Retinal Imaging-Based Oculomics: Artificial Intelligence as a Tool in the Diagnosis of Cardiovascular and Metabolic Diseases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
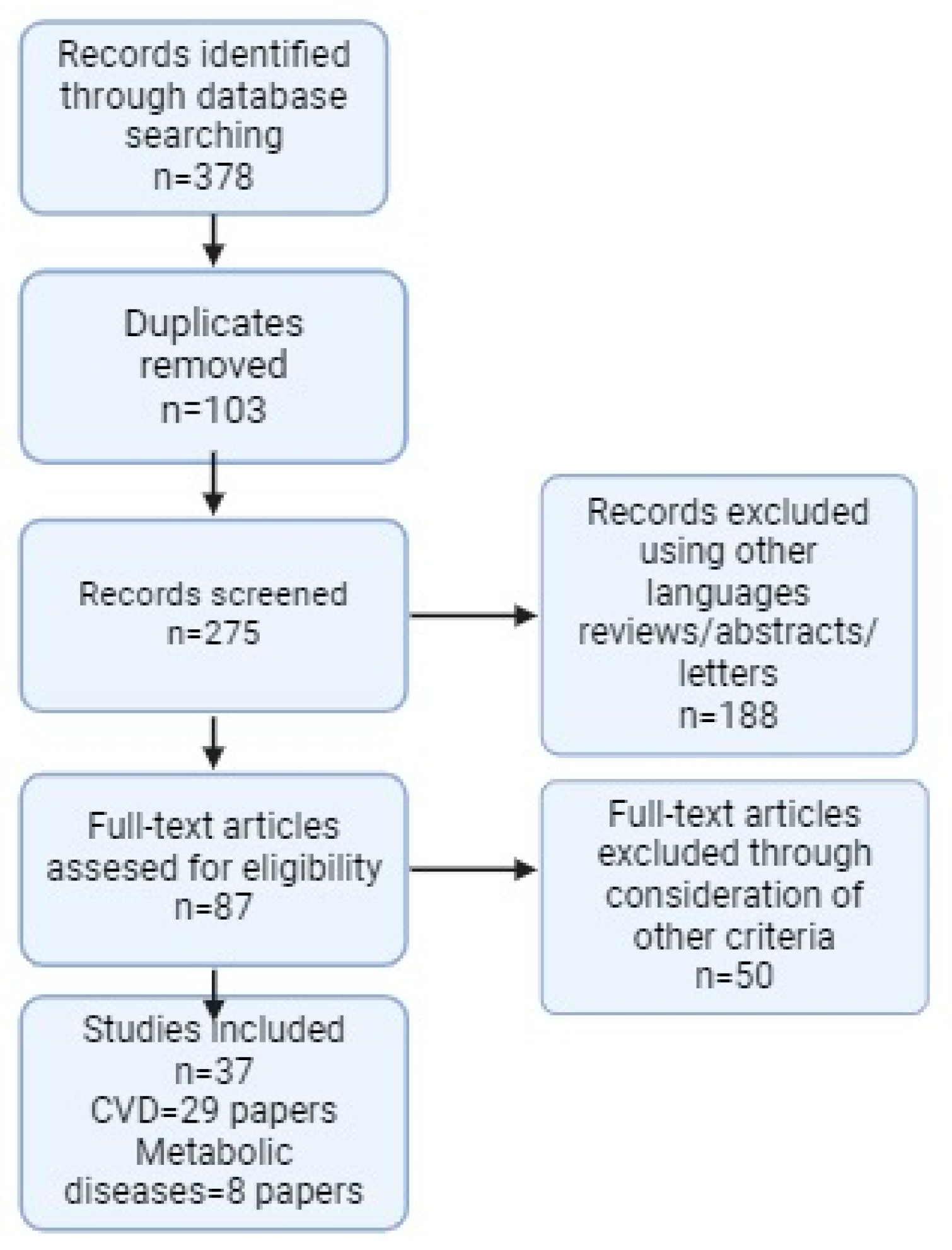
2.2. Indentification and Selection of Studies
2.3. Data Extraction Process
2.4. Overview of Studies Included in the Review
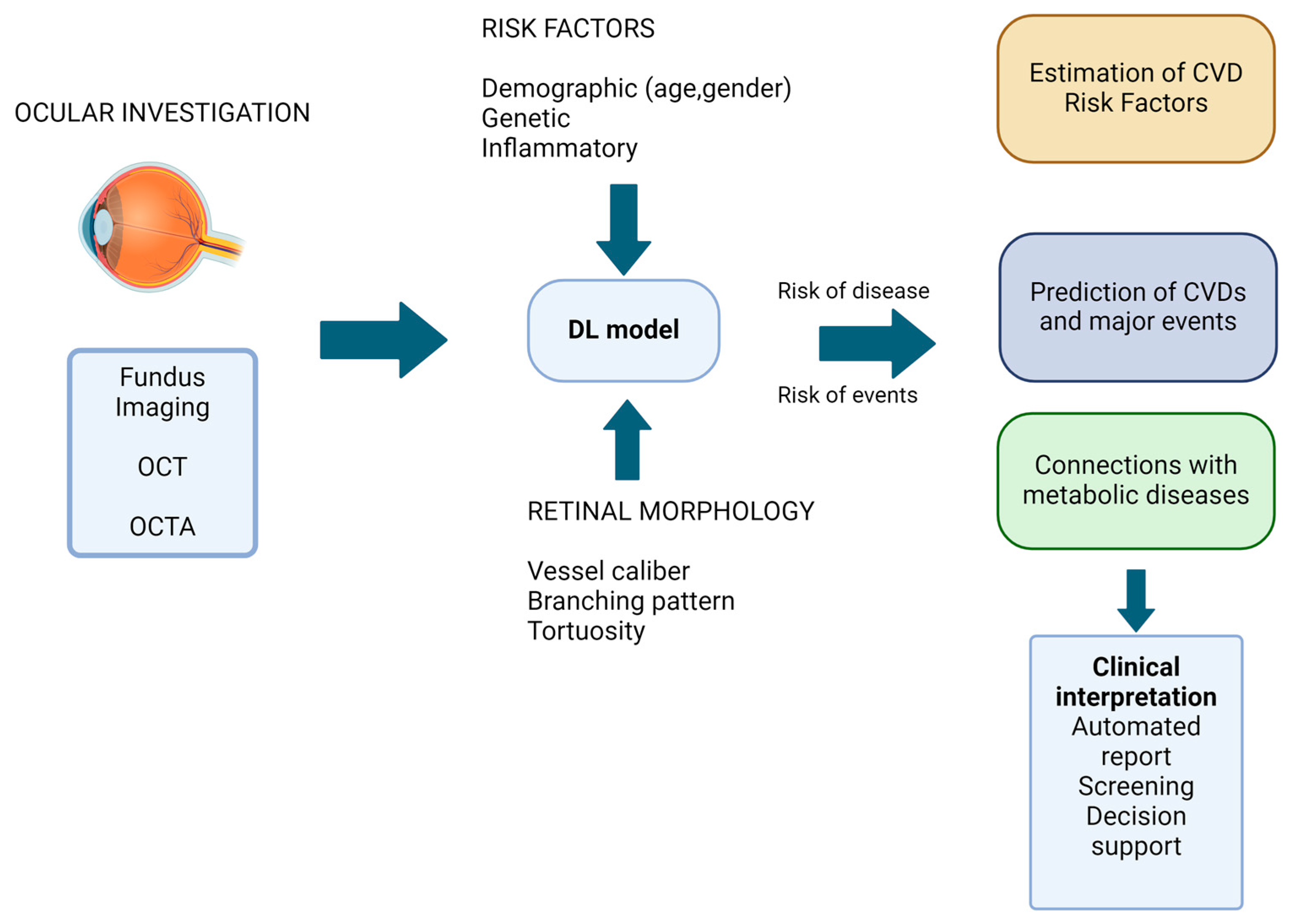
3. Technological Advances in Oculomics
4. Ocular Biomarkers in Cardiovascular Diseases
4.1. Retinal Morphology Variation in Cardiovascular Disease
4.2. Automated Idenfication and Prediction of Cardiovascular Diseases
| Study | Disease | Method | Dataset | Recruitment | Task | Outcome | Results | Sensitivity/Specificity |
|---|---|---|---|---|---|---|---|---|
| Aschauer et al., 2021 [43] | CAD | Fundus imaging, OCTA, AO | 45 patients | Prospective | Identification | Clinical diagnosis | Related | - |
| Chang et al., 2020 [40] | CAD | Fundus imaging | 15,408 images | Retrospective | Prediction | Atherosclerosis Score | AUC = 0.713 | 89.1%/40.4% |
| Cheung et al., 2021 [44] | CAD | Fundus imaging | 5309 images | Retrospective | Prediction | Retinal-vessel morphological parameters | Related | - |
| Huang et al., 2022 [45] | CAD | Fundus imaging | 145 patients | Prospective | Prediction | Vascular biomarkers and CAD-RADS | AUC = 0.739 | 71.1%/69.7% |
| Lee et al., 2023 [46] | CAD | Fundus imaging | 2954 images | Retrospective | Prediction | Clinical diagnosis | AUC = 0.872 | 87.1%/50.8% |
| Matuleviciute et al., 2022 [47] | CAD | OCT/ OCTA | 184 patients | Retrospective | Prediction | Retinal and choroidal thickness, retinal vascular parameters | Related | - |
| Nusinovici et al., 2022 [33] | CAD | Fundus imaging | 129,236 images | Retrospective | Prediction | Biological age | Related | 76%/55% |
| Ren et al., 2023 [48] | CAD | OCTA | 185 patients | Retrospective | Prediction | Clinical diagnosis | AUC = 0.840/0.830 | - |
| Son et al., 2020 [49] | CAD | Fundus imaging | 44,184 images | Retrospective | Prediction | Coronary artery calcium score | AUC = 0.832 | - |
| Wang et al., 2019 [50] | CAD | OCTA | 316 patients | Prospective | Identification | Clinical diagnosis | Related | - |
| Cao et al., 2021 [51] | IS | OCTA | 86 patients | Retrospective | Identification | Retinal-vessel morphological parameters | Related | - |
| Duan et al., 2022 [52] | IS | OCTA | 60 patients | Retrospective | Identification | Retinal-vessel morphological parameters | Related | - |
| Liang et al., 2022 [53] | IS | OCTA | 268 patients | Retrospective | Identification | Retinal-vessel morphological parameters | Related | - |
| Molero-Senosiain et al., 2022 [54] | IS | OCT/ OCTA | 65 patients | Retrospective | Identification | Retinal-vessel morphological parameters | Related | - |
| Zhang et al., 2020 [55] | IS | OCTA | 150 patients | Retrospective | Prediction | Retinal-vessel morphological parameters | Related | - |
| Ye et al., 2022 [56] | IS | OCT/ OCTA | 66 patients | Retrospective | Identification | Retinal-vessel morphological parameters | Related | - |
| Arnould et al., 2020 [57] | MI | OCTA | 30 patients | Prospective | Prediction | Retinal-vessel morphological parameters | Not related | 89% |
| Diaz-Pinto et al., 2022 [39] | MI | Fundus imaging | 87,476 participants | Retrospective | Prediction | Cardiac indices | AUC = 0.80 | 74%/71% |
| Sideri et al., 2023 [58] | MI | OCTA | 165 patients | Prospective | Prediction | Cardiac indices | Related | - |
| Zhong et al., 2021 [59] | MI | OCTA | 218 patients | Prospective | Prediction | Retinal-vessel morphological parameters | AUC = 0.812 | 65.9%/89% |
| Rakusiewicz et al., 2021[60] | CHF | OCTA | 60 patients | Retrospective | Prediction | Clinical diagnosis | Related | - |
| Topaloglu et al., 2023 [61] | CHF | OCTA | 50 patients | Retrospective | Prediction | Clinical diagnosis | Related | - |
| Zekavat et al., 2022 [62] | CHF | Fundus imaging | 97,895 images | Retrospective | Prediction | Retinal-vessel morphological parameters | Related | 97.4%/100% ** |
| Chua et al., 2019 [20] | HTN | Fundus imaging/OCTA | 77 patients | Prospective | Prediction | Clinical diagnosis | Related | - |
| Hua et al., 2019 [63] | HTN | OCTA | 97 patients | Prospective | Prediction | Clinical diagnosis | Related | - |
| Pascual-Prieto et al., 2020 [64] | HTN | OCTA | 73 patients | Prospective | Identification | Retinal-vessel morphological parameters | Related | 78.3%/66.7% and 56.5%/79.2% |
| Peng et al., 2020 [65] | HTN | Fundus imaging /OCTA | 169 patients | Prospective | Prediction | Retinal-vessel morphological parameters | Related | - |
| Sargues et al., 2023 [66] | HTN | OCTA | 89 patients | Retrospective | Prediction | Retinal-vessel morphological parameters | Related | - |
| Zeng et al., 2022 [67] | HTN | OCTA | 32 patients | Prospective | Identification | Retinal-vessel morphological parameters | Related | - |
4.3. Automated Idenfication and Prediction of Cardiovascular Risk Factors
4.4. Other Biomarkers in Cardiovascular Assesement
5. Linking Automated Prediction and Identification of Metabolic and Cardiovascular Diseases Using AI Models
| Study | Disease | Method | Dataset | Recruitment | Task | Outcome | Results | Sensitivity/Specificity |
