Relationship between Infant Feeding and the Microbiome: Implications for Allergies and Food Intolerances
Abstract
:1. Introduction
1.1. Overview of Infant Feeding Practices and Recommendations
1.1.1. Breastfeeding
1.1.2. Formula Feeding
1.1.3. Introduction of Solid Foods
1.2. Importance of the Gut Microbiome and Its Early Development
2. Infant Feeding and the Microbiome Development
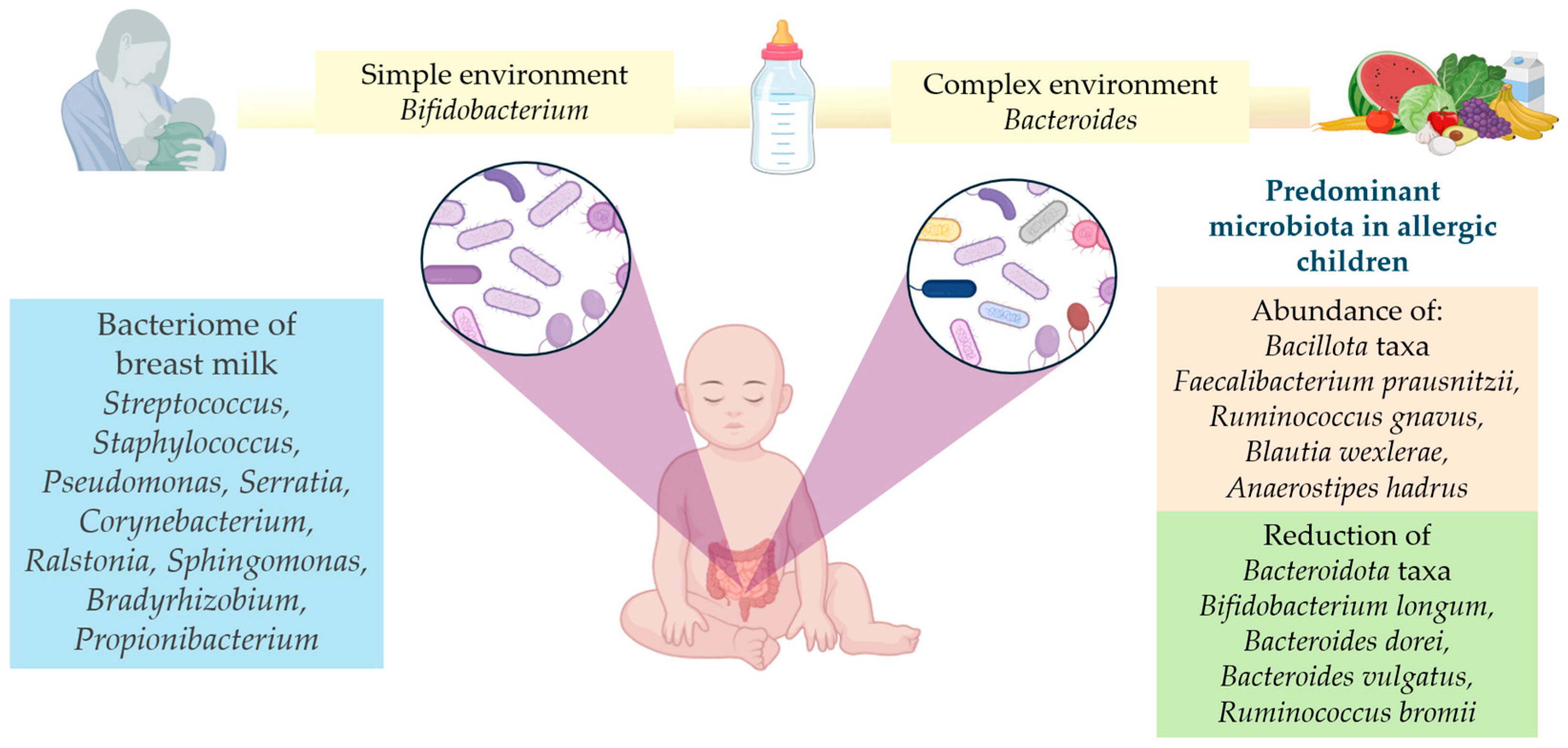
2.1. The Human Milk Microbiome: Composition and Impact on the Organism
2.2. Benefits of Breastfeeding on Gut Health
2.3. Formula Feeding: Implications in Microbiome and Related Possibilities of Formulation
2.4. Timing and Types of First Foods and Their Impact on Microbiome Diversity
3. Microbiome and Allergies
3.1. Allergies: Underlying Mechanisms and Links with the Microbiome
3.2. Childhood Allergies and Microbiome
3.3. Microbiome Signature in Food Allergies
3.4. Impact of Early Feeding Choices on Allergy Development
4. Microbiome and Food Intolerances
4.1. Understanding Food Intolerances
4.2. Food Intolerances and Microbiome
5. Practical Implications
5.1. Recommendations for Infant Feeding
5.2. Breastfeeding Encouragement and Support
5.3. Choosing the Right Formula Based on Its Components
5.4. Guidelines for Introducing Solid Foods
5.5. Future Research Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berti, C.; Socha, P. Infant and Young Child Feeding Practices and Health. Nutrients 2023, 15, 1184. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Infant and Young Child Feeding. Available online: https://www.who.int/news-room/fact-sheets/detail/infant-and-young-child-feeding (accessed on 24 July 2024).
- Rollins, N.C.; Bhandari, N.; Hajeebhoy, N.; Horton, S.; Lutter, C.K.; Martines, J.C.; Piwoz, E.G.; Richter, L.M.; Victora, C.G. Why invest, and what it will take to improve breastfeeding practices? Lancet 2016, 387, 491–504. [Google Scholar] [CrossRef]
- Laxmi, A.; Amanda, L.; Dolly, D.; Rubina, G. Infant Feeding Practices: A Global Perspective; IntechOpen: London, UK, 2023. [Google Scholar]
- Hirani, S.A.A.; Richter, S.; Salami, B.; Vallianatos, H. Sociocultural Factors Affecting Breastfeeding Practices of Mothers During Natural Disasters: A Critical Ethnography in Rural Pakistan. Glob. Qual. Nurs. Res. 2023, 10, 23333936221148808. [Google Scholar] [CrossRef]
- Reinsma, K.; Bolima, N.; Fonteh, F.; Okwen, P.; Yota, D.; Montgomery, S. Incorporating cultural beliefs in promoting exclusive breastfeeding. Afr. J. Midwifery Womens Health 2012, 6, 65–70. [Google Scholar] [CrossRef]
- UNICEF. Infant and Young Child Feeding. Available online: https://data.unicef.org/topic/nutrition/infant-and-young-child-feeding/ (accessed on 24 July 2024).
- World Health Organization (WHO). Breastfeeding. Available online: https://www.who.int/health-topics/breastfeeding#tab=tab_1 (accessed on 24 July 2024).
- World Health Organization (WHO). Exclusively Breastfeed for 6 Months. Available online: https://www.emro.who.int/nutrition/breastfeeding/index.html (accessed on 25 July 2024).
- Pan American Health Organization (PAHO). Exclusive Breastfeeding in Infant under Six Months of Age. Available online: https://www.paho.org/en/enlace/exclusive-breastfeeding-infant-under-six-months-age (accessed on 25 July 2024).
