GastricAITool: A Clinical Decision Support Tool for the Diagnosis and Prognosis of Gastric Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Helicobacter Pylori Diagnosis
2.3. Genetic Study: Selection of Polymorphisms and Genotyping
2.4. Building GastricAITool
2.4.1. Data Preprocessing
2.4.2. Data Analysis
2.4.3. Genetic Risk Score
2.4.4. Data Modelling
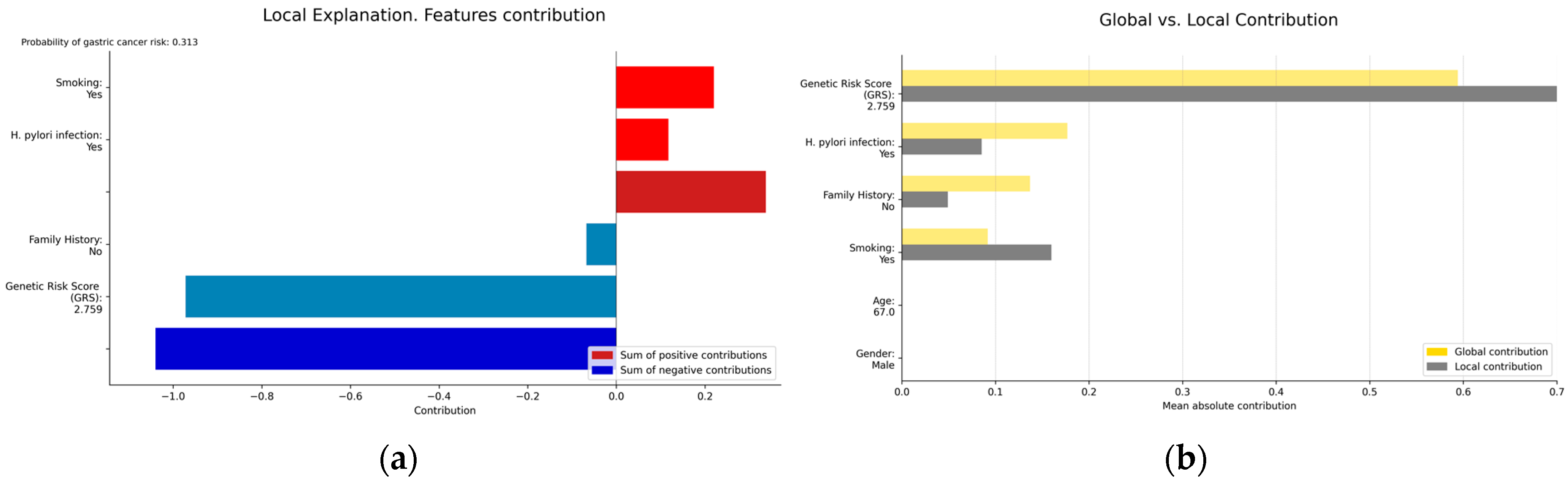
2.4.5. Interpretability and Explainability
2.4.6. Clinical Decision Support Tool: GastricAITool
- GastricAITool Model Executor/Manager: This component is responsible for processing input data, applying the model, and generating corresponding results. It is programmed in Python.
- PostgreSQL Database: Responsible for storing and managing the data necessary for the tool’s operation, including user input data and the results generated by the model.
