Assessment of the Circulating PD-1 and PD-L1 Levels and P53 Expression as a Predictor of Relapse in Pediatric Patients with Wilms Tumor and Hypernephroma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Sampling Method
2.3. Methods
2.3.1. Flow Cytometry Investigations
Sample Preparations
2.3.2. Flow Cytometric Parameters
2.3.3. Antioxidant Parameters
2.3.4. Statistical Analysis
3. Results
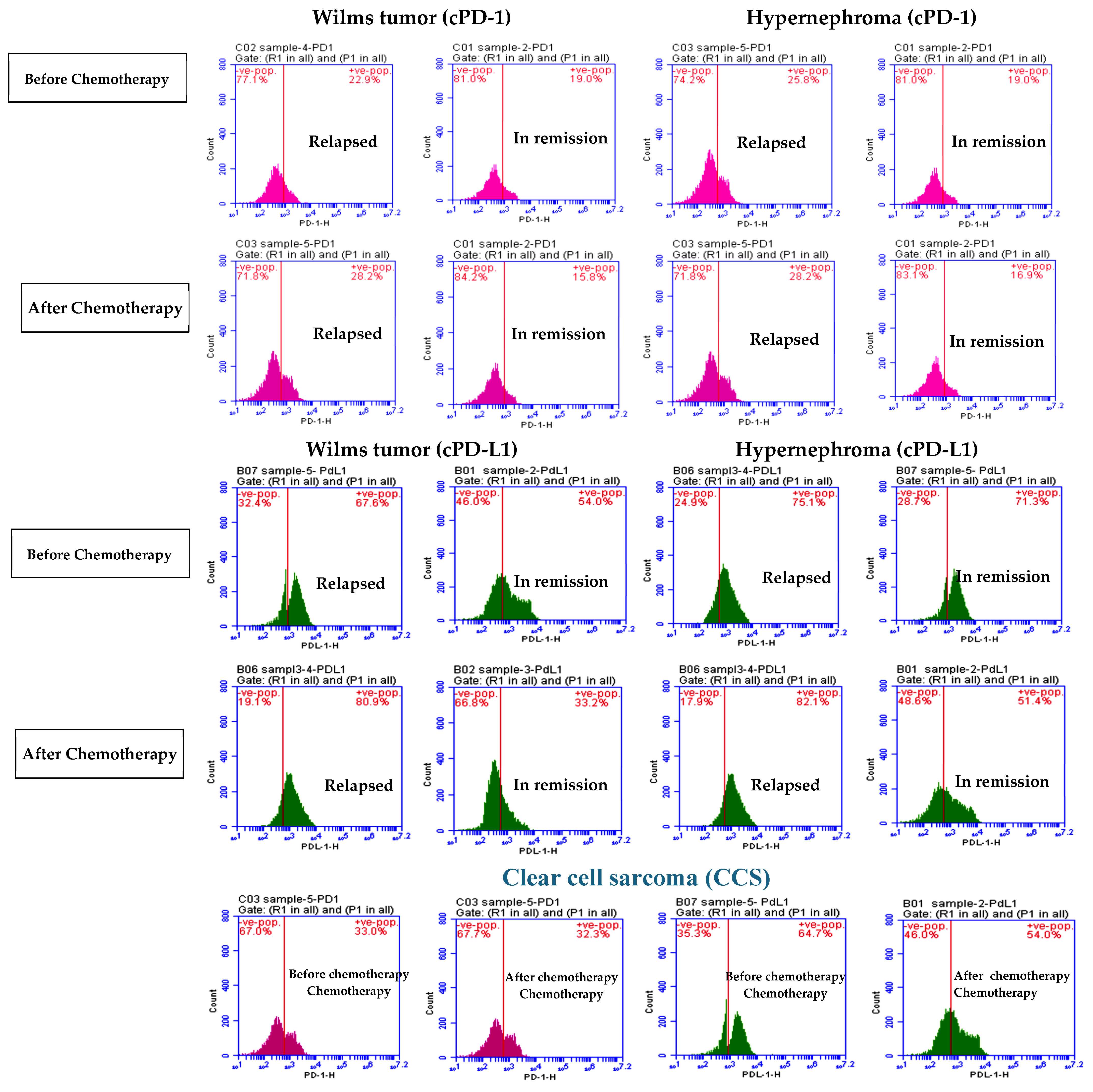
3.1. Elevated cPD1 and cPD-L1 Levels Were Correlated to Relapsed Patients
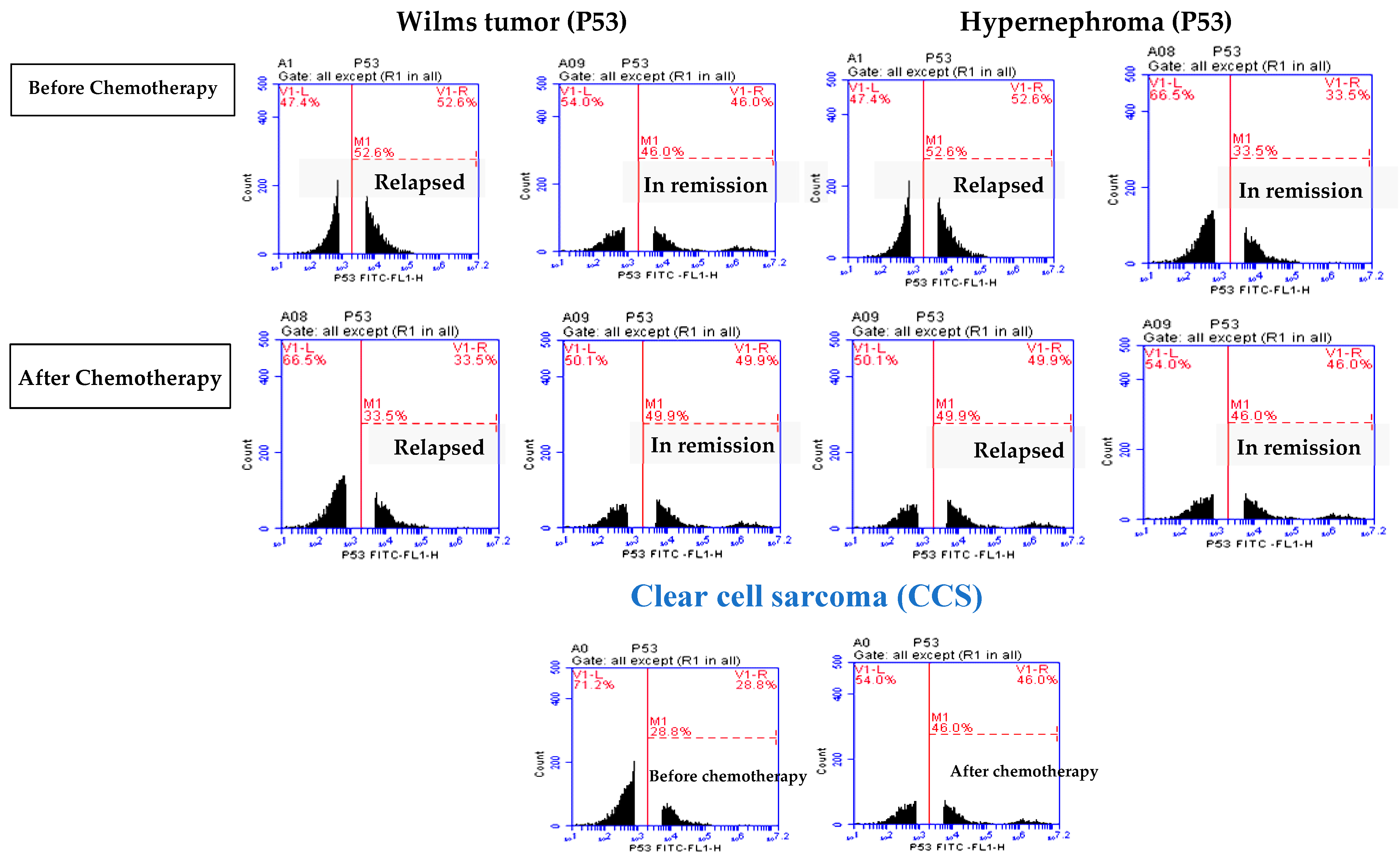
3.2. Elevated P53 Concentration in Relapsed Patients
3.3. Oxidative Stress Markers in Relapsed Pediatric Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carter, N.; Avery, A.; Libes, J.; Lovvorn, H.; Hansen, E. Pediatric Solid Tumors in Resource-Constrained Settings: A Review of Available Evidence on Management, Outcomes, and Barriers to Care. Children 2018, 5, 143. [Google Scholar] [CrossRef] [PubMed]
- Pater, L.; Melchior, P.; Rübe, C.; Cooper, B.T.; McAleer, M.F.; Kalapurakal, J.A.; Paulino, A.C. Wilms Tumor. Pediatr. Blood Cancer 2021, 68, e28257. [Google Scholar] [CrossRef]
- Groenendijk, A.; Spreafico, F.; de Krijger, R.R.; Drost, J.; Brok, J.; Perotti, D.; van Tinteren, H.; Venkatramani, R.; Godziński, J.; Rübe, C.; et al. Prognostic Factors for Wilms Tumor Recurrence: A Review of the Literature. Cancers 2021, 13, 3142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-J.; Liu, W.; Gao, Y.-M.; Qin, Y.-J.; Wu, R.-D. The Expression of IL-6 and STAT3 Might Predict Progression and Unfavorable Prognosis in Wilms’ Tumor. Biochem. Biophys. Res. Commun. 2013, 435, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, L.; Wu, R. Differential Expression of STAT1 and IFN-γ in Primary and Invasive or Metastatic Wilms Tumors. J. Surg. Oncol. 2013, 108, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Spiegelberg, J. Renal Cell Carcinoma. memo-Mag. Eur. Med. Oncol. 2023, 16, 230–233. [Google Scholar] [CrossRef]
- Baine, M.J.; Menning, M.; Smith, L.M.; Mallya, K.; Kaur, S.; Rachagani, S.; Chakraborty, S.; Sasson, A.R.; Brand, R.E.; Batra, S.K. Differential Gene Expression Analysis of Peripheral Blood Mononuclear Cells Reveals Novel Test for Early Detection of Pancreatic Cancer. Cancer Biomark. 2012, 11, 1–14. [Google Scholar] [CrossRef]