- Yan, J.; Liu, L.; Zhu, Y.; Huang, G.; Wang, P.P. The association between breastfeeding and childhood obesity: A meta-analysis. BMC Public Health 2014, 14, 1267. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, S.; Paswan, V.K.; Yadav, S.P.; Bhinchhar, B.K.; Kharkwal, S.; Rose, H.; Kanetkar, P.; Kumar, V.; Al-Zamani, Z.A.S.; Bunkar, D.S. A comprehensive review on infant formula: Nutritional and functional constituents, recent trends in processing and its impact on infants’ gut microbiota. Front. Nutr. 2023, 10, 1194679. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine. Feeding Infants and Children from Birth to 24 Months: Summarizing Existing Guidance; National Academies Press: Washington, DC, USA, 2020. [Google Scholar]
- Health Canada; Canadian Paediatric Society; Dietitians of Canada; Breastfeeding Committee for Canada. Nutrition for healthy term infants: Recommendations from birth to six months. Can. J. Diet. Pract. Res. 2012, 73, 204. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, C.; Scholtens, P.A.; Lalanne, A.; Weenen, H.; Nicklaus, S. Development of healthy eating habits early in life. Review of recent evidence and selected guidelines. Appetite 2011, 57, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Klerks, M.; Roman, S.; Bernal, M.J.; Haro-Vicente, J.F.; Sanchez-Siles, L.M. Complementary Feeding Practices and Parental Pressure to Eat among Spanish Infants and Toddlers: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2021, 18, 1982. [Google Scholar] [CrossRef]
- Low, F.M.; Gluckman, P.D.; Hanson, M.A. Maternal and child health: Is making ‘healthy choices’ an oxymoron? Glob. Health Promot. 2021, 28, 66–69. [Google Scholar] [CrossRef]
- Maier-Noth, A.; Schaal, B.; Leathwood, P.; Issanchou, S. The Lasting Influences of Early Food-Related Variety Experience: A Longitudinal Study of Vegetable Acceptance from 5 Months to 6 Years in Two Populations. PLoS ONE 2016, 11, e0151356. [Google Scholar] [CrossRef] [PubMed]
- Nicklaus, S. The role of food experiences during early childhood in food pleasure learning. Appetite 2016, 104, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Faber, M.; Malan, L.; Kruger, H.S.; Asare, H.; Visser, M.; Mukwevho, T.; Ricci, C.; Smuts, C.M. Potential of Egg as Complementary Food to Improve Nutrient Intake and Dietary Diversity. Nutrients 2022, 14, 3396. [Google Scholar] [CrossRef]
- Huang, H.; Gao, Y.; Zhu, N.; Yuan, G.; Li, X.; Feng, Y.; Gao, L.; Yu, J. The Effects of Breastfeeding for Four Months on Thinness, Overweight, and Obesity in Children Aged 3 to 6 Years: A Retrospective Cohort Study from National Physical Fitness Surveillance of Jiangsu Province, China. Nutrients 2022, 14, 4154. [Google Scholar] [CrossRef]
- Gillman, M.W.; Ludwig, D.S. How early should obesity prevention start? N. Engl. J. Med. 2013, 369, 2173–2175. [Google Scholar] [CrossRef]
- Birch, L.L.; Doub, A.E. Learning to eat: Birth to age 2 y. Am. J. Clin. Nutr. 2014, 99, 723S–728S. [Google Scholar] [CrossRef] [PubMed]
- Hermoso, M.; Tabacchi, G.; Iglesia-Altaba, I.; Bel-Serrat, S.; Moreno-Aznar, L.A.; García-Santos, Y.; García-Luzardo, M.d.R.; Santana-Salguero, B.; Peña-Quintana, L.; Serra-Majem, L. The nutritional requirements of infants. Towards EU alignment of reference values: The EURRECA network. Matern. Child Nutr. 2010, 6, 55–83. [Google Scholar] [CrossRef]
- Pearce, J.; Taylor, M.; Langley-Evans, S. Timing of the introduction of complementary feeding and risk of childhood obesity: A systematic review. Int. J. Obes. 2013, 37, 1295–1306. [Google Scholar] [CrossRef]
- Sun, C.; Foskey, R.J.; Allen, K.J.; Dharmage, S.C.; Koplin, J.J.; Ponsonby, A.-L.; Lowe, A.J.; Matheson, M.C.; Tang, M.L.; Gurrin, L. The impact of timing of introduction of solids on infant body mass index. J. Pediatr. 2016, 179, 104–110.e1. [Google Scholar] [CrossRef]
- Brophy, S.; Cooksey, R.; Gravenor, M.B.; Mistry, R.; Thomas, N.; Lyons, R.A.; Williams, R. Risk factors for childhood obesity at age 5: Analysis of the millennium cohort study. BMC Public Health 2009, 9, 467. [Google Scholar] [CrossRef]
- Huh, S.Y.; Rifas-Shiman, S.L.; Taveras, E.M.; Oken, E.; Gillman, M.W. Timing of solid food introduction and risk of obesity in preschool-aged children. Pediatrics 2011, 127, e544–e551. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.-S.; Liu, H.; Zhao, Y.-M.; Li, J.; Chen, Y.; Zhu, S.; Chen, H.; Huang, T.; Li, D. Complementary feeding and childhood adiposity in preschool-aged children in a large Chinese cohort. J. Pediatr. 2015, 166, 326–331.e2. [Google Scholar] [CrossRef] [PubMed]
- Gartner, L.M.; Morton, J.; Lawrence, R.A.; Naylor, A.J.; O’Hare, D.; Schanler, R.J.; Eidelman, A.I. Breastfeeding and the use of human milk. Pediatrics 2005, 115, 496–506. [Google Scholar]
- Critch, J.N.; Canadian Paediatric Society, Nutrition and Gastroenterology Committee. Nutrition for healthy term infants, birth to six months: An overview. Paediatr. Child. Health 2013, 18, 206–207. [Google Scholar] [PubMed]
- Boland, M. Exclusive breastfeeding should continue to six months. Paediatr. Child Health 2005, 10, 148. [Google Scholar] [CrossRef]
- D’Hollander, C.J.; Keown-Stoneman, C.D.; Birken, C.S.; O’Connor, D.L.; Maguire, J.L.; Cohn, R.; Lau, E.; Laupacis, A.; Parkin, P.C.; Salter, M. Timing of introduction to solid food, growth, and nutrition risk in later childhood. J. Pediatr. 2022, 240, 102–109.e3. [Google Scholar] [CrossRef]
- Kramer, M.S.; Kakuma, R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst. Rev. 2012, 2012, CD003517. [Google Scholar] [CrossRef] [PubMed]
- Fewtrell, M.; Bronsky, J.; Campoy, C.; Domellöf, M.; Embleton, N.; Mis, N.F.; Hojsak, I.; Hulst, J.M.; Indrio, F.; Lapillonne, A. Complementary feeding: A position paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 119–132. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Vilchez-Padial, L.M.; Gil, A. Evidence of the Anti-Inflammatory Effects of Probiotics and Synbiotics in Intestinal Chronic Diseases. Nutrients 2017, 9, 555. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Immune-Mediated Mechanisms of Action of Probiotics and Synbiotics in Treating Pediatric Intestinal Diseases. Nutrients 2018, 10, 42. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Gomez-Fernandez, A.; Chueca, N.; Torre-Aguilar, M.J.; Gil, A.; Perez-Navero, J.L.; Flores-Rojas, K.; Martin-Borreguero, P.; Solis-Urra, P.; Ruiz-Ojeda, F.J.; et al. Autism Spectrum Disorder (ASD) with and without Mental Regression is Associated with Changes in the Fecal Microbiota. Nutrients 2019, 11, 337. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Mercado, A.I.; Navarro-Oliveros, M.; Robles-Sanchez, C.; Plaza-Diaz, J.; Saez-Lara, M.J.; Munoz-Quezada, S.; Fontana, L.; Abadia-Molina, F. Microbial Population Changes and Their Relationship with Human Health and Disease. Microorganisms 2019, 7, 68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sparks, J.B.; Karyala, S.V.; Settlage, R.; Luo, X.M. Host adaptive immunity alters gut microbiota. ISME J. 2015, 9, 770–781. [Google Scholar] [CrossRef]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef]
- Hakansson, A.; Molin, G. Gut microbiota and inflammation. Nutrients 2011, 3, 637–682. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.L.; Patterson, A.D. The gut microbiome: An orchestrator of xenobiotic metabolism. Acta Pharm. Sin. B 2020, 10, 19–32. [Google Scholar] [CrossRef]
- Wong, J.M.; De Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Wen, L.; Duffy, A. Factors Influencing the Gut Microbiota, Inflammation, and Type 2 Diabetes. J. Nutr. 2017, 147, 1468S–1475S. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Kumbhare, S.V.; Patangia, D.V.; Patil, R.H.; Shouche, Y.S.; Patil, N.P. Factors influencing the gut microbiome in children: From infancy to childhood. J. Biosci. 2019, 44, 49. [Google Scholar] [CrossRef]
- Raspini, B.; Vacca, M.; Porri, D.; De Giuseppe, R.; Calabrese, F.M.; Chieppa, M.; Liso, M.; Cerbo, R.M.; Civardi, E.; Garofoli, F.; et al. Early Life Microbiota Colonization at Six Months of Age: A Transitional Time Point. Front. Cell Infect. Microbiol. 2021, 11, 590202. [Google Scholar] [CrossRef] [PubMed]