3. Results
3.1. Data Analysis
3.2. Genetic Risk Scores
3.3. Data Modelling
3.3.1. Interpretability and Explainability
3.3.2. Clinical Decision Support Tool: GastricAITool
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guan, Y.; Zhang, H.; Quang, D.; Wang, Z.; Parker, S.C.; Pappas, D.A.; Kremer, J.M.; Zhu, F. Machine learning to predict anti–tumor necrosis factor drug responses of rheumatoid arthritis patients by integrating clinical and genetic markers. Arthritis Rheumatol. 2019, 71, 1987–1996. [Google Scholar] [CrossRef] [PubMed]
- Grigore, B.; Lewis, R.; Peters, J.; Robinson, S.; Hyde, C.J. Development, validation and effectiveness of diagnostic prediction tools for colorectal cancer in primary care: A systematic review. BMC Cancer 2020, 20, 1084. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Miyagami, T.; Kunitomo, K.; Shimizu, T. Clinical decision support systems for diagnosis in primary care: A scoping review. Int. J. Environ. Res. Public Health 2021, 18, 8435. [Google Scholar] [CrossRef] [PubMed]
- Simmons, C.P.; McMillan, D.C.; McWilliams, K.; Sande, T.A.; Fearon, K.C.; Tuck, S.; Fallon, T.; Laird, B.J. Prognostic tools in patients with advanced cancer: A systematic review. J. Pain Symptom Manag. 2017, 53, 962–970. [Google Scholar] [CrossRef]
- Liu, C.A.; Zhang, Q.; Ruan, G.T.; Shen, L.Y.; Xie, H.L.; Liu, T.; Tang, M.; Zhang, X.; Yang, M.; Hu, C.L.; et al. Novel diagnostic and prognostic tools for lung cancer cachexia: Based on nutritional and inflammatory status. Front. Oncol. 2022, 12, 890745. [Google Scholar] [CrossRef]
- Casal-Guisande, M.; Álvarez-Pazó, A.; Cerqueiro-Pequeño, J.; Bouza-Rodríguez, J.B.; Peláez-Lourido, G.; Comesaña-Campos, A. Proposal and definition of an intelligent clinical decision support system applied to the screening and early diagnosis of breast cancer. Cancers 2023, 15, 1711. [Google Scholar] [CrossRef]
- Mozumder, S.I.; Dickman, P.W.; Rutherford, M.J.; Lambert, P.C. InterPreT cancer survival: A dynamic web interactive prediction cancer survival tool for health-care professionals and cancer epidemiologists. Cancer Epidemiol. 2018, 56, 46–52. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Uemura, N.; Okamoto, S.; Yamamoto, S.; Matsumura, N.; Yamaguchi, S.; Yamakido, M.; Taniyama, K.; Sasaki, N.; Schlemper, R.J. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 2001, 345, 784–789. [Google Scholar] [CrossRef]
- Tsugane, S.; Sasazuki, S. Diet and the risk of gastric cancer: Review of epidemiological evidence. Gastric Cancer 2007, 10, 75–83. [Google Scholar] [CrossRef]
- Figueiredo, C.; Garcia-Gonzalez, M.A.; Machado, J.C. Molecular pathogenesis of gastric cancer. Helicobacter 2013, 18, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Yasui, W.; Oue, N.; Aung, P.P.; Matsumura, S.; Shutoh, M.; Nakayama, H. Molecular-pathological prognostic factors of gastric cancer: A review. Gastric Cancer 2005, 8, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, H.; Yoshimura, K.; Saeki, N.; Katai, H.; Shimoda, T.; Matsuno, Y.; Saito, D.; Sugimura, H.; Tanioka, F.; Kato, S.; et al. Genetic variation in psca is associated with susceptibility to diffuse-type gastric cancer. Nat. Genet. 2008, 40, 730–740. [Google Scholar] [CrossRef]