- Twine, N.C.; Stover, J.A.; Marshall, B.; Dukart, G.; Hidalgo, M.; Stadler, W.; Logan, T.; Dutcher, J.; Hudes, G.; Dorner, A.J.; et al. Disease-Associated Expression Profiles in Peripheral Blood Mononuclear Cells from Patients with Advanced Renal Cell Carcinoma. Cancer Res. 2003, 63, 6069–6075. [Google Scholar]
- Tsimafeyeu, I.; Volkova, M.; Olshanskaia, A.; Raskin, G.; Aschuba, S.; Khochenkova, Y.; Bondarenko, A.; Khochenkov, D. Expression of Receptor Tyrosine Kinases on Peripheral Blood Mononuclear Cells and Tumor-Infiltrating Lymphocytes in Patients with Renal Cell Carcinoma and Healthy Donors. Oncology 2020, 98, 252–258. [Google Scholar] [CrossRef]
- Hensler, M.; Vančurová, I.; Becht, E.; Palata, O.; Strnad, P.; Tesařová, P.; Čabiňaková, M.; Švec, D.; Kubista, M.; Bartůňková, J.; et al. Gene Expression Profiling of Circulating Tumor Cells and Peripheral Blood Mononuclear Cells from Breast Cancer Patients. Oncoimmunology 2016, 5, e1102827. [Google Scholar] [CrossRef]
- Baine, M.J.; Chakraborty, S.; Smith, L.M.; Mallya, K.; Sasson, A.R.; Brand, R.E.; Batra, S.K. Transcriptional Profiling of Peripheral Blood Mononuclear Cells in Pancreatic Cancer Patients Identifies Novel Genes with Potential Diagnostic Utility. PLoS ONE 2011, 6, e17014. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Obermann, S.; Von Wasielewski, R.; Haile, L.; Manns, M.P.; Korangy, F.; Greten, T.F. Increase in Frequency of Myeloid-derived Suppressor Cells in Mice with Spontaneous Pancreatic Carcinoma. Immunology 2009, 128, 141–149. [Google Scholar] [CrossRef]
- Said, S.S.; Ibrahim, W.N. Cancer Resistance to Immunotherapy: Comprehensive Insights with Future Perspectives. Pharmaceutics 2023, 15, 1143. [Google Scholar] [CrossRef]
- Abaza, A.; Sid Idris, F.; Anis Shaikh, H.; Vahora, I.; Moparthi, K.P.; Al Rushaidi, M.T.; Muddam, M.R.; Obajeun, O.A.; Jaramillo, A.P.; Khan, S. Programmed Cell Death Protein 1 (PD-1) and Programmed Cell Death Ligand 1 (PD-L1) Immunotherapy: A Promising Breakthrough in Cancer Therapeutics. Cureus 2023, 15, e44582. [Google Scholar] [CrossRef]