- Bogaert, D.; van Beveren, G.J.; de Koff, E.M.; Lusarreta Parga, P.; Balcazar Lopez, C.E.; Koppensteiner, L.; Clerc, M.; Hasrat, R.; Arp, K.; Chu, M.; et al. Mother-to-infant microbiota transmission and infant microbiota development across multiple body sites. Cell Host Microbe 2023, 31, 447–460.e6. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.L.; Monteagudo-Mera, A.; Cadenas, M.B.; Lampl, M.L.; Azcarate-Peril, M.A. Milk-and solid-feeding practices and daycare attendance are associated with differences in bacterial diversity, predominant communities, and metabolic and immune function of the infant gut microbiome. Front. Cell. Infect. Microbiol. 2015, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.J.; Ajami, N.J.; O’Brien, J.L.; Hutchinson, D.S.; Smith, D.P.; Wong, M.C.; Ross, M.C.; Lloyd, R.E.; Doddapaneni, H.; Metcalf, G.A. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature 2018, 562, 583–588. [Google Scholar] [CrossRef]
- Moore, R.E.; Townsend, S.D. Temporal development of the infant gut microbiome. Open Biol. 2019, 9, 190128. [Google Scholar] [CrossRef]
- Leyva, L.L.; Brereton, N.J.; Koski, K.G. Emerging frontiers in human milk microbiome research and suggested primers for 16S rRNA gene analysis. Comput. Struct. Biotechnol. J. 2021, 19, 121–133. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yi, D.Y. Analysis of the human breast milk microbiome and bacterial extracellular vesicles in healthy mothers. Exp. Mol. Med. 2020, 52, 1288–1297. [Google Scholar] [CrossRef]
- Moubareck, C.A. Human milk microbiota and oligosaccharides: A glimpse into benefits, diversity, and correlations. Nutrients 2021, 13, 1123. [Google Scholar] [CrossRef]
- Demmelmair, H.; Jiménez, E.; Collado, M.C.; Salminen, S.; McGuire, M.K. Maternal and perinatal factors associated with the human milk microbiome. Curr. Dev. Nutr. 2020, 4, nzaa027. [Google Scholar]
- Enaud, R.; Prevel, R.; Ciarlo, E.; Beaufils, F.; Wieërs, G.; Guery, B.; Delhaes, L. The gut-lung axis in health and respiratory diseases: A place for inter-organ and inter-kingdom crosstalks. Front. Cell. Infect. Microbiol. 2020, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C.; Franco, L.; Regal, P.; Lamas, A.; Cepeda, A.; Fente, C. Breast milk: A source of functional compounds with potential application in nutrition and therapy. Nutrients 2021, 13, 1026. [Google Scholar] [CrossRef]
- Oikonomou, G.; Addis, M.F.; Chassard, C.; Nader-Macias, M.E.F.; Grant, I.; Delbès, C.; Bogni, C.I.; Le Loir, Y.; Even, S. Milk microbiota: What are we exactly talking about? Front. Microbiol. 2020, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Chung, J.; Battaglia, T.; Henderson, N.; Jay, M.; Li, H.; Lieber, A.D.; Wu, F.; Perez-Perez, G.I.; Chen, Y. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci. Transl. Med. 2016, 8, 343ra82. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef]
- Shao, Y.; Forster, S.C.; Tsaliki, E.; Vervier, K.; Strang, A.; Simpson, N.; Kumar, N.; Stares, M.D.; Rodger, A.; Brocklehurst, P. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature 2019, 574, 117–121. [Google Scholar] [CrossRef]
- Reyman, M.; van Houten, M.A.; van Baarle, D.; Bosch, A.A.; Man, W.H.; Chu, M.L.J.; Arp, K.; Watson, R.L.; Sanders, E.A.; Fuentes, S. Impact of delivery mode-associated gut microbiota dynamics on health in the first year of life. Nat. Commun. 2019, 10, 4997. [Google Scholar] [CrossRef]
- Chu, D.M.; Ma, J.; Prince, A.L.; Antony, K.M.; Seferovic, M.D.; Aagaard, K.M. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat. Med. 2017, 23, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Alvarez, A.S.; de Vos, W.M. The Gut Microbiota in the First Decade of Life. Trends Microbiol. 2019, 27, 997–1010. [Google Scholar] [CrossRef]
- Huang, H.; Jiang, J.; Wang, X.; Jiang, K.; Cao, H. Exposure to prescribed medication in early life and impacts on gut microbiota and disease development. EClinicalMedicine 2024, 68, 102428. [Google Scholar] [CrossRef]
- van den Elsen, L.W.J.; Garssen, J.; Burcelin, R.; Verhasselt, V. Shaping the Gut Microbiota by Breastfeeding: The Gateway to Allergy Prevention? Front. Pediatr. 2019, 7, 47. [Google Scholar] [CrossRef]
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K. Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatr. 2017, 171, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Roger, L.C.; Costabile, A.; Holland, D.T.; Hoyles, L.; McCartney, A.L. Examination of faecal Bifidobacterium populations in breast-and formula-fed infants during the first 18 months of life. Microbiology 2010, 156, 3329–3341. [Google Scholar] [CrossRef]
- Roger, L.C.; McCartney, A.L. Longitudinal investigation of the faecal microbiota of healthy full-term infants using fluorescence in situ hybridization and denaturing gradient gel electrophoresis. Microbiology 2010, 156, 3317–3328. [Google Scholar] [CrossRef] [PubMed]
- Bridgman, S.L.; Azad, M.B.; Field, C.J.; Haqq, A.M.; Becker, A.B.; Mandhane, P.J.; Subbarao, P.; Turvey, S.E.; Sears, M.R.; Scott, J.A. Fecal short-chain fatty acid variations by breastfeeding status in infants at 4 months: Differences in relative versus absolute concentrations. Front. Nutr. 2017, 4, 11. [Google Scholar] [CrossRef]
- Ma, J.; Li, Z.; Zhang, W.; Zhang, C.; Zhang, Y.; Mei, H.; Zhuo, N.; Wang, H.; Wang, L.; Wu, D. Comparison of gut microbiota in exclusively breast-fed and formula-fed babies: A study of 91 term infants. Sci. Rep. 2020, 10, 15792. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.A.; Iyengar, R.S. Breast milk, microbiota, and intestinal immune homeostasis. Pediatr. Res. 2015, 77, 220–228. [Google Scholar] [CrossRef]
- Levin, A.M.; Sitarik, A.R.; Havstad, S.L.; Fujimura, K.E.; Wegienka, G.; Cassidy-Bushrow, A.E.; Kim, H.; Zoratti, E.M.; Lukacs, N.W.; Boushey, H.A. Joint effects of pregnancy, sociocultural, and environmental factors on early life gut microbiome structure and diversity. Sci. Rep. 2016, 6, 31775. [Google Scholar] [CrossRef] [PubMed]
- Donald, K.; Petersen, C.; Turvey, S.E.; Finlay, B.B.; Azad, M.B. Secretory IgA: Linking microbes, maternal health, and infant health through human milk. Cell Host Microbe 2022, 30, 650–659. [Google Scholar] [CrossRef]
- Dzidic, M.; Mira, A.; Artacho, A.; Abrahamsson, T.R.; Jenmalm, M.C.; Collado, M.C. Allergy development is associated with consumption of breastmilk with a reduced microbial richness in the first month of life. Pediatr. Allergy Immunol. 2020, 31, 250–257. [Google Scholar] [CrossRef]
- Orndorff, P.E.; Devapali, A.; Palestrant, S.; Wyse, A.; Everett, M.L.; Bollinger, R.R.; Parker, W. Immunoglobulin-mediated agglutination of and biofilm formation by Escherichia coli K-12 require the type 1 pilus fiber. Infect. Immun. 2004, 72, 1929–1938. [Google Scholar] [CrossRef]
- Pannaraj, P.S.; Ly, M.; Cerini, C.; Saavedra, M.; Aldrovandi, G.M.; Saboory, A.A.; Johnson, K.M.; Pride, D.T. Shared and distinct features of human milk and infant stool viromes. Front. Microbiol. 2018, 9, 1162. [Google Scholar] [CrossRef] [PubMed]
- Milani, C.; Casey, E.; Lugli, G.A.; Moore, R.; Kaczorowska, J.; Feehily, C.; Mangifesta, M.; Mancabelli, L.; Duranti, S.; Turroni, F. Tracing mother-infant transmission of bacteriophages by means of a novel analytical tool for shotgun metagenomic datasets: METAnnotatorX. Microbiome 2018, 6, 145. [Google Scholar] [CrossRef]
- Davis, E.C.; Castagna, V.P.; Sela, D.A.; Hillard, M.A.; Lindberg, S.; Mantis, N.J.; Seppo, A.E.; Jarvinen, K.M. Gut microbiome and breast-feeding: Implications for early immune development. J. Allergy Clin. Immunol. 2022, 150, 523–534. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.C.; Ferdaus, M.J. Technological Advances in Infant Formula Ingredients; IntechOpen: London, UK, 2023. [Google Scholar]
- de Almagro Garcia, M.C.; JA, M.M. New ingredients in infant formula. Health and functional benefits. Nutr. Hosp. 2017, 34, 8–12. [Google Scholar] [PubMed]
- Hedrick, J.; Yeiser, M.; Harris, C.L.; Wampler, J.L.; London, H.E.; Patterson, A.C.; Wu, S.S. Infant formula with added bovine milk fat globule membrane and modified iron supports growth and normal iron status at one year of age: A randomized controlled trial. Nutrients 2021, 13, 4541. [Google Scholar] [CrossRef]