- Shi, Y.; Hu, Z.; Wu, C.; Dai, J.; Li, H.; Dong, J.; Wang, M.; Miao, X.; Zhou, Y.; Lu, F.; et al. A genome-wide association study identifies new susceptibility loci for non-cardia gastric cancer at 3q13. 31 and 5p13. 1. Nat. Genet. 2011, 43, 1215–1218. [Google Scholar] [CrossRef]
- García-González, M.A.; Bujanda, L.; Quintero, E.; Santolaria, S.; Benito, R.; Strunk, M.; Sopeña, F.; Thomson, C.; Pérez-Aisa, A.; Nicolás-Pérez, D.; et al. Association of PSCA rs2294008 gene variants with poor prognosis and increased susceptibility to gastric cancer and decreased risk of duodenal ulcer disease. Int. J. Cancer 2015, 137, 1362–1373. [Google Scholar] [CrossRef]
- Hess, T.; Maj, C.; Gehlen, J.; Borisov, O.; Haas, S.L.; Gockel, I.; Vieth, M.; Piessen, G.; Alakus, H.; Vashist, Y.; et al. Dissecting the genetic heterogeneity of gastric cancer. EBioMedicine 2023, 92, 104616. [Google Scholar] [CrossRef]
- Cheng, L.; Qiu, L.X.; Jia, M.; Zhou, F.; Wang, M.Y.; Zhang, R.X.; Yang, Y.; Wang, X.; Wang, J.; Jin, J.; et al. Is there a dose-dependent effect of genetic susceptibility loci for gastric cancer on prognosis of the patients? Oncotarget 2017, 8, 18435. [Google Scholar] [CrossRef] [PubMed]
- Duan, F.; Liu, L.; Chen, X.; Yang, Q.; Wang, Y.; Zhang, Y.; Wang, K. Genetic risk and gastric cancer: Polygenic risk scores in population-based case-control study. Expert Rev. Mol. Diagn. 2023, 23, 545–554. [Google Scholar] [CrossRef]
- Feng, Q.X.; Liu, C.; Qi, L.; Sun, S.W.; Song, Y.; Yang, G.; Zhang, Y.; Liu, X.S. An intelligent clinical decision support system for preoperative prediction of lymph node metastasis in gastric cancer. J. Am. Coll. Radiol. 2019, 16, 952–960. [Google Scholar] [CrossRef]
- Hao, D.; Li, Q.; Feng, Q.X.; Qi, L.; Liu, X.S.; Arefan, D.; Zhang, Y.; Wu, S. SurvivalCNN: A deep learning-based method for gastric cancer survival prediction using radiological imaging data and clinicopathological variables. Artif. Intell. Med. 2022, 134, 102424. [Google Scholar] [CrossRef]
- Charvat, H.; Sasazuki, S.; Inoue, M.; Iwasaki, M.; Sawada, N.; Shimazu, T.; Yamaji, T.; Tsugane, S.; JPHC Study Group. Prediction of the 10-year probability of gastric cancer occurrence in the Japanese population: The JPHC study cohort II. Int. J. Cancer 2016, 138, 320–331. [Google Scholar] [CrossRef]
- Mahmoodi, S.A.; Mirzaie, K.; Mahmoodi, M.S.; Mahmoudi, S.M. A medical decision support system to assess risk factors for gastric cancer based on fuzzy cognitive map. Comput. Math. Methods Med. 2020, 2020, 1016284. [Google Scholar] [CrossRef] [PubMed]
- Niu, P.H.; Zhao, L.L.; Wu, H.L.; Zhao, D.B.; Chen, Y.T. Artificial intelligence in gastric cancer: Application and future perspectives. World J. Gastroenterol. 2020, 26, 5408. [Google Scholar] [CrossRef]
- Cabitza, F.; Rasoini, R.; Gensini, G.F. Unintended consequences of machine learning in medicine. JAMA 2017, 318, 517–518. [Google Scholar] [CrossRef]
