A.; Korf, H.-W.; von Gall, C.; Ali, A.A.H. Hepatocellular Carcinoma in Mice Affects Neuronal Activity and Glia Cells in the Suprachiasmatic Nucleus. Biomedicines 2024, 12, 2202. https://doi.org/10.3390/biomedicines12102202
Yassine M, Hassan SA, Yücel LA, Purath FFA, Korf H-W, von Gall C, Ali AAH. Hepatocellular Carcinoma in Mice Affects Neuronal Activity and Glia Cells in the Suprachiasmatic Nucleus. Biomedicines. 2024; 12(10):2202. https://doi.org/10.3390/biomedicines12102202
Chicago/Turabian StyleYassine, Mona, Soha A. Hassan, Lea Aylin Yücel, Fathima Faiba A. Purath, Horst-Werner Korf, Charlotte von Gall, and Amira A. H. Ali. 2024. "Hepatocellular Carcinoma in Mice Affects Neuronal Activity and Glia Cells in the Suprachiasmatic Nucleus" Biomedicines 12, no. 10: 2202. https://doi.org/10.3390/biomedicines12102202