- Takeuchi, H.; Konnai, S.; Maekawa, N.; Minato, E.; Ichikawa, Y.; Kobayashi, A.; Okagawa, T.; Murata, S.; Ohashi, K. Expression Analysis of Canine CMTM6 and CMTM4 as Potential Regulators of the PD-L1 Protein in Canine Cancers. Front. Vet. Sci. 2020, 7, 330. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Yang, K.; Wang, Z.; Zhao, M.; Deng, Y.; Ji, W.; Zou, Y.; Qian, C.; Liu, Y.; Xiao, H.; et al. CD44-Mediated Poor Prognosis in Glioma Is Associated With M2-Polarization of Tumor-Associated Macrophages and Immunosuppression. Front. Surg. 2022, 8, 775194. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Du, J.; Gao, Z.; Sun, H.; Mei, M.; Wang, Y.; Ren, Y.; Zhou, X. Evolving Landscape of PD-L2: Bring New Light to Checkpoint Immunotherapy. Br. J. Cancer 2023, 128, 1196–1207. [Google Scholar] [CrossRef]
- Huang, W.; Liu, J.; Xu, K.; Chen, H.; Bian, C. PD-1/PD-L1 Inhibitors for Advanced or Metastatic Cervical Cancer: From Bench to Bed. Front. Oncol. 2022, 12, 849352. [Google Scholar] [CrossRef]
- Gu, X.; Dong, M.; Liu, Z.; Mi, Y.; Yang, J.; Zhang, Z.; Liu, K.; Jiang, L.; Zhang, Y.; Dong, S.; et al. Elevated PD-L1 Expression Predicts Poor Survival Outcomes in Patients with Cervical Cancer. Cancer Cell Int. 2019, 19, 146. [Google Scholar] [CrossRef]
- Cui, P.; Jing, P.; Liu, X.; Xu, W. Prognostic Significance of Pd-L1 Expression and Its Tumor-Intrinsic Functions in Hypopharyngeal Squamous Cell Carcinoma. Cancer Manag. Res. 2020, 12, 5893–5902. [Google Scholar] [CrossRef]
- Tuminello, S.; Sikavi, D.; Veluswamy, R.; Gamarra, C.; Lieberman-Cribbin, W.; Flores, R.; Taioli, E. PD-L1 as a Prognostic Biomarker in Surgically Resectable Nonsmall Cell Lung Cancer: A Meta-Analysis. Transl. Lung Cancer Res. 2020, 9, 1343–1360. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, X.; Sun, L.; Mao, Y. Correlation Between PD-L2 Expression and Clinical Outcome in Solid Cancer Patients: A Meta-Analysis. Front. Oncol. 2019, 9, 47. [Google Scholar] [CrossRef]
- Broecker, B. Commentary to Clinical Significance of Tumoral PD-L1 Expression in Wilms Tumor. J. Pediatr. Urol. 2022, 18, 15. [Google Scholar] [CrossRef]
- Zhang, L.; Jiao, H.; Shen, M.; Liu, W.; Li, Z.; Lin, J. Clinical Significance of Tumoral PD-L1 Expression in Wilms Tumors. J. Pediatr. Urol. 2022, 18, 14.e1–14.e8. [Google Scholar] [CrossRef]
- Routh, J.C.; Grundy, P.E.; Anderson, J.R.; Retik, A.B.; Kurek, K.C. B7-H1 as a Biomarker for Therapy Failure in Patients with Favorable Histology Wilms Tumor. J. Urol. 2013, 189, 1487–1492. [Google Scholar] [CrossRef]