- Lauren, P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma: An attempt at a histo-clinical classification. Acta Pathol. Microbiol. Scand. 1965, 64, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Santolaria, S.; Lanas, A.; Benito, R.; Pérez-Aísa, A.; Montoro, M.; Sainz, R. Helicobacter pylori infection is a protective factor for bleeding gastric ulcers but not for bleeding duodenal ulcers in NSAID users. Aliment. Pharmacol. Ther. 1999, 13, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gonzalez, M.A.; Lanas, A.; Santolaria, S.; Crusius, J.B.A.; Serrano, M.T.; Pena, A.S. The polymorphic IL-1B and IL-1RN genes in the aetiopathogenesis of peptic ulcer. Clin. Exp. Immunol. 2001, 125, 368–375. [Google Scholar] [CrossRef]
- Arand, M.; Mühlbauer, R.; Hengstler, J.; Jäger, E.; Fuchs, J.; Winkler, L.; Oesch, F. A multiplex polymerase chain reaction protocol for the simultaneous analysis of the glutathioneS-transferase GSTM1 and GSTT1 polymorphisms. Anal. Biochem. 1996, 236, 184–186. [Google Scholar] [CrossRef]
- Peto, R.; Peto, J. Asymptotically efficient rank invariant test procedures. J. R. Stat. Soc. Ser. A 1972, 135, 185–198. [Google Scholar] [CrossRef]
- Gehan, E.A. A generalized Wilcoxon test for comparing arbitrarily singly-censored samples. Biometrika 1965, 52, 203–224. [Google Scholar] [CrossRef]
- Hastle, T.; Tibshirani, R.; Wainwright, M. Statistical Learning with Sparsity: The Lasso and Generalizations; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Cristianini, N.; Shawe-Taylor, J. An Introduction to Support Vector Machines and Other Kernel-Based Learning Methods; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. Xgboost: A scalable tree boosting system. In Proceedings of the 22nd Acm Sigkdd International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar] [CrossRef]
- Rumelhart, D.E.; Hinton, G.E.; Williams, R.J. Learning representations by back-propagating errors. Nature 1986, 323, 533–536. [Google Scholar] [CrossRef]
- Cox, D.R. Regression models and life-tables. J. R. Stat. Soc. Series B Stat. Methodol. 1972, 34, 187–220. [Google Scholar] [CrossRef]
- Ishwaran, H.; Kogalur, U.B.; Blackstone, E.H.; Lauer, M.S. Random survival forests. Ann. Appl. Stat. 2008, 2, 841–860. [Google Scholar] [CrossRef]
- Pölsterl, S.; Navab, N.; Katouzian, A. Fast training of support vector machines for survival analysis. In Machine Learning and Knowledge Discovery in Databases: European Conference, ECML PKDD 2015, Porto, Portugal, September 7–11, 2015, Proceedings, Part II 15; Springer International Publishing: Cham, Switzerland, 2015; pp. 243–259. [Google Scholar] [CrossRef]
- Katzman, J.L.; Shaham, U.; Cloninger, A.; Bates, J.; Jiang, T.; Kluger, Y. DeepSurv: Personalized treatment recommender system using a Cox proportional hazards deep neural network. BMC Med. Res. Methodol. 2018, 18, 24. [Google Scholar] [CrossRef]
- Akiba, T.; Sano, S.; Yanase, T.; Ohta, T.; Koyama, M. Optuna: A next-generation hyperparameter optimization framework. In Proceedings of the 25th ACM SIGKDD International Conference on Knowledge Discovery & Data Mining, Anchorage, AK, USA, 4–8 August 2019; pp. 2623–2631. [Google Scholar] [CrossRef]
- Bergstra, J.; Bardenet, R.; Bengio, Y.; Kégl, B. Algorithms for hyper-parameter optimization. Adv. Neural Inf. Process Syst. 2011, 24, 2546–2554. [Google Scholar]