|---|---|---|---|---|---|---|---|---|
| Ale-Chilet et al., 2022 [93] | type I DM | OCTA | 425 patients | Prospective | Identification | Clinical diagnosis | AUC = 0.58 | - |
| Barriada et al., 2022 [32] | DM | Fundus Imaging | 76 patients | Retrospective | Prediction | Coronary Artery Calcium Score | Related | 91% (sensitivity) |
| Bernal-Morales et al., 2021 [87] | type I DM | OCT/OCTA | 593 patients | Prospective | Prediction | Clinical diagnosis | Related | - |
| Cui et al., 2020 [94] | type II DM | Fluoroscein Angiography/OCTA/Fundus imaging | 101 patients | Prospective | Identification | Clinical diagnosis | Related | 96.5%/94.7% |
| DuPont et al., 2024 [95] | type II DM | Fluoroscein Angiography/OCTA | 15 patients | Prospective | Prediction | Clinical diagnosis | Related | - |
| Li et al., 2020 [83] | type II DM/ prediabetes | Fundus imaging | 2876 patients | Prospective | Prediction | Clinical diagnosis/risk variables | Related | - |
| Torabi et al., 2019 [85] | type II DM | OCT | 115 patients | Prospective | Prediction | Choroidal thickness | Related | - |
| Zhang et al., 2020 [88] | HTN Hyperglycemia, dyslipidemia | Fundus imaging | 625 patients | Prospective | Prediction | Clinical diagnosis/risk variables | AUC = 0.766; AUC = 0.880; AUC = 0.703 | - |
6. Artificial Intelligence-Driven Development of Automated Retinal Vessel Measurement Models
7. Traditional Diagnostic Methods for Cardiovascular Diseases Versus AI Retinal-Based Approaches
8. Challenges and Future Perspectives
9. Novel Contributions
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Basu, K.; Sinha, R.; Ong, A.; Basu, T. Artificial Intelligence: How Is It Changing Medical Sciences and Its Future? Indian J. Dermatol. 2020, 65, 365. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cao, J.; Grzybowski, A.; Jin, K.; Lou, L.; Ye, J. Diagnosing Systemic Disorders with AI Algorithms Based on Ocular Images. Healthcare 2023, 11, 1739. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Salowe, R.; Chow, C.; Li, S.; Bastani, O.; O’Brien, J.M. Advancing Glaucoma Care: Integrating Artificial Intelligence in Diagnosis, Management, and Progression Detection. Bioengineering 2024, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.K.; Fu, D.J.; Faes, L.; Liu, X.; Huemer, J.; Khalid, H.; Ferraz, D.; Korot, E.; Kelly, C.; Balaskas, K.; et al. Insights into Systemic Disease through Retinal Imaging-Based Oculomics. Transl. Vis. Sci. Technol. 2020, 9, 6. [Google Scholar] [CrossRef]
- Muharram, F.R.; Multazam, C.E.C.Z.; Mustofa, A.; Socha, W.; Andrianto; Martini, S.; Aminde, L.; Yi-Li, C. The 30 Years of Shifting in The Indonesian Cardiovascular Burden—Analysis of The Global Burden of Disease Study. J. Epidemiol. Glob. Health 2024, 14, 193–212. [Google Scholar] [CrossRef]
- Magliano, D.; Boyko, E.J. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; ISBN 978-2-930229-98-0. [Google Scholar]
- Blaha, M.J.; DeFilippis, A.P. Multi-Ethnic Study of Atherosclerosis (MESA): JACC Focus Seminar 5/8. J. Am. Coll. Cardiol. 2021, 77, 3195–3216. [Google Scholar] [CrossRef]
- ACCORD Study Group; Buse, J.B.; Bigger, J.T.; Byington, R.P.; Cooper, L.S.; Cushman, W.C.; Friedewald, W.T.; Genuth, S.; Gerstein, H.C.; Ginsberg, H.N.; et al. Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial: Design and Methods. Am. J. Cardiol. 2007, 99, 21i–33i. [Google Scholar] [CrossRef]
- Chandra, A.; Seidelmann, S.B.; Claggett, B.L.; Klein, B.E.; Klein, R.; Shah, A.M.; Solomon, S.D. The Association of Retinal Vessel Calibres with Heart Failure and Long-term Alterations in Cardiac Structure and Function: The Atherosclerosis Risk in Communities (ARIC) Study. Eur. J. Heart Fail. 2019, 21, 1207–1215. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wong, T.Y.; Klein, R.; Couper, D.J.; Cooper, L.S.; Shahar, E.; Hubbard, L.D.; Wofford, M.R.; Sharrett, A.R. Retinal Microvascular Abnormalities and Incident Stroke: The Atherosclerosis Risk in Communities Study. The Lancet 2001, 358, 1134–1140. [Google Scholar] [CrossRef]
- Seifert, B.-U.; Vilser, W. Retinal Vessel Analyzer (Rva)—Design and Function. Biomed. Tech. Eng. 2002, 47, 678–681. [Google Scholar] [CrossRef] [PubMed]
- Harazny, J.M.; Ritt, M.; Baleanu, D.; Ott, C.; Heckmann, J.; Schlaich, M.P.; Michelson, G.; Schmieder, R.E. Increased Wall:Lumen Ratio of Retinal Arterioles in Male Patients with a History of a Cerebrovascular Event. Hypertension 2007, 50, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Rizzoni, D.; Mengozzi, A.; Masi, S.; Agabiti Rosei, C.; De Ciuceis, C.; Virdis, A. New Noninvasive Methods to Evaluate Microvascular Structure and Function. Hypertension 2022, 79, 874–886. [Google Scholar] [CrossRef] [PubMed]
- Ghenciu, L.A.; Șișu, A.M.; Stoicescu, E.R.; Dănilă, A.-I.; Iacob, R.; Săndesc, M.-A.; Hațegan, O.A. Thyroid Eye Disease and Glaucoma: A Cross-Sectional Study Comparing Clinical Characteristics and Disease Severity. Medicina 2024, 60, 1430. [Google Scholar] [CrossRef]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical Coherence Tomography Angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef]