- Routh, J.C.; Ashley, R.A.; Sebo, T.J.; Lohse, C.M.; Husmann, D.A.; Kramer, S.A.; Kwon, E.D. B7-H1 Expression in Wilms Tumor: Correlation With Tumor Biology and Disease Recurrence. J. Urol. 2008, 179, 1954–1960. [Google Scholar] [CrossRef]
- Duffy, M.J.; Synnott, N.C.; O’Grady, S.; Crown, J. Targeting P53 for the Treatment of Cancer. Semin. Cancer Biol. 2022, 79, 58–67. [Google Scholar] [CrossRef]
- Ssenkumba, B.; Atwiine, B.; Mitala, Y.; Adongo, J.; Olowo, S.; Nabulya, R.; Diaz Anaya, A.; Atwine, R. High Expression of WT1 and Low Expression of P53 in Archived Blocks of Children with Wilms Tumor in South Western Uganda. Cancer Manag. Res. 2024, 16, 127–136. [Google Scholar] [CrossRef]
- Salama, A.; Kamel, A. Evaluation of Nuclear Unrest and P53 Immunostaining in Wilms’ Tumor. J. Egypt. Natl. Cancer Inst. 2011, 23, 31–39. [Google Scholar] [CrossRef]
- Ooms, A.H.A.G.; Gadd, S.; Gerhard, D.S.; Smith, M.A.; Guidry Auvil, J.M.; Meerzaman, D.; Chen, Q.-R.; Hsu, C.H.; Yan, C.; Nguyen, C.; et al. Significance of TP53 Mutation in Wilms Tumors with Diffuse Anaplasia: A Report from the Children’s Oncology Group. Clin. Cancer Res. 2016, 22, 5582–5591. [Google Scholar] [CrossRef]
- Krishna, O.H.R.; Kayla, G.; Abdul Aleem, M.; Malleboyina, R.; Reddy Kota, R. Immunohistochemical Expression of Ki67 and P53 in Wilms Tumor and Its Relationship with Tumor Histology and Stage at Presentation. Pathol. Res. Int. 2016, 2016, 6123951. [Google Scholar] [CrossRef]
- Atwa, A.M.; Hafez, A.T.; Abdelhameed, M.; Dawaba, M.; Nabeeh, A.; Helmy, T.E. Does Immunohistochemical Staining of P53, Ki 67 and Cyclin A Accurately Predict Wilms Tumor Recurrence and Survival? Arab J. Urol. 2022, 20, 107–114. [Google Scholar] [CrossRef]
- Morgan, E.D.; Yahaya, J.J.; Ngaiza, A.I.; Othieno, E.; Livex, O.A. Immunohistochemical Expression of P53 Protein in Nephroblastoma: A Predictor of Unfavorable Prognosis. J. Egypt. Natl. Cancer Inst. 2023, 35, 23. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, S.; Jiang, N.; Wang, A.; Liu, S.; Xie, H.; Guo, L.; Cai, Q.; Niu, Y. Prognostic and Clinicopathological Value of P53 Expression in Renal Cell Carcinoma: A Meta-Analysis. Oncotarget 2017, 8, 102361–102370. [Google Scholar] [CrossRef]