- Pepe, M.S.; Longton, G.; Janes, H. Estimation and comparison of receiver operating characteristic curves. Stata J. 2009, 9, 1–16. [Google Scholar] [CrossRef]
- Štrumbelj, E.; Kononenko, I. Explaining prediction models and individual predictions with feature contributions. Knowl. Inf. Syst. 2014, 41, 647–665. [Google Scholar] [CrossRef]
- Okd. Available online: https://www.okd.io/ (accessed on 6 August 2024).
- Nodejs. Available online: https://nodejs.org/en/ (accessed on 6 August 2024).
- Postgresql. Available online: https://www.postgresql.org/ (accessed on 6 August 2024).
- Vue.js. Available online: https://vuejs.org/ (accessed on 6 August 2024).
- Nuxt.js. Available online: https://nuxt.com/ (accessed on 6 August 2024).
- Vuetify. Available online: https://vuetifyjs.com/en/ (accessed on 6 August 2024).
- Etemadi, A.; Safiri, S.; Sepanlou, S.G.; Ikuta, K.; Bisignano, C.; Shakeri, R.; Amani, M.; Fitzmaurice, C.; Nixon, M.; Abbasi, N.; et al. The global, regional, and national burden of stomach cancer in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 42–54. [Google Scholar] [CrossRef]
- Jin, G.; Lv, J.; Yang, M.; Wang, M.; Zhu, M.; Wang, T.; Yan, C.; Yu, C.; Ding, Y.; Li, G.; et al. Genetic risk, incident gastric cancer, and healthy lifestyle: A meta-analysis of genome-wide association studies and prospective cohort study. Lancet Oncol. 2020, 21, 1378–1386. [Google Scholar] [CrossRef]
- Xin, J.; Jiang, X.; Li, H.; Chen, S.; Zhang, Z.; Wang, M.; Gu, D.; Du, M.; Christiani, D.C. Prognostic evaluation of polygenic risk score underlying pan-cancer analysis: Evidence from two large-scale cohorts. EBioMedicine 2023, 89, 104454. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Y.; Han, Y.; Xu, H.M.; Wang, Z.N.; Xu, Y.; Song, Y.; Xu, H.; Yin, S.C.; Liu, X.Y.; Miao, Z.F. Smoking status and subsequent gastric cancer risk in men compared with women: A meta-analysis of prospective observational studies. BMC Cancer 2019, 19, 377. [Google Scholar] [CrossRef] [PubMed]
- Bernini, M.; Barbi, S.; Roviello, F.; Scarpa, A.; Moore, P.; Pedrazzani, C.; Beghelli, S.; Marrelli, D.; de Manzoni, G. Family history of gastric cancer: A correlation between epidemiologic findings and clinical data. Gastric Cancer 2006, 9, 9–13. [Google Scholar] [CrossRef]
- Luo, G.; Zhang, Y.; Guo, P.; Wang, L.; Huang, Y.; Li, K. Global patterns and trends in stomach cancer incidence: Age, period and birth cohort analysis. Int. J. Cancer 2017, 141, 1333–1344. [Google Scholar] [CrossRef]
- Sala, P.L.; Etxeberria, M.L.; Iguíñiz, E.I.; Rodríguez, A.A.; Oteiza, M.A.; Etxaniz, M.Z. Gastric adenocarcinoma: A review of the TNM classification system and ways of spreading. Radiol. (Engl. Ed.) 2023, 65, 66–80. [Google Scholar]
- Shiraishi, N.; Sato, K.; Yasuda, K.; Inomata, M.; Kitano, S. Multivariate prognostic study on large gastric cancer. J. Surg. Oncol. 2007, 96, 14–18. [Google Scholar] [CrossRef]
- Ilic, M.; Ilic, I. Epidemiology of stomach cancer. World J. Gastroenterol. 2022, 28, 1187. [Google Scholar] [CrossRef]
- Ni, J.; Wang, M.; Wang, T.; Yan, C.; Ren, C.; Li, G.; Ding, Y.; Li, H.; Du, L.; Jiang, Y.; et al. Construction and evaluation of a polygenic hazard score for prognostic assessment in localized gastric cancer. Fundam. Res. 2022; in press. [Google Scholar] [CrossRef]