- Mukherjee, C.; Al-Fahad, Q.; Elsherbiny, S. The Role of Optical Coherence Tomography in Therapeutics and Conditions, Which Primarily Have Systemic Manifestations: A Narrative Review. Ther. Adv. Ophthalmol. 2019, 11, 251584141983115. [Google Scholar] [CrossRef]
- DeBuc, D.C.; Rege, A.; Smiddy, W.E. Use of XyCAM RI for Noninvasive Visualization and Analysis of Retinal Blood Flow Dynamics During Clinical Investigations. Expert Rev. Med. Devices 2021, 18, 225–237. [Google Scholar] [CrossRef]
- Bakker, E.; Dikland, F.A.; Van Bakel, R.; Andrade De Jesus, D.; Sánchez Brea, L.; Klein, S.; Van Walsum, T.; Rossant, F.; Farías, D.C.; Grieve, K.; et al. Adaptive Optics Ophthalmoscopy: A Systematic Review of Vascular Biomarkers. Surv. Ophthalmol. 2022, 67, 369–387. [Google Scholar] [CrossRef]
- Chua, J.; Le, T.; Sim, Y.C.; Chye, H.Y.; Tan, B.; Yao, X.; Wong, D.; Ang, B.W.Y.; Toh, D.; Lim, H.; et al. Relationship of Quantitative Retinal Capillary Network and Myocardial Remodeling in Systemic Hypertension. J. Am. Heart Assoc. 2022, 11, e024226. [Google Scholar] [CrossRef]
- Zhang, P.; Wahl, D.J.; Mocci, J.; Miller, E.B.; Bonora, S.; Sarunic, M.V.; Zawadzki, R.J. Adaptive Optics Scanning Laser Ophthalmoscopy and Optical Coherence Tomography (AO-SLO-OCT) System for In Vivo Mouse Retina Imaging. Biomed. Opt. Express 2023, 14, 299. [Google Scholar] [CrossRef]
- Mujat, M.; Ferguson, R.D.; Hammer, D.X.; Patel, A.H.; Iftimia, N. High-Resolution Retinal Imaging: Technology Overview and Applications. Photonics 2024, 11, 522. [Google Scholar] [CrossRef]
- Luís Dean-Ben, X.; Razansky, D. Localization Optoacoustic Tomography. Light Sci. Appl. 2018, 7, 18004. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Hadoux, X.; Van Wijngaarden, P. Hyperspectral Retinal Imaging Biomarkers of Ocular and Systemic Diseases. Eye 2024, 6, 2. [Google Scholar] [CrossRef]
- Arnould, L.; Meriaudeau, F.; Guenancia, C.; Germanese, C.; Delcourt, C.; Kawasaki, R.; Cheung, C.Y.; Creuzot-Garcher, C.; Grzybowski, A. Using Artificial Intelligence to Analyse the Retinal Vascular Network: The Future of Cardiovascular Risk Assessment Based on Oculomics? A Narrative Review. Ophthalmol. Ther. 2023, 12, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Levy, B.I.; Schiffrin, E.L.; Mourad, J.-J.; Agostini, D.; Vicaut, E.; Safar, M.E.; Struijker-Boudier, H.A.J. Impaired Tissue Perfusion: A Pathology Common to Hypertension, Obesity, and Diabetes Mellitus. Circulation 2008, 118, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y. Quantitative Retinal Venular Caliber and Risk of Cardiovascular Disease in Older Persons: The Cardiovascular Health Study. Arch. Intern. Med. 2006, 166, 2388. [Google Scholar] [CrossRef]
- Tabatabaee, A.; Asharin, M.; Dehghan, M.; Pourbehi, M.; Nasiri-Ahmadabadi, M.; Assadi, M. Retinal Vessel Abnormalities Predict Coronary Artery Diseases. Perfusion 2013, 28, 232–237. [Google Scholar] [CrossRef]
- Wong, T.Y.; Klein, R.; Sharrett, A.R.; Duncan, B.B.; Couper, D.J.; Tielsch, J.M.; Klein, B.E.K.; Hubbard, L.D. Retinal Arteriolar Narrowing and Risk of Coronary Heart Disease in Men and Women: The Atherosclerosis Risk in Communities Study. JAMA 2002, 287, 1153–1159. [Google Scholar] [CrossRef]
- Hanssen, H.; Streese, L.; Vilser, W. Retinal Vessel Diameters and Function in Cardiovascular Risk and Disease. Prog. Retin. Eye Res. 2022, 91, 101095. [Google Scholar] [CrossRef]
- Chew, S.K.H.; Xie, J.; Wang, J.J. Retinal Arteriolar Diameter and the Prevalence and Incidence of Hypertension: A Systematic Review and Meta-Analysis of Their Association. Curr. Hypertens. Rep. 2012, 14, 144–151. [Google Scholar] [CrossRef]
- Barriada, R.G.; Simó-Servat, O.; Planas, A.; Hernández, C.; Simó, R.; Masip, D. Deep Learning of Retinal Imaging: A Useful Tool for Coronary Artery Calcium Score Prediction in Diabetic Patients. Appl. Sci. 2022, 12, 1401. [Google Scholar] [CrossRef]
- Nusinovici, S.; Rim, T.H.; Yu, M.; Lee, G.; Tham, Y.-C.; Cheung, N.; Chong, C.C.Y.; Da Soh, Z.; Thakur, S.; Lee, C.J.; et al. Retinal Photograph-Based Deep Learning Predicts Biological Age, and Stratifies Morbidity and Mortality Risk. Age Ageing 2022, 51, afac065. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, Y.; Wang, W.; Wang, Y.; Hu, W.; Shang, X.; Liao, H.; Shi, D.; Huang, Y.; Ha, J.; et al. Association of Retinal Age Gap with Arterial Stiffness and Incident Cardiovascular Disease. Stroke 2022, 53, 3320–3328. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Hu, W.; Chen, R.; Xiong, R.; Wang, W.; Shang, X.; Chen, Y.; Kiburg, K.; Shi, D.; He, S.; et al. Retinal Age Gap as a Predictive Biomarker of Stroke Risk. BMC Med. 2022, 20, 466. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Klein, B.E.K.; Moss, S.E.; Wong, T.Y.; Sharrett, A.R. Retinal Vascular Caliber in Persons with Type 2 Diabetes: The Wisconsin Epidemiological Study of Diabetic Retinopathy: XX. Ophthalmology 2006, 113, 1488–1498. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.; Lim, Z.W.; Xu, D.; Ting, D.S.W.; Wong, T.Y.; Lee, M.L.; Hsu, W. Feature Isolation for Hypothesis Testing in Retinal Imaging: An Ischemic Stroke Prediction Case Study. Proc. AAAI Conf. Artif. Intell. 2019, 33, 9510–9515. [Google Scholar] [CrossRef]