- Martin, C.R.; Ling, P.R.; Blackburn, G.L. Review of Infant Feeding: Key Features of Breast Milk and Infant Formula. Nutrients 2016, 8, 279. [Google Scholar] [CrossRef]
- Ferreres-Serafini, L.; Martin-Orue, S.M.; Sadurni, M.; Jimenez, J.; Moreno-Munoz, J.A.; Castillejos, L. Supplementing infant milk formula with a multi-strain synbiotic and osteopontin enhances colonic microbial colonization and modifies jejunal gene expression in lactating piglets. Food Funct. 2024, 15, 6536–6552. [Google Scholar] [CrossRef] [PubMed]
- Chouraqui, J.P.; Grathwohl, D.; Labaune, J.M.; Hascoet, J.M.; de Montgolfier, I.; Leclaire, M.; Giarre, M.; Steenhout, P. Assessment of the safety, tolerance, and protective effect against diarrhea of infant formulas containing mixtures of probiotics or probiotics and prebiotics in a randomized controlled trial. Am. J. Clin. Nutr. 2008, 87, 1365–1373. [Google Scholar] [CrossRef]
- Braegger, C.; Chmielewska, A.; Decsi, T.; Kolacek, S.; Mihatsch, W.; Moreno, L.; Piescik, M.; Puntis, J.; Shamir, R.; Szajewska, H.; et al. Supplementation of infant formula with probiotics and/or prebiotics: A systematic review and comment by the ESPGHAN committee on nutrition. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 238–250. [Google Scholar] [CrossRef]
- Fallani, M.; Amarri, S.; Uusijarvi, A.; Adam, R.; Khanna, S.; Aguilera, M.; Gil, A.; Vieites, J.M.; Norin, E.; Young, D. Determinants of the human infant intestinal microbiota after the introduction of first complementary foods in infant samples from five European centres. Microbiology 2011, 157, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, J.; Lange, B.; Frick, J.-S.; Sauer, H.; Zimmermann, K.; Schwiertz, A.; Rusch, K.; Klosterhalfen, S.; Enck, P. A vegan or vegetarian diet substantially alters the human colonic faecal microbiota. Eur. J. Clin. Nutr. 2012, 66, 53–60. [Google Scholar] [CrossRef]
- Johnson, C.L.; Versalovic, J. The human microbiome and its potential importance to pediatrics. Pediatrics 2012, 129, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Ku, H.J.; Kim, Y.T.; Lee, J.H. Microbiome Study of Initial Gut Microbiota from Newborn Infants to Children Reveals that Diet Determines Its Compositional Development. J. Microbiol. Biotechnol. 2020, 30, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Borowitz, S.M. First Bites-Why, When, and What Solid Foods to Feed Infants. Front. Pediatr. 2021, 9, 654171. [Google Scholar] [CrossRef]
- Homann, C.M.; Rossel, C.A.J.; Dizzell, S.; Bervoets, L.; Simioni, J.; Li, J.; Gunn, E.; Surette, M.G.; de Souza, R.J.; Mommers, M.; et al. Infants’ First Solid Foods: Impact on Gut Microbiota Development in Two Intercontinental Cohorts. Nutrients 2021, 13, 2639. [Google Scholar] [CrossRef]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- Arrieta, M.-C.; Stiemsma, L.T.; Amenyogbe, N.; Brown, E.M.; Finlay, B. The intestinal microbiome in early life: Health and disease. Front. Immunol. 2014, 5, 427. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, J.; Liang, H.; Ye, L.; Lan, L.; Lu, F.; Wang, Q.; Lei, T.; Yang, X.; Cui, P.; et al. Differences in Alpha Diversity of Gut Microbiota in Neurological Diseases. Front. Neurosci. 2022, 16, 879318. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Zhang, H.; Hu, L.; Liu, J.; Wang, L.; Wang, T.; Zhang, H.; Cong, L.; Wang, Q. Pathogenesis of allergic diseases and implications for therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 138. [Google Scholar] [CrossRef]
- Wanka, L.; Jappe, U. Trained immunity and allergy: State of the art and future perspectives. Allergy 2021, 76, 1265–1267. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, K.E.; Lynch, S.V. Microbiota in allergy and asthma and the emerging relationship with the gut microbiome. Cell Host Microbe 2015, 17, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Stark, J.F. Bacteriology in the service of sanitation: The factory environment and the regulation of industrial anthrax in late-victorian Britain. Social Hist. Med. 2012, 25, 343–361. [Google Scholar] [CrossRef]
- Zhao, H.; Liao, X.; Kang, Y. Tregs: Where we are and what comes next? Front. Immunol. 2017, 8, 1578. [Google Scholar] [CrossRef]
- Chaudhary, B.; Elkord, E. Regulatory T cells in the tumor microenvironment and cancer progression: Role and therapeutic targeting. Vaccines 2016, 4, 28. [Google Scholar] [CrossRef]
- Miyara, M.; Yoshioka, Y.; Kitoh, A.; Shima, T.; Wing, K.; Niwa, A.; Parizot, C.; Taflin, C.; Heike, T.; Valeyre, D. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 2009, 30, 899–911. [Google Scholar] [CrossRef]
- Duhen, T.; Duhen, R.; Lanzavecchia, A.; Sallusto, F.; Campbell, D.J. Functionally distinct subsets of human FOXP3+ Treg cells that phenotypically mirror effector Th cells. Blood J. Am. Soc. Hematol. 2012, 119, 4430–4440. [Google Scholar] [CrossRef]
- Dong, S.; Maiella, S.; Xhaard, A.; Pang, Y.; Wenandy, L.; Larghero, J.; Becavin, C.; Benecke, A.; Bianchi, E.; Socié, G. Multiparameter single-cell profiling of human CD4+ FOXP3+ regulatory T-cell populations in homeostatic conditions and during graft-versus-host disease. Blood J. Am. Soc. Hematol. 2013, 122, 1802–1812. [Google Scholar] [CrossRef]
- Mason, G.M.; Lowe, K.; Melchiotti, R.; Ellis, R.; de Rinaldis, E.; Peakman, M.; Heck, S.; Lombardi, G.; Tree, T.I. Phenotypic complexity of the human regulatory T cell compartment revealed by mass cytometry. J. Immunol. 2015, 195, 2030–2037. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Wing, J.B.; Sakaguchi, S. Two modes of immune suppression by Foxp3+ regulatory T cells under inflammatory or non-inflammatory conditions. Semin. Immunol. 2011, 23, 424–430. [Google Scholar] [CrossRef]
- Vignali, D.A. Mechanisms of Treg suppression: Still a long way to go. Front. Immunol. 2012, 3, 191. [Google Scholar] [CrossRef] [PubMed]
- Markov, A.G.; Aschenbach, J.R.; Amasheh, S. The epithelial barrier and beyond: Claudins as amplifiers of physiological organ functions. IUBMB Life 2017, 69, 290–296. [Google Scholar] [CrossRef]
- Snoeck, V.; Goddeeris, B.; Cox, E. The role of enterocytes in the intestinal barrier function and antigen uptake. Microbes Infect. 2005, 7, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Lechuga, S.; Braga-Neto, M.B.; Naydenov, N.G.; Rieder, F.; Ivanov, A.I. Understanding disruption of the gut barrier during inflammation: Should we abandon traditional epithelial cell lines and switch to intestinal organoids? Front. Immunol. 2023, 14, 1108289. [Google Scholar] [CrossRef] [PubMed]
- Buckley, A.; Turner, J.R. Cell biology of tight junction barrier regulation and mucosal disease. Cold Spring Harb. Perspect. Biol. 2018, 10, a029314. [Google Scholar] [CrossRef] [PubMed]
- Laukoetter, M.G.; Bruewer, M.; Nusrat, A. Regulation of the intestinal epithelial barrier by the apical junctional complex. Curr. Opin. Gastroenterol. 2006, 22, 85–89. [Google Scholar] [CrossRef]
- Guo, C.; Shen, J. Cytoskeletal organization and cell polarity in the pathogenesis of Crohn’s disease. Clin. Rev. Allergy Immunol. 2021, 60, 164–174. [Google Scholar] [CrossRef]
- Lechuga, S.; Ivanov, A.I. Disruption of the epithelial barrier during intestinal inflammation: Quest for new molecules and mechanisms. Biochim. Et Biophys. Acta Mol. Cell Res. 2017, 1864, 1183–1194. [Google Scholar] [CrossRef]
- Ivanov, A.I.; Parkos, C.A.; Nusrat, A. Cytoskeletal regulation of epithelial barrier function during inflammation. Am. J. Pathol. 2010, 177, 512–524. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.-D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal permeability–a new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef]
- Lopetuso, L.R.; Scaldaferri, F.; Bruno, G.; Petito, V.; Franceschi, F.; Gasbarrini, A. The therapeutic management of gut barrier leaking: The emerging role for mucosal barrier protectors. Eur. Rev. Med. Pharmacol. Sci. 2015, 9, 1068–1076. [Google Scholar]
- Iweala, O.I.; Nagler, C.R. The microbiome and food allergy. Annu. Rev. Immunol. 2019, 37, 377–403. [Google Scholar] [CrossRef] [PubMed]
- Odenwald, M.A.; Turner, J.R. The intestinal epithelial barrier: A therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 9–21. [Google Scholar] [CrossRef]