- Weigl, K.; Thomsen, H.; Balavarca, Y.; Hellwege, J.N.; Shrubsole, M.J.; Brenner, H. Genetic risk score is associated with prevalence of advanced neoplasms in a colorectal cancer screening population. Gastroenterology 2018, 155, 88–98. [Google Scholar] [CrossRef]
- Seibert, T.M.; Fan, C.C.; Wang, Y.; Zuber, V.; Karunamuni, R.; Parsons, J.K.; Eeles, R.A.; Easton, D.; Kote-Jarai, S.; Al-Olama, A.A.; et al. Polygenic hazard score to guide screening for aggressive prostate cancer: Development and validation in large scale cohorts. BMJ 2018, 360, j5757. [Google Scholar] [CrossRef]
- Wolfson, M.; Gribble, S.; Pashayan, N.; Easton, D.F.; Antoniou, A.C.; Lee, A.; van Katwyk, S.; Simard, J. Potential of polygenic risk scores for improving population estimates of women’s breast cancer genetic risks. Genet. Med. 2021, 23, 2114–2121. [Google Scholar] [CrossRef]
- Gargallo-Puyuelo, C.J.; Aznar-Gimeno, R.; Carrera-Lasfuentes, P.; Lanas, Á.; Ferrández, Á.; Quintero, E.; Carrillo, M.; Alonso-Abreu, I.; Esteban, L.M.; de la Vega Rodrigálvarez-Chamarro, M.; et al. Predictive Value of Genetic Risk Scores in the Development of Colorectal Adenomas. Dig. Dis. Sci. 2022, 67, 4049–4058. [Google Scholar] [CrossRef]
- Lennon, N.J.; Kottyan, L.C.; Kachulis, C.; Abul-Husn, N.S.; Arias, J.; Belbin, G.; Below, J.E.; Berndt, S.I.; Chung, W.K.; Cimino, J.J.; et al. Selection, optimization and validation of ten chronic disease polygenic risk scores for clinical implementation in diverse US populations. Nat. Med. 2024, 30, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Collister, J.A.; Liu, X.; Littlejohns, T.J.; Cuzick, J.; Clifton, L.; Hunter, D.J. Assessing the Value of Incorporating a Polygenic Risk Score with Nongenetic Factors for Predicting Breast Cancer Diagnosis in the UK Biobank. Cancer Epidemiol. Biomark. Prev. 2024, 33, 812–820. [Google Scholar] [CrossRef]
- Jiang, S.; Gao, H.; He, J.; Shi, J.; Tong, Y.; Wu, J. Machine learning: A non-invasive prediction method for gastric cancer based on a survey of lifestyle behaviors. Front. Artif. Intell. 2022, 5, 956385. [Google Scholar] [CrossRef]
- Afrash, M.R.; Shafiee, M.; Kazemi-Arpanahi, H. Establishing machine learning models to predict the early risk of gastric cancer based on lifestyle factors. BMC Gastroenterol. 2023, 23, 6. [Google Scholar] [CrossRef]
- Kuo, H.Y.; Chang, W.L.; Yeh, Y.C.; Tsai, Y.C.; Wu, C.T.; Cheng, H.C.; Yang, H.B.; Lu, C.C.; Sheu, B.S. Serum level of trefoil factor 2 can predict the extent of gastric spasmolytic polypeptide-expressing metaplasia in the H. pylori-infected gastric cancer relatives. Helicobacter 2017, 22, e12320. [Google Scholar] [CrossRef]
- Zhu, S.L.; Dong, J.; Zhang, C.; Huang, Y.B.; Pan, W. Application of machine learning in the diagnosis of gastric cancer based on noninvasive characteristics. PLoS ONE 2020, 15, e0244869. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, C.; Hu, Z.; Li, G.; Wang, C.; Yang, C.; Huang, D.; Chen, X.; Zhang, H.; Zhuang, R.; et al. A five-microRNA signature identified from genome-wide serum microRNA expression profiling serves as a fingerprint for gastric cancer diagnosis. Eur. J. Cancer 2011, 47, 784–791. [Google Scholar] [CrossRef]