- Szegedy, C.; Ioffe, S.; Vanhoucke, V.; Alemi, A. Inception-v4, Inception-ResNet and the Impact of Residual Connections on Learning. Proc. AAAI Conf. Artif. Intell. 2017, 31, 1. [Google Scholar] [CrossRef]
- Diaz-Pinto, A.; Ravikumar, N.; Attar, R.; Suinesiaputra, A.; Zhao, Y.; Levelt, E.; Dall’Armellina, E.; Lorenzi, M.; Chen, Q.; Keenan, T.D.L.; et al. Predicting Myocardial Infarction through Retinal Scans and Minimal Personal Information. Nat. Mach. Intell. 2022, 4, 55–61. [Google Scholar] [CrossRef]
- Chang, J.; Ko, A.; Park, S.M.; Choi, S.; Kim, K.; Kim, S.M.; Yun, J.M.; Kang, U.; Shin, I.H.; Shin, J.Y.; et al. Association of Cardiovascular Mortality and Deep Learning-Funduscopic Atherosclerosis Score Derived from Retinal Fundus Images. Am. J. Ophthalmol. 2020, 217, 121–130. [Google Scholar] [CrossRef]
- Chaitanuwong, P.; Singhanetr, P.; Chainakul, M.; Arjkongharn, N.; Ruamviboonsuk, P.; Grzybowski, A. Potential Ocular Biomarkers for Early Detection of Alzheimer’s Disease and Their Roles in Artificial Intelligence Studies. Neurol. Ther. 2023, 12, 1517–1532. [Google Scholar] [CrossRef]
- Huang, Y.; Li, C.; Shi, D.; Wang, H.; Shang, X.; Wang, W.; Zhang, X.; Zhang, X.; Hu, Y.; Tang, S.; et al. Integrating Oculomics with Genomics Reveals Imaging Biomarkers for Preventive and Personalized Prediction of Arterial Aneurysms. EPMA J. 2023, 14, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Aschauer, J.; Aschauer, S.; Pollreisz, A.; Datlinger, F.; Gatterer, C.; Mylonas, G.; Egner, B.; Hofer, D.; Steiner, I.; Hengstenberg, C.; et al. Identification of Subclinical Microvascular Biomarkers in Coronary Heart Disease in Retinal Imaging. Transl. Vis. Sci. Technol. 2021, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.Y.; Xu, D.; Cheng, C.-Y.; Sabanayagam, C.; Tham, Y.-C.; Yu, M.; Rim, T.H.; Chai, C.Y.; Gopinath, B.; Mitchell, P.; et al. A Deep-Learning System for the Assessment of Cardiovascular Disease Risk via the Measurement of Retinal-Vessel Calibre. Nat. Biomed. Eng. 2020, 5, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Lian, J.; Ng, K.-S.; Shih, K.; Vardhanabhuti, V. Predicting CT-Based Coronary Artery Disease Using Vascular Biomarkers Derived from Fundus Photographs with a Graph Convolutional Neural Network. Diagnostics 2022, 12, 1390. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Cha, J.; Shim, I.; Park, W.-Y.; Kang, S.W.; Lim, D.H.; Won, H.-H. Multimodal Deep Learning of Fundus Abnormalities and Traditional Risk Factors for Cardiovascular Risk Prediction. Npj Digit. Med. 2023, 6, 14. [Google Scholar] [CrossRef]
- Matulevičiūtė, I.; Sidaraitė, A.; Tatarūnas, V.; Veikutienė, A.; Dobilienė, O.; Žaliūnienė, D. Retinal and Choroidal Thinning—A Predictor of Coronary Artery Occlusion? Diagnostics 2022, 12, 2016. [Google Scholar] [CrossRef]
- Ren, Y.; Hu, Y.; Li, C.; Zhong, P.; Liu, H.; Wang, H.; Kuang, Y.; Fu, B.; Wang, Y.; Zhao, H.; et al. Impaired Retinal Microcirculation in Patients with Non-Obstructive Coronary Artery Disease. Microvasc. Res. 2023, 148, 104533. [Google Scholar] [CrossRef]
- Son, J.; Shin, J.Y.; Chun, E.J.; Jung, K.-H.; Park, K.H.; Park, S.J. Predicting High Coronary Artery Calcium Score from Retinal Fundus Images with Deep Learning Algorithms. Transl. Vis. Sci. Technol. 2020, 9, 28. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, J.; Zhang, Y.; Qian, Y.W.; Zhang, J.F.; Wang, Z.L. Retinal and Choroidal Vascular Changes in Coronary Heart Disease: An Optical Coherence Tomography Angiography Study. Biomed. Opt. Express 2019, 10, 1532. [Google Scholar] [CrossRef]
- Cao, Y.; Yan, J.; Zhan, Z.; Liang, Y.; Han, Z. Macula Structure and Microvascular Changes in Recent Small Subcortical Infarct Patients. Front. Neurol. 2021, 11, 615252. [Google Scholar] [CrossRef]
- Duan, H.; Xie, J.; Zhou, Y.; Zhang, H.; Liu, Y.; Tang, C.; Zhao, Y.; Qi, H. Characterization of the Retinal Microvasculature and FAZ Changes in Ischemic Stroke and Its Different Types. Transl. Vis. Sci. Technol. 2022, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Liu, B.; Xiao, Y.; Zeng, X.; Wu, G.; Du, Z.; Fang, Y.; Hu, Y.; Yang, X.; Yu, H. Retinal Neurovascular Changes in Patients with Ischemic Stroke Investigated by Optical Coherence Tomography Angiography. Front. Aging Neurosci. 2022, 14, 834560. [Google Scholar] [CrossRef] [PubMed]
- Molero-Senosiain, M.; Vidal-Villegas, B.; Pascual-Prieto, J.; Valor-Suarez, C.; Saenz-Frances, F.; Santos-Bueso, E. Correlation Between Retrograde Trans-Synaptic Degeneration of Ganglion Cells and Optical Coherence Tomography Angiography Following Ischemic Stroke. Cureus 2021, 13, e19788. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shi, C.; Chen, Y.; Wang, W.; Huang, S.; Han, Z.; Lin, X.; Lu, F.; Shen, M. Retinal Structural and Microvascular Alterations in Different Acute Ischemic Stroke Subtypes. J. Ophthalmol. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Ye, C.; Kwapong, W.R.; Tao, W.; Lu, K.; Pan, R.; Wang, A.; Liu, J.; Liu, M.; Wu, B. Characterization of Macular Structural and Microvascular Changes in Thalamic Infarction Patients: A Swept-Source Optical Coherence Tomography–Angiography Study. Brain Sci. 2022, 12, 518. [Google Scholar] [CrossRef]