- Zimmermann, P.; Messina, N.; Mohn, W.W.; Finlay, B.B.; Curtis, N. Association between the intestinal microbiota and allergic sensitization, eczema, and asthma: A systematic review. J. Allergy Clin. Immunol. 2019, 143, 467–485. [Google Scholar] [CrossRef]
- Liang, L.; Saunders, C.; Sanossian, N. Food, gut barrier dysfunction, and related diseases: A new target for future individualized disease prevention and management. Food Sci. Nutr. 2023, 11, 1671–1704. [Google Scholar] [CrossRef]
- Maeda, K.; Caldez, M.J.; Akira, S. Innate immunity in allergy. Allergy 2019, 74, 1660–1674. [Google Scholar] [CrossRef]
- Loh, W.; Tang, M.L. The epidemiology of food allergy in the global context. Int. J. Environ. Res. Public Health 2018, 15, 2043. [Google Scholar] [CrossRef] [PubMed]
- Berni Canani, R.; Paparo, L.; Nocerino, R.; Di Scala, C.; Della Gatta, G.; Maddalena, Y.; Buono, A.; Bruno, C.; Voto, L.; Ercolini, D. Gut microbiome as target for innovative strategies against food allergy. Front. Immunol. 2019, 10, 191. [Google Scholar] [CrossRef]
- De Filippis, F.; Paparo, L.; Nocerino, R.; Della Gatta, G.; Carucci, L.; Russo, R.; Pasolli, E.; Ercolini, D.; Berni Canani, R. Specific gut microbiome signatures and the associated pro-inflammatory functions are linked to pediatric allergy and acquisition of immune tolerance. Nat. Commun. 2021, 12, 5958. [Google Scholar] [CrossRef]
- Wilkins, L.J.; Monga, M.; Miller, A.W. Defining dysbiosis for a cluster of chronic diseases. Sci. Rep. 2019, 9, 12918. [Google Scholar] [CrossRef]
- Lee, K.H.; Song, Y.; Wu, W.; Yu, K.; Zhang, G. The gut microbiota, environmental factors, and links to the development of food allergy. Clin. Mol. Allergy 2020, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Cait, A.; Cardenas, E.; Dimitriu, P.A.; Amenyogbe, N.; Dai, D.; Cait, J.; Sbihi, H.; Stiemsma, L.; Subbarao, P.; Mandhane, P.J. Reduced genetic potential for butyrate fermentation in the gut microbiome of infants who develop allergic sensitization. J. Allergy Clin. Immunol. 2019, 144, 1638–1647.e3. [Google Scholar] [CrossRef]
- Feehley, T.; Plunkett, C.H.; Bao, R.; Choi Hong, S.M.; Culleen, E.; Belda-Ferre, P.; Campbell, E.; Aitoro, R.; Nocerino, R.; Paparo, L. Healthy infants harbor intestinal bacteria that protect against food allergy. Nat. Med. 2019, 25, 448–453. [Google Scholar] [CrossRef]
- Berni Canani, R.; Sangwan, N.; Stefka, A.T.; Nocerino, R.; Paparo, L.; Aitoro, R.; Calignano, A.; Khan, A.A.; Gilbert, J.A.; Nagler, C.R. Lactobacillus rhamnosus GG-supplemented formula expands butyrate-producing bacterial strains in food allergic infants. ISME J. 2016, 10, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Chua, H.-H.; Chou, H.-C.; Tung, Y.-L.; Chiang, B.-L.; Liao, C.-C.; Liu, H.-H.; Ni, Y.-H. Intestinal dysbiosis featuring abundance of Ruminococcus gnavus associates with allergic diseases in infants. Gastroenterology 2018, 154, 154–167. [Google Scholar] [CrossRef]
- Galazzo, G.; van Best, N.; Bervoets, L.; Dapaah, I.O.; Savelkoul, P.H.; Hornef, M.W.; Hutton, E.K.; Morrison, K.; Holloway, A.C.; McDonald, H. Development of the microbiota and associations with birth mode, diet, and atopic disorders in a longitudinal analysis of stool samples, collected from infancy through early childhood. Gastroenterology 2020, 158, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Bao, R.; Hesser, L.A.; He, Z.; Zhou, X.; Nadeau, K.C.; Nagler, C.R. Fecal microbiome and metabolome differ in healthy and food-allergic twins. J. Clin. Investig. 2021, 131, e141935. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Paparo, L.; Nocerino, R.; Ciaglia, E.; Di Scala, C.; De Caro, C.; Russo, R.; Trinchese, G.; Aitoro, R.; Amoroso, A.; Bruno, C. Butyrate as a bioactive human milk protective component against food allergy. Allergy 2021, 76, 1398–1415. [Google Scholar] [CrossRef]
- Depner, M.; Taft, D.H.; Kirjavainen, P.V.; Kalanetra, K.M.; Karvonen, A.M.; Peschel, S.; Schmausser-Hechfellner, E.; Roduit, C.; Frei, R.; Lauener, R. Maturation of the gut microbiome during the first year of life contributes to the protective farm effect on childhood asthma. Nat. Med. 2020, 26, 1766–1775. [Google Scholar] [CrossRef]
- Goldberg, M.R.; Mor, H.; Magid Neriya, D.; Magzal, F.; Muller, E.; Appel, M.Y.; Nachshon, L.; Borenstein, E.; Tamir, S.; Louzoun, Y. Microbial signature in IgE-mediated food allergies. Genome Med. 2020, 12, 92. [Google Scholar] [CrossRef] [PubMed]
- Berni Canani, R.; De Filippis, F.; Nocerino, R.; Paparo, L.; Di Scala, C.; Cosenza, L.; Della Gatta, G.; Calignano, A.; De Caro, C.; Laiola, M. Gut microbiota composition and butyrate production in children affected by non-IgE-mediated cow’s milk allergy. Sci. Rep. 2018, 8, 12500. [Google Scholar] [CrossRef]
- Shim, J.A.; Ryu, J.H.; Jo, Y.; Hong, C. The role of gut microbiota in T cell immunity and immune mediated disorders. Int. J. Biol. Sci. 2023, 19, 1178. [Google Scholar] [CrossRef]
- Round, J.L.; Mazmanian, S.K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. USA 2010, 107, 12204–12209. [Google Scholar] [CrossRef]
- Stefka, A.T.; Feehley, T.; Tripathi, P.; Qiu, J.; McCoy, K.; Mazmanian, S.K.; Tjota, M.Y.; Seo, G.-Y.; Cao, S.; Theriault, B.R. Commensal bacteria protect against food allergen sensitization. Proc. Natl. Acad. Sci. USA 2014, 111, 13145–13150. [Google Scholar] [CrossRef] [PubMed]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert. Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef]
- Savage, J.H.; Lee-Sarwar, K.A.; Sordillo, J.; Bunyavanich, S.; Zhou, Y.; O’Connor, G.; Sandel, M.; Bacharier, L.B.; Zeiger, R.; Sodergren, E.; et al. A prospective microbiome-wide association study of food sensitization and food allergy in early childhood. Allergy 2018, 73, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Liu, X.; Chen, F.; Rolnik, B.M.; Chleilat, F.; Ling, Z.; Snyder, M.P.; Zhou, X. The Roles and Mechanisms of Gut Microbiota in Food Allergy. Adv. Gut Microbiome Res. 2023, 2023, 9575410. [Google Scholar] [CrossRef]
- Yang, H.; Qu, Y.; Gao, Y.; Sun, S.; Wu, R.; Wu, J. Research Progress on the Correlation between the Intestinal Microbiota and Food Allergy. Foods 2022, 11, 2913. [Google Scholar] [CrossRef]
- Madhogaria, B.; Bhowmik, P.; Kundu, A. Correlation between human gut microbiome and diseases. Infect. Med. 2022, 1, 180–191. [Google Scholar] [CrossRef]
- Wu, H.J.; Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012, 3, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Bunyavanich, S.; Shen, N.; Grishin, A.; Wood, R.; Burks, W.; Dawson, P.; Jones, S.M.; Leung, D.Y.M.; Sampson, H.; Sicherer, S.; et al. Early-life gut microbiome composition and milk allergy resolution. J. Allergy Clin. Immunol. 2016, 138, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Thompson-Chagoyan, O.C.; Vieites, J.M.; Maldonado, J.; Edwards, C.; Gil, A. Changes in faecal microbiota of infants with cow’s milk protein allergy—A Spanish prospective case-control 6-month follow-up study. Pediatr. Allergy Immunol. 2010, 21, e394–e400. [Google Scholar] [CrossRef]
- Fazlollahi, M.; Chun, Y.; Grishin, A.; Wood, R.A.; Burks, A.W.; Dawson, P.; Jones, S.M.; Leung, D.Y.M.; Sampson, H.A.; Sicherer, S.H.; et al. Early-life gut microbiome and egg allergy. Allergy 2018, 73, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Fric, P.; Gabrovska, D.; Nevoral, J. Celiac disease, gluten-free diet, and oats. Nutr. Rev. 2011, 69, 107–115. [Google Scholar] [CrossRef]
- Aljada, B.; Zohni, A.; El-Matary, W. The Gluten-Free Diet for Celiac Disease and Beyond. Nutrients 2021, 13, 3993. [Google Scholar] [CrossRef] [PubMed]
- De Palma, G.; Nadal, I.; Collado, M.C.; Sanz, Y. Effects of a gluten-free diet on gut microbiota and immune function in healthy adult human subjects. Br. J. Nutr. 2009, 102, 1154–1160. [Google Scholar] [CrossRef]
- Hemarajata, P.; Versalovic, J. Effects of probiotics on gut microbiota: Mechanisms of intestinal immunomodulation and neuromodulation. Ther. Adv. Gastroenterol. 2013, 6, 39–51. [Google Scholar] [CrossRef]
- Yang, B.; Xiao, L.; Liu, S.; Liu, X.; Luo, Y.; Ji, Q.; Yang, P.; Liu, Z. Exploration of the effect of probiotics supplementation on intestinal microbiota of food allergic mice. Am. J. Transl. Res. 2017, 9, 376. [Google Scholar]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef]