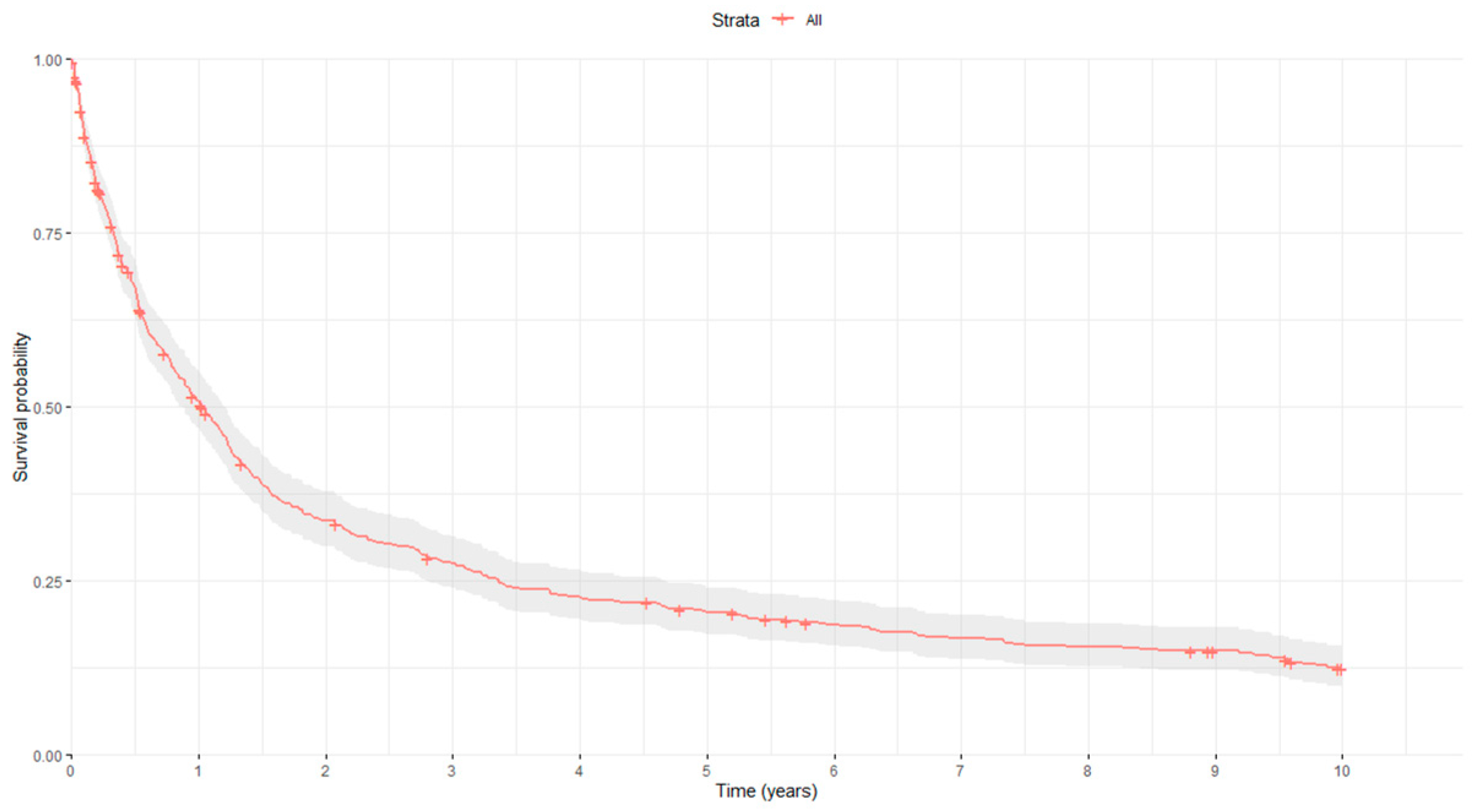










| Characteristics | Controls n = 533 | GC Patients n = 490 | p-Value | |
|---|---|---|---|---|
| Age (years) | Mean (SD) | 69.96 (12.61) | 70.6 (12.58) | 0.32 |
| Median (IQR) | 72 (63–79) | 73 (64–80) | ||
| Sex | Female | 171 (32.08%) | 154 (31.43%) | 0.875 |
| Smoking | Never/Former | 452 (84.8%) | 382 (77.96%) | 0.006 |
| Current smoker | 81 (15.2%) | 108 (22.04%) | ||
| H. pylori infection | Positive | 325 (60.98%) | 349 (71.22%) | 0.001 |
| Family history of GC | Positive | 33 (6.19%) | 72 (14.69%) | <0.001 |
| CarrierIlra2 * | rs1060826 | rs10759932 | rs17655 | rs20417 | rs2074522 | rs2228000 |
| rs2345060 | rs4072037 | rs4150416 | rs4986764 | rs569143 | rs5788 | rs6679677 |
| rs909253 | rs9894946 |
| Variables | p-Value (1.5 Years) | p-Value (3 Years) | p-Value (5 Years) | p-Value (10 Years) |
|---|---|---|---|---|
| H. Pylori Infection | 0.442 | 0.728 | 0.735 | 0.71 |
| Sex | 0.21 | 0.112 | 0.067 | 0.069 |
| Age > 50 years | 0.027 | 0.142 | 0.144 | 0.088 |
| Smoking (current) | 0.511 | 0.858 | 0.528 | 0.776 |
| Family History of GC | 0.572 | 0.348 | 0.262 | 0.169 |
| Charlson index ≥ 3 | 0.742 | 0.633 | 0.373 | 0.265 |
| Cardial Tumour location | 0.011 | 0.002 | 0.001 | 0.001 |
| Lauren’s Histological type | 0.215 | 0.362 | 0.608 | 0.896 |
| TNM staging | <0.001 | <0.001 | <0.001 | <0.001 |
| Metastasis at Diagnosis | <0.001 | <0.001 | <0.001 | <0.001 |
| T1–T2 vs. T3–T4 | <0.001 | <0.001 | <0.001 | <0.001 |
| N0 vs. N1–N2–N3 | <0.001 | <0.001 | <0.001 | <0.001 |
| Chemotherapy | <0.001 | <0.001 | <0.001 | <0.001 |
| Radiotherapy | <0.001 | <0.001 | <0.001 | <0.001 |
| Surgery | <0.001 | <0.001 | <0.001 | <0.001 |
| CarrierIlra2 * | rs1052133 | rs11086565 | rs12711521 | rs13181 | rs144848 | rs1799796 |
| rs1800470 | rs1898830 | rs2074522 | rs207906 | rs26779 | rs2738120 | rs2738169 |
| rs293794 | rs3088074 | rs4072037 | rs4234259 | rs4986790 | rs4987876 | rs6151662 |
| rs7744 | rs7797466 | rs7932766 | rs8305 | rs9841504 |
| GRS Values | OR | Lower CI | Upper CI | p-Value |
|---|---|---|---|---|
| ≤15 | Ref | |||
| 16 | 1.40 | 0.33 | 6.48 | 0.657 |
| 17 | 1.03 | 0.29 | 4.25 | 0.966 |
| 18 | 1.47 | 0.46 | 5.65 | 0.537 |
| 19 | 2.01 | 0.67 | 7.46 | 0.243 |
| 20 | 2.52 | 0.86 | 9.17 | 0.117 |
| 21 | 3.24 | 1.11 | 11.83 | 0.046 |
| 22 | 4.59 | 1.56 | 16.81 | 0.01 |
| 23 | 5.21 | 1.75 | 19.28 | 0.006 |
| 24 | 5.87 | 1.94 | 21.91 | 0.003 |
| 25 | 5.69 | 1.71 | 22.92 | 0.007 |
| ≥ 26 | 12.25 | 3.17 | 58.04 | 0.001 |
| Continuous value | 1.25 | 1.19 | 1.33 | <0.001 |
| GRS Values | OR | Lower CI | Upper CI | p-Value |
|---|---|---|---|---|
| 1 | Ref | |||
| 2 | 1.99 | 1.38 | 2.88 | <0.001 |
| 3 | 3.62 | 2.51 | 5.25 | <0.001 |
| 4 | 4.03 | 2.79 | 5.86 | <0.001 |
| GRS Values | HR | Lower CI | Upper CI | p-Value |
|---|---|---|---|---|
| ≤15 | Ref | |||
| 16 | 0.84 | 0.45 | 1.50 | 0.566 |
| 17 | 1.33 | 0.81 | 2.20 | 0.258 |
| 18 | 1.22 | 0.74 | 2.00 | 0.438 |
| 19 | 1.12 | 0.69 | 1.80 | 0.012 |
| 20 | 1.79 | 1.13 | 2.80 | <0.001 |