- Arnould, L.; Guenancia, C.; Azemar, A.; Alan, G.; Pitois, S.; Bichat, F.; Zeller, M.; Gabrielle, P.-H.; Bron, A.M.; Creuzot-Garcher, C.; et al. The EYE-MI Pilot Study: A Prospective Acute Coronary Syndrome Cohort Evaluated with Retinal Optical Coherence Tomography Angiography. Investig. Opthalmology Vis. Sci. 2018, 59, 4299. [Google Scholar] [CrossRef]
- Sideri, A.-M.; Kanakis, M.; Katsimpris, A.; Karamaounas, A.; Brouzas, D.; Petrou, P.; Papakonstaninou, E.; Droutsas, K.; Kandarakis, S.; Giannopoulos, G.; et al. Correlation Between Coronary and Retinal Microangiopathy in Patients with STEMI. Transl. Vis. Sci. Technol. 2023, 12, 8. [Google Scholar] [CrossRef]
- Zhong, P.; Hu, Y.; Jiang, L.; Peng, Q.; Huang, M.; Li, C.; Kuang, Y.; Tan, N.; Yu, H.; Yang, X. Retinal Microvasculature Changes in Patients with Coronary Total Occlusion on Optical Coherence Tomography Angiography. Front. Med. 2021, 8, 708491. [Google Scholar] [CrossRef]
- Rakusiewicz, K.; Kanigowska, K.; Hautz, W.; Ziółkowska, L. The Impact of Chronic Heart Failure on Retinal Vessel Density Assessed by Optical Coherence Tomography Angiography in Children with Dilated Cardiomyopathy. J. Clin. Med. 2021, 10, 2659. [Google Scholar] [CrossRef]
- Topaloglu, C.; Bekmez, S. Retinal Vascular Density Change in Patients with Heart Failure. Photodiagnosis Photodyn. Ther. 2023, 42, 103621. [Google Scholar] [CrossRef]
- Zekavat, S.M.; Raghu, V.K.; Trinder, M.; Ye, Y.; Koyama, S.; Honigberg, M.C.; Yu, Z.; Pampana, A.; Urbut, S.; Haidermota, S.; et al. Deep Learning of the Retina Enables Phenome- and Genome-Wide Analyses of the Microvasculature. Circulation 2022, 145, 134–150. [Google Scholar] [CrossRef] [PubMed]
- Hua, D.; Xu, Y.; Zeng, X.; Yang, N.; Jiang, M.; Zhang, X.; Yang, J.; He, T.; Xing, Y. Use of Optical Coherence Tomography Angiography for Assessment of Microvascular Changes in the Macula and Optic Nerve Head in Hypertensive Patients without Hypertensive Retinopathy. Microvasc. Res. 2020, 129, 103969. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Prieto, J.; Burgos-Blasco, B.; Ávila Sánchez-Torija, M.; Fernández-Vigo, J.I.; Arriola-Villalobos, P.; Barbero Pedraz, M.A.; García-Feijoo, J.; Martínez-de-la-Casa, J.M. Utility of Optical Coherence Tomography Angiography in Detecting Vascular Retinal Damage Caused by Arterial Hypertension. Eur. J. Ophthalmol. 2020, 30, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Hu, Y.; Huang, M.; Wu, Y.; Zhong, P.; Dong, X.; Wu, Q.; Liu, B.; Li, C.; Xie, J.; et al. Retinal Neurovascular Impairment in Patients with Essential Hypertension: An Optical Coherence Tomography Angiography Study. Investig. Opthalmology Vis. Sci. 2020, 61, 42. [Google Scholar] [CrossRef]
- Remolí Sargues, L.; Monferrer Adsuara, C.; Castro Navarro, V.; Navarro Palop, C.; Montero Hernández, J.; Cervera Taulet, E. Swept-Source Optical Coherence Tomography Angiography Automatic Analysis of Microvascular Changes Secondary to Systemic Hypertension. Eur. J. Ophthalmol. 2023, 33, 1452–1458. [Google Scholar] [CrossRef]
- Zeng, R.; Garg, I.; Bannai, D.; Kasetty, M.; Katz, R.; Park, J.Y.; Lizano, P.; Miller, J.B. Retinal Microvasculature and Vasoreactivity Changes in Hypertension Using Optical Coherence Tomography-Angiography. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 3505–3515. [Google Scholar] [CrossRef]
- Poplin, R.; Varadarajan, A.V.; Blumer, K.; Liu, Y.; McConnell, M.V.; Corrado, G.S.; Peng, L.; Webster, D.R. Prediction of Cardiovascular Risk Factors from Retinal Fundus Photographs via Deep Learning. Nat. Biomed. Eng. 2018, 2, 158–164. [Google Scholar] [CrossRef]
- Vaghefi, E.; Yang, S.; Hill, S.; Humphrey, G.; Walker, N.; Squirrell, D. Detection of Smoking Status from Retinal Images; a Convolutional Neural Network Study. Sci. Rep. 2019, 9, 7180. [Google Scholar] [CrossRef]
- Betzler, B.K.; Yang, H.H.S.; Thakur, S.; Yu, M.; Quek, T.C.; Soh, Z.D.; Lee, G.; Tham, Y.-C.; Wong, T.Y.; Rim, T.H.; et al. Gender Prediction for a Multiethnic Population via Deep Learning Across Different Retinal Fundus Photograph Fields: Retrospective Cross-Sectional Study. JMIR Med. Inform. 2021, 9, e25165. [Google Scholar] [CrossRef]
- Korot, E.; Pontikos, N.; Liu, X.; Wagner, S.K.; Faes, L.; Huemer, J.; Balaskas, K.; Denniston, A.K.; Khawaja, A.; Keane, P.A. Predicting Sex from Retinal Fundus Photographs Using Automated Deep Learning. Sci. Rep. 2021, 11, 10286. [Google Scholar] [CrossRef]
- Rim, T.H.; Lee, G.; Kim, Y.; Tham, Y.-C.; Lee, C.J.; Baik, S.J.; Kim, Y.A.; Yu, M.; Deshmukh, M.; Lee, B.K.; et al. Prediction of Systemic Biomarkers from Retinal Photographs: Development and Validation of Deep-Learning Algorithms. Lancet Digit. Health 2020, 2, e526–e536. [Google Scholar] [CrossRef] [PubMed]
- Gerrits, N.; Elen, B.; Craenendonck, T.V.; Triantafyllidou, D.; Petropoulos, I.N.; Malik, R.A.; De Boever, P. Age and Sex Affect Deep Learning Prediction of Cardiometabolic Risk Factors from Retinal Images. Sci. Rep. 2020, 10, 9432. [Google Scholar] [CrossRef] [PubMed]
- Budoff, M.J.; Raggi, P.; Beller, G.A.; Berman, D.S.; Druz, R.S.; Malik, S.; Rigolin, V.H.; Weigold, W.G.; Soman, P. Noninvasive Cardiovascular Risk Assessment of the Asymptomatic Diabetic Patient. JACC Cardiovasc. Imaging 2016, 9, 176–192. [Google Scholar] [CrossRef] [PubMed]