- Caminero, A.; Meisel, M.; Jabri, B.; Verdu, E.F. Mechanisms by which gut microorganisms influence food sensitivities. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Caminero, A.; Herrán, A.R.; Nistal, E.; Pérez-Andrés, J.; Vaquero, L.; Vivas, S.; Ruiz de Morales, J.M.G.; Albillos, S.M.; Casqueiro, J. Diversity of the cultivable human gut microbiome involved in gluten metabolism: Isolation of microorganisms with potential interest for coeliac disease. FEMS Microbiol. Ecol. 2014, 88, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Caminero, A.; Nistal, E.; Herrán, A.R.; Pérez-Andrés, J.; Ferrero, M.A.; Ayala, L.V.; Vivas, S.; de Morales, J.M.R.; Albillos, S.M.; Casqueiro, F.J. Differences in gluten metabolism among healthy volunteers, coeliac disease patients and first-degree relatives. Br. J. Nutr. 2015, 114, 1157–1167. [Google Scholar] [CrossRef]
- Herrán, A.R.; Pérez-Andrés, J.; Caminero, A.; Nistal, E.; Vivas, S.; de Morales, J.M.R.; Casqueiro, J. Gluten-degrading bacteria are present in the human small intestine of healthy volunteers and celiac patients. Res. Microbiol. 2017, 168, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Helmerhorst, E.J.; Zamakhchari, M.; Schuppan, D.; Oppenheim, F.G. Discovery of a novel and rich source of gluten-degrading microbial enzymes in the oral cavity. PLoS ONE 2010, 5, e13264. [Google Scholar] [CrossRef]
- Fernandez-Feo, M.; Wei, G.; Blumenkranz, G.; Dewhirst, F.E.; Schuppan, D.; Oppenheim, F.G.; Helmerhorst, E.J. The cultivable human oral gluten-degrading microbiome and its potential implications in coeliac disease and gluten sensitivity. Clin. Microbiol. Infect. 2013, 19, E386–E394. [Google Scholar] [CrossRef]
- Dowling, D.J.; Levy, O. Ontogeny of early life immunity. Trends Immunol. 2014, 35, 299–310. [Google Scholar] [CrossRef]
- Julia, V.; Macia, L.; Dombrowicz, D. The impact of diet on asthma and allergic diseases. Nat. Rev. Immunol. 2015, 15, 308–322. [Google Scholar] [CrossRef]
- Wang, S.; Yin, P.; Yu, L.; Tian, F.; Chen, W.; Zhai, Q. Effects of Early Diet on the Prevalence of Allergic Disease in Children: A Systematic Review and Meta-Analysis. Adv. Nutr. 2024, 15, 100128. [Google Scholar] [CrossRef]
- Sansotta, N.; Piacentini, G.L.; Mazzei, F.; Minniti, F.; Boner, A.L.; Peroni, D.G. Timing of introduction of solid food and risk of allergic disease development: Understanding the evidence. Allergol. Immunopathol. 2013, 41, 337–345. [Google Scholar] [CrossRef]
- Scarpone, R.; Kimkool, P.; Ierodiakonou, D.; Leonardi-Bee, J.; Garcia-Larsen, V.; Perkin, M.R.; Boyle, R.J. Timing of allergenic food introduction and risk of immunoglobulin E–mediated food allergy: A systematic review and meta-analysis. JAMA Pediatr. 2023, 177, 489–497. [Google Scholar] [CrossRef]
- Skjerven, H.O.; Lie, A.; Vettukattil, R.; Rehbinder, E.M.; LeBlanc, M.; Asarnoj, A.; Carlsen, K.-H.; Despriee, Å.W.; Färdig, M.; Gerdin, S.W. Early food intervention and skin emollients to prevent food allergy in young children (PreventADALL): A factorial, multicentre, cluster-randomised trial. Lancet 2022, 399, 2398–2411. [Google Scholar] [CrossRef] [PubMed]
- Halken, S.; Muraro, A.; de Silva, D.; Khaleva, E.; Angier, E.; Arasi, S.; Arshad, H.; Bahnson, H.T.; Beyer, K.; Boyle, R. EAACI guideline: Preventing the development of food allergy in infants and young children (2020 update). Pediatr. Allergy Immunol. 2021, 32, 843–858. [Google Scholar] [CrossRef]
- Perkin, M.R.; Logan, K.; Tseng, A.; Raji, B.; Ayis, S.; Peacock, J.; Brough, H.; Marrs, T.; Radulovic, S.; Craven, J. Randomized trial of introduction of allergenic foods in breast-fed infants. N. Engl. J. Med. 2016, 374, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- Perkin, M.R.; Logan, K.; Marrs, T.; Radulovic, S.; Craven, J.; Flohr, C.; Lack, G.; Young, L.; Offord, V.; DeSousa, M. Enquiring About Tolerance (EAT) study: Feasibility of an early allergenic food introduction regimen. J. Allergy Clin. Immunol. 2016, 137, 1477–1486.e8. [Google Scholar] [CrossRef] [PubMed]
- Adam, T.; Divaret-Chauveau, A.; Roduit, C.; Adel-Patient, K.; Deschildre, A.; Raherison, C.; Charles, M.A.; Nicklaus, S.; de Lauzon-Guillain, B. Complementary feeding practices are related to the risk of food allergy in the ELFE cohort. Allergy 2023, 78, 2456–2466. [Google Scholar] [CrossRef] [PubMed]
- Cleveland Clinic. Food Allergy vs. Intolerance: What’s the Difference? Available online: https://health.clevelandclinic.org/allergy-or-intolerance-how-to-tell-the-difference (accessed on 27 July 2024).
- Boyce, J.A.; Assa’ad, A.; Burks, A.W.; Jones, S.M.; Sampson, H.A.; Wood, R.A.; Plaut, M.; Cooper, S.F.; Fenton, M.J.; Arshad, S.H.; et al. Guidelines for the diagnosis and management of food allergy in the United States: Report of the NIAID-sponsored expert panel. J. Allergy Clin. Immunol. 2010, 126, S1–S58. [Google Scholar] [CrossRef]
- AllergyUK. Allergy vs. Intolerance. Available online: https://www.allergyuk.org/about-allergy/allergy-vs-intolerance/ (accessed on 27 July 2024).
- Zopf, Y.; Hahn, E.G.; Raithel, M.; Baenkler, H.-W.; Silbermann, A. The differential diagnosis of food intolerance. Dtsch. Ärzteblatt Int. 2009, 106, 359. [Google Scholar] [CrossRef]
- Young, E.; Stoneham, M.D.; Petruckevitch, A.; Barton, J.; Rona, R. A population study of food intolerance. Lancet 1994, 343, 1127–1130. [Google Scholar] [CrossRef]
- Bohn, L.; Storsrud, S.; Tornblom, H.; Bengtsson, U.J.; Simren, M. Tu2079 Food-Related Gastrointestinal Symptoms in IBS Are Common and Associated With More Severe Symptoms and Reduced Quality of Life. Gastroenterology 2013, 108, 634–641. [Google Scholar] [CrossRef]
- Hayes, P.A.; Fraher, M.H.; Quigley, E.M. Irritable bowel syndrome: The role of food in pathogenesis and management. Gastroenterol. Hepatol. 2014, 10, 164. [Google Scholar]
- Monsbakken, K.W.; Vandvik, P.O.; Farup, P.G. Perceived food intolerance in subjects with irritable bowel syndrome–etiology, prevalence and consequences. Eur. J. Clin. Nutr. 2006, 60, 667–672. [Google Scholar] [CrossRef]
- Boyce, J.A.; Assa’ad, A.; Burks, A.W.; Jones, S.M.; Sampson, H.A.; Wood, R.A.; Plaut, M.; Cooper, S.F.; Fenton, M.J.; Arshad, S.H.; et al. Guidelines for the diagnosis and management of food allergy in the United States: Summary of the NIAID-sponsored expert panel report. Nutr. Res. 2011, 31, 61–75. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gargano, D.; Appanna, R.; Santonicola, A.; De Bartolomeis, F.; Stellato, C.; Cianferoni, A.; Casolaro, V.; Iovino, P. Food Allergy and Intolerance: A Narrative Review on Nutritional Concerns. Nutrients 2021, 13, 1638. [Google Scholar] [CrossRef] [PubMed]
- Caminero, A.; Galipeau, H.J.; McCarville, J.L.; Johnston, C.W.; Bernier, S.P.; Russell, A.K.; Jury, J.; Herran, A.R.; Casqueiro, J.; Tye-Din, J.A. Duodenal bacteria from patients with celiac disease and healthy subjects distinctly affect gluten breakdown and immunogenicity. Gastroenterology 2016, 151, 670–683. [Google Scholar] [CrossRef]
- Galipeau, H.J.; McCarville, J.L.; Huebener, S.; Litwin, O.; Meisel, M.; Jabri, B.; Sanz, Y.; Murray, J.A.; Jordana, M.; Alaedini, A. Intestinal microbiota modulates gluten-induced immunopathology in humanized mice. Am. J. Pathol. 2015, 185, 2969–2982. [Google Scholar] [CrossRef] [PubMed]
- Halmos, E.P.; Power, V.A.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology 2014, 146, 67–75.e5. [Google Scholar] [CrossRef] [PubMed]
- Tuck, C.J.; Biesiekierski, J.R.; Schmid-Grendelmeier, P.; Pohl, D. Food Intolerances. Nutrients 2019, 11, 1684. [Google Scholar] [CrossRef] [PubMed]
- Lomer, M.C.; Parkes, G.; Sanderson, J. Lactose intolerance in clinical practice–myths and realities. Aliment. Pharmacol. Ther. 2008, 27, 93–103. [Google Scholar] [CrossRef]
- Bonder, M.J.; Kurilshikov, A.; Tigchelaar, E.F.; Mujagic, Z.; Imhann, F.; Vila, A.V.; Deelen, P.; Vatanen, T.; Schirmer, M.; Smeekens, S.P. The effect of host genetics on the gut microbiome. Nat. Genet. 2016, 48, 1407–1412. [Google Scholar] [CrossRef]
- Brandao Gois, M.F.; Sinha, T.; Spreckels, J.E.; Vich Vila, A.; Bolte, L.A.; Weersma, R.K.; Wijmenga, C.; Fu, J.; Zhernakova, A.; Kurilshikov, A. Role of the gut microbiome in mediating lactose intolerance symptoms. Gut 2022, 71, 215–217. [Google Scholar] [CrossRef]