- El Far, M.A.; Atwa, M.A.; Yahya, R.S.; El Basuni, M.A. Evaluation of Serum Levels of P53 in Hepatocellular Carcinoma in Egypt. Clin. Chem. Lab. Med. 2006, 44, 653–656. [Google Scholar] [CrossRef]
- Mostaid, M.S.; Mumu, S.B.; Haque, M.A.; Sharmin, S.; Jamiruddin, M.R.; Sayedur Rahman, G.M.; Reza, H.M. Elevated Serum Expression of P53 and Association of TP53 Codon 72 Polymorphisms with Risk of Cervical Cancer in Bangladeshi Women. PLoS ONE 2021, 16, e0261984. [Google Scholar] [CrossRef]
- Balogh, G.A.; Mailo, D.A.; Corte, M.M.; Roncoroni, P.; Nardi, H.; Vincent, E.; Martinez, D.; Cafasso, M.E.; Frizza, A.; Ponce, G.; et al. Mutant P53 Protein in Serum Could Be Used as a Molecular Marker in Human Breast Cancer. Int. J. Oncol. 2006, 28, 995–1002. [Google Scholar] [CrossRef]
- Abou-Shousha, S.A.; Salah, E.; Wagdy, E. Study of P53 in Peripheral Blood and Synovial Mononuclear Cells of Rheumatoid Arthritis and Osteoarthritis Patients and Its Relation to the Degree of Disease Activity. Egypt. J. Immunol. 2005, 12, 61–70. [Google Scholar]
- Zhang, F.; Sriram, S. Identification and Characterization of the Interferon-Beta-Mediated P53 Signal Pathway in Human Peripheral Blood Mononuclear Cells. Immunology 2009, 128, e905–e918. [Google Scholar] [CrossRef]
- Budanov, A.V. The Role of Tumor Suppressor P53 in the Antioxidant Defense and Metabolism. In Sub-Cellular Biochemistry; Plenum Publishers: New York, NY, USA, 2014; pp. 337–358. [Google Scholar]
- Bailly, C. Regulation of PD-L1 Expression on Cancer Cells with ROS-Modulating Drugs. Life Sci. 2020, 246, 117403. [Google Scholar] [CrossRef]
- Dai, S.; Jia, R.; Zhang, X.; Fang, Q.; Huang, L. The PD-1/PD-Ls Pathway and Autoimmune Diseases. Cell. Immunol. 2014, 290, 72–79. [Google Scholar] [CrossRef]
- Du, Y.; Nie, L.; Xu, L.; Wu, X.; Zhang, S.; Xue, J. Serum Levels of Soluble Programmed Death-1 (SPD-1) and Soluble Programmed Death Ligand 1(SPD-L1) in Systemic Lupus Erythematosus: Association with Activity and Severity. Scand. J. Immunol. 2020, 92, e12884. [Google Scholar] [CrossRef]