- Tseng, R.M.W.W.; Rim, T.H.; Cheung, C.Y.; Wong, T.Y. Artificial Intelligence Using the Eye as a Biomarker of Systemic Risk. In Artificial Intelligence in Ophthalmology; Grzybowski, A., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 243–255. ISBN 978-3-030-78600-7. [Google Scholar]
- Tseng, R.M.W.W.; Rim, T.H.; Shantsila, E.; Yi, J.K.; Park, S.; Kim, S.S.; Lee, C.J.; Thakur, S.; Nusinovici, S.; Peng, Q.; et al. Validation of a Deep-Learning-Based Retinal Biomarker (Reti-CVD) in the Prediction of Cardiovascular Disease: Data from UK Biobank. BMC Med. 2023, 21, 28. [Google Scholar] [CrossRef]
- Touboul, P.-J. Intima-Media Thickness of Carotid Arteries. In Frontiers of Neurology and Neuroscience; Alonso, A., Hennerici, M.G., Meairs, S., Eds.; S. Karger AG: Basel, Switzerland, 2015; Volume 36, pp. 31–39. ISBN 978-3-318-02790-7. [Google Scholar]
- Simó, R.; Bañeras, J.; Hernández, C.; Rodríguez-Palomares, J.; Valente, F.; Gutierrez, L.; González-Alujas, T.; Ferreira, I.; Aguadé-Bruix, S.; Montaner, J.; et al. Diabetic Retinopathy as an Independent Predictor of Subclinical Cardiovascular Disease: Baseline Results of the PRECISED Study. BMJ Open Diabetes Res. Care 2019, 7, e000845. [Google Scholar] [CrossRef]
- Grzybowski, A.; Rao, D.P.; Brona, P.; Negiloni, K.; Krzywicki, T.; Savoy, F.M. Diagnostic Accuracy of Automated Diabetic Retinopathy Image Assessment Softwares: IDx-DR and Medios Artificial Intelligence. Ophthalmic Res. 2023, 66, 1286–1292. [Google Scholar] [CrossRef]
- Gulshan, V.; Peng, L.; Coram, M.; Stumpe, M.C.; Wu, D.; Narayanaswamy, A.; Venugopalan, S.; Widner, K.; Madams, T.; Cuadros, J.; et al. Development and Validation of a Deep Learning Algorithm for Detection of Diabetic Retinopathy in Retinal Fundus Photographs. JAMA 2016, 316, 2402. [Google Scholar] [CrossRef]
- Breiman, L.; Friedman, J.H.; Olshen, R.A.; Stone, C.J. Classification And Regression Trees, 1st ed.; Routledge: London, UK, 2017; ISBN 978-1-315-13947-0. [Google Scholar]
- Shetkar, A.; Mai, C.K.; Yamini, C. Diabetic Symptoms Prediction Through Retinopathy. In Machine Learning Technologies and Applications; Mai, C.K., Reddy, A.B., Raju, K.S., Eds.; Algorithms for Intelligent Systems; Springer: Singapore, 2021; pp. 13–20. ISBN 978-981-334-045-9. [Google Scholar]
- Li, W.; Schram, M.T.; Berendschot, T.T.J.M.; Webers, C.A.B.; Kroon, A.A.; Van Der Kallen, C.J.H.; Henry, R.M.A.; Schaper, N.C.; Huang, F.; Dashtbozorg, B.; et al. Type 2 Diabetes and HbA1c Are Independently Associated with Wider Retinal Arterioles: The Maastricht Study. Diabetologia 2020, 63, 1408–1417. [Google Scholar] [CrossRef]
- Ting, D.S.W.; Cheung, C.Y.-L.; Lim, G.; Tan, G.S.W.; Quang, N.D.; Gan, A.; Hamzah, H.; Garcia-Franco, R.; San Yeo, I.Y.; Lee, S.Y.; et al. Development and Validation of a Deep Learning System for Diabetic Retinopathy and Related Eye Diseases Using Retinal Images from Multiethnic Populations with Diabetes. JAMA 2017, 318, 2211. [Google Scholar] [CrossRef]
- Torabi, H.; Saberi Isfeedvajani, M.; Ramezani, M.; Daryabari, S.-H. Choroidal Thickness and Hemoglobin A1c Levels in Patients with Type 2 Diabetes Mellitus. J. Ophthalmic Vis. Res. 2019, 14, 285–290. [Google Scholar] [CrossRef]
- Subrayan, V.; Menon, S.; Peyman, M.; Loo Voon Pei, A. Correlation between Optical Coherence Tomography-Derived Macular Measurements and Glycosylated Haemoglobin, Age, Visual Acuity and Diabetes Duration in Patients with Type 2 Diabetes Mellitus. Int. J. Diabetes Dev. Ctries. 2015, 35, 385–388. [Google Scholar] [CrossRef]
- Bernal-Morales, C.; Alé-Chilet, A.; Martín-Pinardel, R.; Barraso, M.; Hernández, T.; Oliva, C.; Vinagre, I.; Ortega, E.; Figueras-Roca, M.; Sala-Puigdollers, A.; et al. Optical Coherence Tomography Angiography in Type 1 Diabetes Mellitus. Report 4: Glycated Haemoglobin. Diagnostics 2021, 11, 1537. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yuan, M.; An, Z.; Zhao, X.; Wu, H.; Li, H.; Wang, Y.; Sun, B.; Li, H.; Ding, S.; et al. Prediction of Hypertension, Hyperglycemia and Dyslipidemia from Retinal Fundus Photographs via Deep Learning: A Cross-Sectional Study of Chronic Diseases in Central China. PLoS ONE 2020, 15, e0233166. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Lin, H.; Zhang, C.; Zhang, Q.; Zhang, D.; Zhang, Y.; Meng, W.; Zhu, Z.; Tang, F.; Xue, F.; et al. Association between Erythrocyte Parameters and Metabolic Syndrome in Urban Han Chinese: A Longitudinal Cohort Study. BMC Public Health 2013, 13, 989. [Google Scholar] [CrossRef]
- Liew, G.; Wang, J.J.; Rochtchina, E.; Wong, T.Y.; Mitchell, P. Complete Blood Count and Retinal Vessel Calibers. PLoS ONE 2014, 9, e102230. [Google Scholar] [CrossRef]
- Gargeya, R.; Leng, T. Automated Identification of Diabetic Retinopathy Using Deep Learning. Ophthalmology 2017, 124, 962–969. [Google Scholar] [CrossRef]
- Babenko, B.; Mitani, A.; Traynis, I.; Kitade, N.; Singh, P.; Maa, A.Y.; Cuadros, J.; Corrado, G.S.; Peng, L.; Webster, D.R.; et al. Detection of Signs of Disease in External Photographs of the Eyes via Deep Learning. Nat. Biomed. Eng. 2022, 6, 1370–1383. [Google Scholar] [CrossRef]