- He, T.; Venema, K.; Priebe, M.; Welling, G.; Brummer, R.J.; Vonk, R. The role of colonic metabolism in lactose intolerance. Eur. J. Clin. Investig. 2008, 38, 541–547. [Google Scholar] [CrossRef]
- He, T.; Priebe, M.G.; Harmsen, H.J.; Stellaard, F.; Sun, X.; Welling, G.W.; Vonk, R.J. Colonic fermentation may play a role in lactose intolerance in humans. J. Nutr. 2006, 136, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.M.; Lomer, M.C.; Farquharson, F.M.; Louis, P.; Fava, F.; Franciosi, E.; Scholz, M.; Tuohy, K.M.; Lindsay, J.O.; Irving, P.M. A diet low in FODMAPs reduces symptoms in patients with irritable bowel syndrome and a probiotic restores Bifidobacterium species: A randomized controlled trial. Gastroenterology 2017, 153, 936–947. [Google Scholar] [CrossRef]
- Mysore Saiprasad, S.; Moreno, O.G.; Savaiano, D.A. A Narrative Review of Human Clinical Trials to Improve Lactose Digestion and Tolerance by Feeding Bifidobacteria or Galacto-Oligosacharides. Nutrients 2023, 15, 3559. [Google Scholar] [CrossRef] [PubMed]
- Wielgosz-Grochowska, J.P.; Domanski, N.; Drywien, M.E. Identification of SIBO Subtypes along with Nutritional Status and Diet as Key Elements of SIBO Therapy. Int. J. Mol. Sci. 2024, 25, 7341. [Google Scholar] [CrossRef]
- Knez, E.; Kadac-Czapska, K.; Grembecka, M. The importance of food quality, gut motility, and microbiome in SIBO development and treatment. Nutrition 2024, 124, 112464. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Chen, Y.; Guo, H.; Liu, Z.; Du, Y.; Duan, L. Differences in clinical manifestations and the fecal microbiome between irritable bowel syndrome and small intestinal bacterial overgrowth. Dig. Liver Dis. 2024. [Google Scholar] [CrossRef] [PubMed]
- Ames, S.R.; Lotoski, L.C.; Azad, M.B. Comparing early life nutritional sources and human milk feeding practices: Personalized and dynamic nutrition supports infant gut microbiome development and immune system maturation. Gut Microbes 2023, 15, 2190305. [Google Scholar] [CrossRef]
- Widstrom, A.M.; Brimdyr, K.; Svensson, K.; Cadwell, K.; Nissen, E. Skin-to-skin contact the first hour after birth, underlying implications and clinical practice. Acta Paediatr. 2019, 108, 1192–1204. [Google Scholar] [CrossRef]
- Davanzo, R.; Strajn, T.; Kennedy, J.; Crocetta, A.; De Cunto, A. From tube to breast: The bridging role of semi-demand breastfeeding. J. Hum. Lact. 2014, 30, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Patangia, D.V.; Anthony Ryan, C.; Dempsey, E.; Paul Ross, R.; Stanton, C. Impact of antibiotics on the human microbiome and consequences for host health. Microbiol. Open 2022, 11, e1260. [Google Scholar] [CrossRef]
- Bzikowska-Jura, A.; Czerwonogrodzka-Senczyna, A.; Oledzka, G.; Szostak-Wegierek, D.; Weker, H.; Wesolowska, A. Maternal Nutrition and Body Composition During Breastfeeding: Association with Human Milk Composition. Nutrients 2018, 10, 1379. [Google Scholar] [CrossRef] [PubMed]
- Savage, J.H.; Lee-Sarwar, K.A.; Sordillo, J.E.; Lange, N.E.; Zhou, Y.; O’Connor, G.T.; Sandel, M.; Bacharier, L.B.; Zeiger, R.; Sodergren, E.; et al. Diet during Pregnancy and Infancy and the Infant Intestinal Microbiome. J. Pediatr. 2018, 203, 47–54.e4. [Google Scholar] [CrossRef]
- van den Akker, C.H.P.; van Goudoever, J.B.; Shamir, R.; Domellof, M.; Embleton, N.D.; Hojsak, I.; Lapillonne, A.; Mihatsch, W.A.; Berni Canani, R.; Bronsky, J.; et al. Probiotics and Preterm Infants: A Position Paper by the European Society for Paediatric Gastroenterology Hepatology and Nutrition Committee on Nutrition and the European Society for Paediatric Gastroenterology Hepatology and Nutrition Working Group for Probiotics and Prebiotics. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 664–680. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Plaza-Diaz, J.; Morales, J.; Alvarez-Calatayud, G.; Climent, E.; Silva, A.; Martinez-Blanch, J.F.; Enrique, M.; Tortajada, M.; Ramon, D.; et al. Effects of a Novel Infant Formula on the Fecal Microbiota in the First Six Months of Life: The INNOVA 2020 Study. Int. J. Mol. Sci. 2023, 24, 3034. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Morales, J.; Martin-Masot, R.; Climent, E.; Silva, A.; Martinez-Blanch, J.F.; Enrique, M.; Tortajada, M.; Ramon, D.; et al. Innova 2020: A Follow-Up Study of the Fecal Microbiota of Infants Using a Novel Infant Formula between 6 Months and 12 Months of Age. Int. J. Mol. Sci. 2023, 24, 7392. [Google Scholar] [CrossRef] [PubMed]
- Martinez Suarez, V. Use of probiotics and prebiotics in infant formulas. Nutr. Hosp. 2015, 31 (Suppl. S1), 72–77. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, I.; Kaliszczak, K.; Skowron, K.; Grudlewska-Buda, K.; Twaruzek, M.; Sinkiewicz-Darol, E. Microbiological status of donor human milk—A single center study from Poland. Food Microbiol. 2024, 122, 104528. [Google Scholar] [CrossRef]
- Aktas Reyhan, F. The effect of breastfeeding education with digital storytelling on fathers’ breastfeeding self-efficacy. J. Eval. Clin. Pract. 2024. early view. [Google Scholar] [CrossRef]
- Bai, R.; Cheng, Y.; Shan, S.; Zhao, X.; Wei, J.; Xia, C. The breastfeeding experience of women with multiple pregnancies: A meta-synthesis of qualitative studies. BMC Pregnancy Childbirth 2024, 24, 492. [Google Scholar] [CrossRef] [PubMed]
- Hiito, E.; Ikonen, R.; Niela-Vilen, H. Internet-based breastfeeding peer support for breastfeeding parents: An integrative review. J. Adv. Nurs. 2024. early view. [Google Scholar] [CrossRef]
- Syahri, I.M.; Laksono, A.D.; Fitria, M.; Rohmah, N.; Masruroh, M.; Ipa, M. Exclusive breastfeeding among Indonesian working mothers: Does early initiation of breastfeeding matter? BMC Public Health 2024, 24, 1225. [Google Scholar] [CrossRef] [PubMed]
- Fair, F.J.; Morison, A.; Soltani, H. Stakeholders’ views of the Baby Friendly Initiative implementation and impact: A mixed methods study. Int. Breastfeed. J. 2024, 19, 49. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.B.; Melo, D.S.; Relvas, G.R.B.; Venancio, S.I.; Silva, R. Promotion, protection, and support of breastfeeding at work, and achieving sustainable development: A scoping review. Cien Saude Colet. 2023, 28, 1059–1072. [Google Scholar] [CrossRef] [PubMed]
- Hill, Z.; Manu, A.; Tawiah-Agyemang, C.; Gyan, T.; Turner, K.; Weobong, B.; Ten Asbroek, A.H.; Kirkwood, B.R. How did formative research inform the development of a home-based neonatal care intervention in rural Ghana? J. Perinatol. 2008, 28 (Suppl. S2), S38–S45. [Google Scholar] [CrossRef]
- Dann, M.H. The lactation consult: Problem solving, teaching, and support for the breastfeeding family. J. Pediatr. Health Care 2005, 19, 12–16. [Google Scholar] [CrossRef]
- Masum, A.K.M.; Chandrapala, J.; Huppertz, T.; Adhikari, B.; Zisu, B. Production and characterization of infant milk formula powders: A review. Dry. Technol. 2021, 39, 1492–1512. [Google Scholar] [CrossRef]
- Hageman, J.H.J.; Danielsen, M.; Nieuwenhuizen, A.G.; Feitsma, A.L.; Dalsgaard, T.K. Comparison of bovine milk fat and vegetable fat for infant formula: Implications for infant health. Int. Dairy J. 2019, 92, 37–49. [Google Scholar] [CrossRef]
- Lund, P.; Bechshoft, M.R.; Ray, C.A.; Lund, M.N. Effect of Processing of Whey Protein Ingredient on Maillard Reactions and Protein Structural Changes in Powdered Infant Formula. J. Agric. Food Chem. 2022, 70, 319–332. [Google Scholar] [CrossRef]
- Imdad, A.; Sherwani, R.; Wall, K. Pediatric Formulas: An Update. Pediatr. Rev. 2024, 45, 394–405. [Google Scholar] [CrossRef]
- Prosser, C.G. Compositional and functional characteristics of goat milk and relevance as a base for infant formula. J. Food Sci. 2021, 86, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Migacheva, N.B.; Mukhametova, E.M.; Makarova, E.G.; Ukraintsev, S.E. The Role and Place of Partially Hydrolysed Protein Infant Formulas in the Nutrition of Full-term Children: Digestive Comfort and Allergy Prevention. Curr. Pediatr. 2020, 19, 279–290. [Google Scholar] [CrossRef]
- Juliano, B.O. Rice: Chemistry and Technology; Woodhead Publishing: Sawston, UK, 1985. [Google Scholar]