| 21 | 2.27 | 1.41 | 3.60 | <0.001 |
| 22 | 2.50 | 1.55 | 4.00 | <0.001 |
| 23 | 3.72 | 2.18 | 6.30 | <0.001 |
| 24 | 7.04 | 3.80 | 13.00 | <0.001 |
| 25 | 7.96 | 3.79 | 16.70 | <0.001 |
| ≥26 | 10.07 | 5.24 | 19.40 | <0.001 |
| Continuous value | 1.20 | 1.20 | 1.30 | <0.001 |
| GRS Values | HR | Lower CI | Upper CI | p-Value |
|---|---|---|---|---|
| 1 | Ref | |||
| 2 | 1.40 | 1.10 | 1.90 | 0.018 |
| 3 | 2.20 | 1.70 | 3.00 | <0.001 |
| 4 | 4.00 | 3.00 | 5.40 | <0.001 |
| Continuous value | 2.70 | 2.30 | 3.20 | <0.001 |
| Models | AUC Mean (SD) |
|---|---|
| (1) Clinical–demographic model | 0.606 (0.034) |
| (2) Univariate model: Unweighted GRS | 0.647 (0.034) |
| (3) Univariate model: Weighted GRS | 0.655 (0.033) |
| (4) Clinical–demographic and unweighted GRS | 0.678 (0.036) |
| (5) Clinical–demographic and weighted GRS | 0.682 (0.036) |
| (6) Model 4 with interactions with unweighted GRS | 0.674 (0.035) |
| (7) Model 5 with interactions with weighted GRS | 0.678 (0.035) |
| (8) Clinical–demographic and SNPs | 0.655 (0.030) |
| Models | AUC Mean (SD) |
|---|---|
| (1) Clinical–demographic model | 0.586 (0.032) |
| (2) TNM model | 0.698 (0.019) |
| (3) Treatments model | 0.623 (0.012) |
| (4) Univariate model: Weighted GRS | 0.664 (0.033) |
| (5) Non-genetic variable model | 0.730 (0.023) |
| (6) Total model (weighted GRS + non-genetic variables) | 0.761 (0.037) |
| (7) Total model (unweighted GRS + non-genetic variables) | 0.674 (0.021) |
| Diagnosis | Prognosis | ||
|---|---|---|---|
| Models | AUC Mean (SD) | Models | C-Index Mean (SD) |
| LR | 0.679 (0.043) | Cox regression | 0.757 (0.011) |
| Lasso LR | 0.679 (0.043) | Lasso Cox | 0.758 (0.012) |
| Ridge LR | 0.680 (0.044) | Ridge Cox | 0.758 (0.013) |
| RF | 0.670 (0.034) | RSF | 0.769 (0.016) |
| SVM | 0.680 (0.040) | SSVM | 0.768 (0.007) |
| XGBoost | 0.684 (0.043) | Survival XGBoost | 0.727 (0.022) |
| MLP (2) * | 0.672 (0.043) | DeepCox | 0.773 (0.016) |
| MLP (3) + | 0.678 (0.044) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aznar-Gimeno, R.; García-González, M.A.; Muñoz-Sierra, R.; Carrera-Lasfuentes, P.; Rodrigálvarez-Chamarro, M.d.l.V.; González-Muñoz, C.; Meléndez-Estrada, E.; Lanas, Á.; del Hoyo-Alonso, R. GastricAITool: A Clinical Decision Support Tool for the Diagnosis and Prognosis of Gastric Cancer. Biomedicines 2024, 12, 2162. https://doi.org/10.3390/biomedicines12092162
Aznar-Gimeno R, García-González MA, Muñoz-Sierra R, Carrera-Lasfuentes P, Rodrigálvarez-Chamarro MdlV, González-Muñoz C, Meléndez-Estrada E, Lanas Á, del Hoyo-Alonso R. GastricAITool: A Clinical Decision Support Tool for the Diagnosis and Prognosis of Gastric Cancer. Biomedicines. 2024; 12(9):2162. https://doi.org/10.3390/biomedicines12092162
Chicago/Turabian StyleAznar-Gimeno, Rocío, María Asunción García-González, Rubén Muñoz-Sierra, Patricia Carrera-Lasfuentes, María de la Vega Rodrigálvarez-Chamarro, Carlos González-Muñoz, Enrique Meléndez-Estrada, Ángel Lanas, and Rafael del Hoyo-Alonso. 2024. "GastricAITool: A Clinical Decision Support Tool for the Diagnosis and Prognosis of Gastric Cancer" Biomedicines 12, no. 9: 2162. https://doi.org/10.3390/biomedicines12092162