- Alé-Chilet, A.; Bernal-Morales, C.; Barraso, M.; Hernández, T.; Oliva, C.; Vinagre, I.; Ortega, E.; Figueras-Roca, M.; Sala-Puigdollers, A.; Esquinas, C.; et al. Optical Coherence Tomography Angiography in Type 1 Diabetes Mellitus—Report 2: Diabetic Kidney Disease. J. Clin. Med. 2021, 11, 197. [Google Scholar] [CrossRef]
- Cui, Y.; Zhu, Y.; Wang, J.C.; Lu, Y.; Zeng, R.; Katz, R.; Vingopoulos, F.; Le, R.; Laíns, I.; Wu, D.M.; et al. Comparison of Widefield Swept-Source Optical Coherence Tomography Angiography with Ultra-Widefield Colour Fundus Photography and Fluorescein Angiography for Detection of Lesions in Diabetic Retinopathy. Br. J. Ophthalmol. 2021, 105, 577–581. [Google Scholar] [CrossRef]
- DuPont, M.; Arthur, E.; Shihab, Y.; Kenny, M.; Ravichandran, S.; Parsons-Wingerter, P.; Vyas, R.; Murray, M.C.; Predovic, M.; Lim, S.; et al. Use of VESsel GENeration with Optical Coherence Tomography Angiography and Fluorescein Angiography for Detection and Quantification of Vascular Changes in Mild and Moderate Diabetic Retinopathy. Life 2024, 14, 893. [Google Scholar] [CrossRef]
- Cheung, C.Y.; Ikram, M.K.; Sabanayagam, C.; Wong, T.Y. Retinal Microvasculature as a Model to Study the Manifestations of Hypertension. Hypertension 2012, 60, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Wong, T. Computer-Assisted Measurement of Retinal Vessel Diameters in the Beaver Dam Eye Study*1methodology, Correlation between Eyes, and Effect of Refractive Errors. Ophthalmology 2004, 111, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Tapp, R.J.; Owen, C.G.; Barman, S.A.; Welikala, R.A.; Foster, P.J.; Whincup, P.H.; Strachan, D.P.; Rudnicka, A.R. For the UK Biobank Eye and Vision Consortium Associations of Retinal Microvascular Diameters and Tortuosity with Blood Pressure and Arterial Stiffness: United Kingdom Biobank. Hypertension 2019, 74, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Rubiś, P.P. Cardiac Disease: Diagnosis, Treatment, and Outcomes. J. Pers. Med. 2022, 12, 1212. [Google Scholar] [CrossRef]
- Detrano, R.; Gianrossi, R.; Froelicher, V. The Diagnostic Accuracy of the Exercise Electrocardiogram: A Meta-Analysis of 22 Years of Research. Prog. Cardiovasc. Dis. 1989, 32, 173–206. [Google Scholar] [CrossRef]
- Hendel, R.C.; Jabbar, A.Y.; Mahata, I. Initial Diagnostic Evaluation of Stable Coronary Artery Disease: The Need for a Patient-Centered Strategy. J. Am. Heart Assoc. 2017, 6, e006863. [Google Scholar] [CrossRef]
- Krittayaphong, R.; Muenkaew, M.; Chiewvit, P.; Ratanasit, N.; Kaolawanich, Y.; Phrommintikul, A.; CORE Investigators. Electrocardiographic Predictors of Cardiovascular Events in Patients at High Cardiovascular Risk: A Multicenter Study. J. Geriatr. Cardiol. 2019, 16, 630–638. [Google Scholar] [CrossRef]
- Lv, Q.; Tang, G.; Zhang, N.; Yu, D.; Sun, H. The Specificity and Accuracy of Echocardiography in Diagnosing Acute Heart Failure: A Systematic Review and Meta-Analysis. Altern. Ther. Health Med. 2024; Epub ahead of print. [Google Scholar]
- Doust, J.A.; Glasziou, P.P.; Pietrzak, E.; Dobson, A.J. A Systematic Review of the Diagnostic Accuracy of Natriuretic Peptides for Heart Failure. Arch. Intern. Med. 2004, 164, 1978–1984. [Google Scholar] [CrossRef]
- Gorenoi, V.; Schönermark, M.P.; Hagen, A. CT Coronary Angiography vs. Invasive Coronary Angiography in CHD. GMS Health Technol. Assess. 2012, 8, Doc02. [Google Scholar] [CrossRef]
- Neves, P.O.; Andrade, J.; Monção, H. Coronary Artery Calcium Score: Current Status. Radiol. Bras. 2017, 50, 182–189. [Google Scholar] [CrossRef]
- Sahoo, R.; Krishna, M.V.; Subrahmaniyan, D.K.S.; Dutta, T.K.; Elangovan, S. Common carotid intima-media thickness in acute ischemic stroke: A case-control study. Neurol. India 2009, 57, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Tedyanto, E.H.; Tini, K.; Pramana, N.A.K. Magnetic Resonance Imaging in Acute Ischemic Stroke. Cureus 2022, 14, e27224. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Yang, Z.; Chen, S.; Wan, J. Diagnostic value of myocardial stress detection based on feature tracking MRI in patients with acute myocardial infarction. J. Thorac. Dis. 2022, 14, 3454–3461. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.M. When and how to use ambulatory blood pressure monitoring and home blood pressure monitoring for managing hypertension. Clin. Hypertens 2024, 30, 10. [Google Scholar] [CrossRef]
- Teoh, C.S.; Wong, K.H.; Xiao, D.; Wong, H.C.; Zhao, P.; Chan, H.W.; Yuen, Y.S.; Naing, T.; Yogesan, K.; Koh, V.T.C. Variability in Grading Diabetic Retinopathy Using Retinal Photography and Its Comparison with an Automated Deep Learning Diabetic Retinopathy Screening Software. Healthcare 2023, 11, 1697. [Google Scholar] [CrossRef]
- Shin, J.H.; Cheong, J.I.; Cheuh, H.W.; Yoo, J.H. Limitations of Current Screening Methods for Lipid Disorders in Korean Adolescents and a Proposal for an Effective Detection Method: A Nationwide, Cross-Sectional Study. Ann. Pediatr. Endocrinol. Metab. 2020, 25, 265–271. [Google Scholar] [CrossRef]