- Dupont, C.; Bocquet, A.; Tome, D.; Bernard, M.; Campeotto, F.; Dumond, P.; Essex, A.; Frelut, M.L.; Guenard-Bilbault, L.; Lack, G.; et al. Hydrolyzed Rice Protein-Based Formulas, a Vegetal Alternative in Cow’s Milk Allergy. Nutrients 2020, 12, 2654. [Google Scholar] [CrossRef]
- Bocquet, A.; Dupont, C.; Chouraqui, J.-P.; Darmaun, D.; Feillet, F.; Frelut, M.-L.; Girardet, J.-P.; Hankard, R.; Lapillonne, A.; Rozé, J.-C. Efficacy and safety of hydrolyzed rice-protein formulas for the treatment of cow’s milk protein allergy. Arch. Pédiatrie 2019, 26, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, A.; Barrio-Torres, J.; Dupont, C.; Howells, H.E.; Shamir, R.; Venter, C.; Meyer, R. Hydrolyzed rice formula for dietary management of infants with cow’s milk allergy. World Allergy Organ. J. 2022, 15, 100717. [Google Scholar] [CrossRef]
- Maryniak, N.Z.; Sancho, A.I.; Hansen, E.B.; Bogh, K.L. Alternatives to Cow’s Milk-Based Infant Formulas in the Prevention and Management of Cow’s Milk Allergy. Foods 2022, 11, 926. [Google Scholar] [CrossRef] [PubMed]
- Milbrandt, T.P. Specialized Infant Formulas. Pediatr. Rev. 2017, 38, 241–242. [Google Scholar] [CrossRef]
- Martinez, J.A.; Ballew, M.P. Infant formulas. Pediatr. Rev. 2011, 32, 179–189, quiz 189. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Fontana, L.; Gil, A. Human Milk Oligosaccharides and Immune System Development. Nutrients 2018, 10, 1038. [Google Scholar] [CrossRef]
- Kunz, C. Historical aspects of human milk oligosaccharides. Adv. Nutr. 2012, 3, 430S–439S. [Google Scholar] [CrossRef] [PubMed]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef]
- Jantscher-Krenn, E.; Bode, L. Human milk oligosaccharides and their potential benefits for the breast-fed neonate. Minerva Pediatr. 2012, 64, 83–99. [Google Scholar]
- Smilowitz, J.T.; Lebrilla, C.B.; Mills, D.A.; German, J.B.; Freeman, S.L. Breast milk oligosaccharides: Structure-function relationships in the neonate. Annu. Rev. Nutr. 2014, 34, 143–169. [Google Scholar] [CrossRef] [PubMed]
- Puccio, G.; Alliet, P.; Cajozzo, C.; Janssens, E.; Corsello, G.; Sprenger, N.; Wernimont, S.; Egli, D.; Gosoniu, L.; Steenhout, P. Effects of Infant Formula With Human Milk Oligosaccharides on Growth and Morbidity: A Randomized Multicenter Trial. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 624–631. [Google Scholar] [CrossRef]
- Dinleyici, M.; Barbieur, J.; Dinleyici, E.C.; Vandenplas, Y. Functional effects of human milk oligosaccharides (HMOs). Gut Microbes 2023, 15, 2186115. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, N.; Tytgat, H.L.; Binia, A.; Austin, S.; Singhal, A. Biology of human milk oligosaccharides: From basic science to clinical evidence. J. Human Nutr. Diet. 2022, 35, 280–299. [Google Scholar] [CrossRef]
- Mosca, F.; Giannì, M.L. Human Milk: Composition and Health Benefits. Pediatr. Med. Chir. 2017, 39, 155. [Google Scholar] [CrossRef]
- Hill, D.R.; Chow, J.M.; Buck, R.H. Multifunctional Benefits of Prevalent HMOs: Implications for Infant Health. Nutrients 2021, 13, 3364. [Google Scholar] [CrossRef]
- European Society for Paediatric Gastroenterology; Hepatology & Nutrition (ESPGHAN); Fewtrell, M.; Baumann, U.; Bronsky, J.; Haiden, N.; Hill, S.; Kivelä, L.; de Koenig, B.; Köglmeier, J.; et al. World Health Organization (WHO) guideline on the complementary feeding of infants and young children aged 6-23 months 2023: A multisociety response. J. Pediatr. Gastroenterol. Nutr. 2024, 79, 181–188. [Google Scholar] [CrossRef]
- Lutter, C.K.; Grummer-Strawn, L.; Rogers, L. Complementary feeding of infants and young children 6 to 23 months of age. Nutr. Rev. 2021, 79, 825–846. [Google Scholar] [CrossRef] [PubMed]
- Clayton, H.B.; Li, R.; Perrine, C.G.; Scanlon, K.S. Prevalence and reasons for introducing infants early to solid foods: Variations by milk feeding type. Pediatrics 2013, 131, e1108–e1114. [Google Scholar] [CrossRef]
- Przyrembel, H. Timing of introduction of complementary food: Short- and long-term health consequences. Ann. Nutr. Metab. 2012, 60 (Suppl. S2), 8–20. [Google Scholar] [CrossRef] [PubMed]
- Coulthard, H.; Harris, G.; Emmett, P. Delayed introduction of lumpy foods to children during the complementary feeding period affects child’s food acceptance and feeding at 7 years of age. Matern. Child. Nutr. 2009, 5, 75–85. [Google Scholar] [CrossRef] [PubMed]
- de Paiva, E.L.; Ali, S.; Vasco, E.R.; Alvito, P.C.; de Oliveira, C.A.F. Bioaccessibility data of potentially toxic elements in complementary foods for infants: A review. Food Res. Int. 2023, 174, 113485. [Google Scholar] [CrossRef]
- Norris, J.M.; Barriga, K.; Hoffenberg, E.J.; Taki, I.; Miao, D.; Haas, J.E.; Emery, L.M.; Sokol, R.J.; Erlich, H.A.; Eisenbarth, G.S.; et al. Risk of celiac disease autoimmunity and timing of gluten introduction in the diet of infants at increased risk of disease. JAMA 2005, 293, 2343–2351. [Google Scholar] [CrossRef]
- Fangupo, L.J.; Heath, A.M.; Williams, S.M.; Erickson Williams, L.W.; Morison, B.J.; Fleming, E.A.; Taylor, B.J.; Wheeler, B.J.; Taylor, R.W. A Baby-Led Approach to Eating Solids and Risk of Choking. Pediatrics 2016, 138, e20160772. [Google Scholar] [CrossRef]
- Turnbull, J.L.; Adams, H.N.; Gorard, D.A. Review article: The diagnosis and management of food allergy and food intolerances. Aliment. Pharmacol. Ther. 2015, 41, 3–25. [Google Scholar] [CrossRef]
- Danielewicz, H. Breastfeeding and Allergy Effect Modified by Genetic, Environmental, Dietary, and Immunological Factors. Nutrients 2022, 14, 3011. [Google Scholar] [CrossRef]
- Wang, S.; Wei, Y.; Liu, L.; Li, Z. Association Between Breastmilk Microbiota and Food Allergy in Infants. Front. Cell Infect. Microbiol. 2021, 11, 770913. [Google Scholar] [CrossRef]
- Jadhav, A.; Bajaj, A.; Xiao, Y.; Markandey, M.; Ahuja, V.; Kashyap, P.C. Role of Diet-Microbiome Interaction in Gastrointestinal Disorders and Strategies to Modulate Them with Microbiome-Targeted Therapies. Annu. Rev. Nutr. 2023, 43, 355–383. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Verges, M.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Addae, H.Y.; Apprey, C.; Kwarteng, A. Gut Microbiome-Targeted Nutrition Interventions and Growth among Children in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis. Curr. Dev. Nutr. 2024, 8, 102085. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, L.; Butcher, J.; Stintzi, A.; Figeys, D. Advancing functional and translational microbiome research using meta-omics approaches. Microbiome 2019, 7, 154. [Google Scholar] [CrossRef]
- Mars, R.A.T.; Frith, M.; Kashyap, P.C. Functional Gastrointestinal Disorders and the Microbiome-What Is the Best Strategy for Moving Microbiome-based Therapies for Functional Gastrointestinal Disorders into the Clinic? Gastroenterology 2021, 160, 538–555. [Google Scholar] [CrossRef]
- Beller, L.; Matthijnssens, J. What is (not) known about the dynamics of the human gut virome in health and disease. Curr. Opin. Virol. 2019, 37, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Wampach, L.; Heintz-Buschart, A.; Hogan, A.; Muller, E.E.L.; Narayanasamy, S.; Laczny, C.C.; Hugerth, L.W.; Bindl, L.; Bottu, J.; Andersson, A.F.; et al. Colonization and Succession within the Human Gut Microbiome by Archaea, Bacteria, and Microeukaryotes during the First Year of Life. Front. Microbiol. 2017, 8, 738. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Quintana, L.; Vázquez-Lorente, H.; Hinojosa-Nogueira, D.; Plaza-Diaz, J. Relationship between Infant Feeding and the Microbiome: Implications for Allergies and Food Intolerances. Children 2024, 11, 1030. https://doi.org/10.3390/children11081030
Herrera-Quintana L, Vázquez-Lorente H, Hinojosa-Nogueira D, Plaza-Diaz J. Relationship between Infant Feeding and the Microbiome: Implications for Allergies and Food Intolerances. Children. 2024; 11(8):1030. https://doi.org/10.3390/children11081030
Chicago/Turabian StyleHerrera-Quintana, Lourdes, Héctor Vázquez-Lorente, Daniel Hinojosa-Nogueira, and Julio Plaza-Diaz. 2024. "Relationship between Infant Feeding and the Microbiome: Implications for Allergies and Food Intolerances" Children 11, no. 8: 1030. https://doi.org/10.3390/children11081030