- Ghenciu, L.A.; Hațegan, O.A.; Stoicescu, E.R.; Iacob, R.; Șișu, A.M. Emerging Therapeutic Approaches and Genetic Insights in Stargardt Disease: A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 8859. [Google Scholar] [CrossRef]
| PICO | Criteria |
|---|---|
| Population | Patients at risk of cardiovascular or metabolic diseases (e.g., coronary artery disease, myocardial infarction, hypertension, diabetes) |
| Intervention | Retinal imaging techniques (fundus photography, OCT, OCTA) analyzed by AI and deep learning models |
| Comparison | Traditional diagnostic methods such as calcium score, angiography, stress test, clinical biomarkers, and ecocardiography |
| Outcome | High diagnostic accuracy, sensitivity, and specificity in predicting cardiovascular and metabolic diseases |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Written in English | Articles in other languages |
| Papers utilizing retinal imaging techniques such as optical coherence tomography (OCT), optical coherence tomography angiography (OCTA), or fundus imaging | Papers with insufficient data for analysis/outcome |
| Papers focused on the application of AI in retinal imaging for diagnosing cardiovascular and/or metabolic diseases | Review/letters/commentaries/abstracts |
| Papers published after January 2018 | Published before 2018 |
| Not relevant to the topic |
| Retinal Parameter | Variation | Associated Outcome |
|---|---|---|
| Retinal Arteriolar Tortuosity | Increased | Linked with current blood pressure and early kidney disease |
| Decreased | Associated with current blood pressure and ischemic heart disease | |
| Retinal Venular Tortuosity | Increased | Connected to current blood pressure |
| Retinal Vascular Fractal Dimension | Increased | Related to acute lacunar stroke |
| Decreased | Correlates with current blood pressure, lacunar and incident stroke | |
| Suboptimal | Associated with chronic kidney disease and coronary heart disease | |
| Retinal Arteriolar Branching Angle | Increased | Associated with current blood pressure |
| Retinal Arteriolar Branching Asymmetry Ratio | Decreased | Linked to current blood pressure |
| Retinal Arteriolar Length-to-Diameter Ratio | Decreased | Connected to current blood pressure, hypertension, and stroke |
| Retinal Arteriolar Branching Coefficient | Decreased | Associated with ischemic heart disease |
| Retinal Arteriolar Optimal Parameter | Increased | Linked to peripheral vascular disease |
| Disease | Retinal Investigation | Comparison |
|---|---|---|
| CAD | Fundus Imaging, OCTA | Comparable AUC and sensitivity to current deep learning-based predictive models for CAD, such as coronary angiography and calcium scoring models with AUC > 0.8 [40,43,44,45,46,47,48,49,50,105,106]. |
| IS | Fundus Imaging, OCTA | Moderate accuracy for ischemic stroke risk prediction compared to established methods like carotid intima-media thickness measurement and brain MRI, which have higher specificity and sensitivity. Retinal imaging offers additional predictive value but often has lower AUC [51,52,53,54,55,56,107,108]. |
| MI | Fundus Imaging, OCTA | Some retinal imaging models show high accuracy in predicting myocardial infarction risk, comparable to traditional methods like ECG and cardiac MRI, with AUC values competitive with advanced predictive models [39,57,100,109]. |
| CHF | Automated Retinal Vessel Measurement, OCTA | High correlation with cardiovascular events and risk factors. Shows promise as a non-invasive tool for early detection, with comparable performance to echocardiography and brain natriuretic peptide testing [60,61,62,103,104]. |
| HTN | Fundus Imaging, OCTA | Slightly lower AUC compared to ambulatory blood pressure monitoring. However, retinal vessel analysis correlates well with cardiovascular risk factors, suggesting potential in improving predictive accuracy [20,63,64,65,66,67,110]. |
| DM | Fundus Imaging, OCTA, Fluorescein Angiography | High sensitivity and specificity in detecting retinal complications of DM, comparable to clinical grading by ophthalmologists [32,88,93,94,111]. |
| Hyperlipidemia | Fundus Imaging | Moderate predictive accuracy for dyslipidemia when using retinal imaging. Studies show that retinal imaging can predict lipid abnormalities but with slightly lower specificity compared to blood lipid panel tests [88,112]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghenciu, L.A.; Dima, M.; Stoicescu, E.R.; Iacob, R.; Boru, C.; Hațegan, O.A. Retinal Imaging-Based Oculomics: Artificial Intelligence as a Tool in the Diagnosis of Cardiovascular and Metabolic Diseases. Biomedicines 2024, 12, 2150. https://doi.org/10.3390/biomedicines12092150
Ghenciu LA, Dima M, Stoicescu ER, Iacob R, Boru C, Hațegan OA. Retinal Imaging-Based Oculomics: Artificial Intelligence as a Tool in the Diagnosis of Cardiovascular and Metabolic Diseases. Biomedicines. 2024; 12(9):2150. https://doi.org/10.3390/biomedicines12092150
Chicago/Turabian StyleGhenciu, Laura Andreea, Mirabela Dima, Emil Robert Stoicescu, Roxana Iacob, Casiana Boru, and Ovidiu Alin Hațegan. 2024. "Retinal Imaging-Based Oculomics: Artificial Intelligence as a Tool in the Diagnosis of Cardiovascular and Metabolic Diseases" Biomedicines 12, no. 9: 2150. https://doi.org/10.3390/biomedicines12092150