- Frigola, X.; Inman, B.A.; Lohse, C.M.; Krco, C.J.; Cheville, J.C.; Thompson, R.H.; Leibovich, B.; Blute, M.L.; Dong, H.; Kwon, E.D. Identification of a Soluble Form of B7-H1 That Retains Immunosuppressive Activity and Is Associated with Aggressive Renal Cell Carcinoma. Clin. Cancer Res. 2011, 17, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Larrinaga, G.; Solano-Iturri, J.D.; Errarte, P.; Unda, M.; Loizaga-Iriarte, A.; Pérez-Fernández, A.; Echevarría, E.; Asumendi, A.; Manini, C.; Angulo, J.C.; et al. Soluble Pd-L1 Is an Independent Prognostic Factor in Clear Cell Renal Cell Carcinoma. Cancers 2021, 13, 667. [Google Scholar] [CrossRef] [PubMed]
- Comel, A.; Sorrentino, G.; Capaci, V.; Del Sal, G. The Cytoplasmic Side of P53’s Oncosuppressive Activities. FEBS Lett. 2014, 588, 2600–2609. [Google Scholar] [CrossRef]
- Cavalcanti, M.B.; da Silva, A.P.G.; de Freitas e Silva, R.; Amaral, A. P53 Protein Expression and Cell Viability in Irradiated Peripheral Blood Mononuclear Cells as Bioindicators of Radiosensitivity. J. Biophys. Chem. 2011, 2, 63–67. [Google Scholar] [CrossRef]
- Abu Hejleh, T.; Furqan, M.; Ballas, Z.; Clamon, G. The Clinical Significance of Soluble PD-1 and PD-L1 in Lung Cancer. Crit. Rev. Oncol. Hematol. 2019, 143, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Guo, H.; Yue, J.; Xu, P. Serum SPD1 and SPDL1 as Biomarkers for Evaluating the Immune State of Lung Adenocarcinoma Patients. J. Immunol. Res. 2022, 2022, 9101912. [Google Scholar] [CrossRef]
- Chivu-Economescu, M.; Herlea, V.; Dima, S.; Sorop, A.; Pechianu, C.; Procop, A.; Kitahara, S.; Necula, L.; Matei, L.; Dragu, D.; et al. Soluble PD-L1 as a Diagnostic and Prognostic Biomarker in Resectable Gastric Cancer Patients. Gastric Cancer 2023, 26, 934–946. [Google Scholar] [CrossRef]
- Shin, K.; Kim, J.; Park, S.J.; Lee, M.A.; Park, J.M.; Choi, M.-G.; Kang, D.; Song, K.Y.; Lee, H.H.; Seo, H.S.; et al. Prognostic Value of Soluble PD-L1 and Exosomal PD-L1 in Advanced Gastric Cancer Patients Receiving Systemic Chemotherapy. Sci. Rep. 2023, 13, 6952. [Google Scholar] [CrossRef]
- Kurosaki, T.; Chamoto, K.; Suzuki, S.; Kanemura, H.; Mitani, S.; Tanaka, K.; Kawakami, H.; Kishimoto, Y.; Haku, Y.; Ito, K.; et al. The Combination of Soluble Forms of PD-1 and PD-L1 as a Predictive Marker of PD-1 Blockade in Patients with Advanced Cancers: A Multicenter Retrospective Study. Front. Immunol. 2023, 14, 1325462. [Google Scholar] [CrossRef] [PubMed]
- Ordukhanian, Z.S.; Gorozhanskaia, E.G.; Saltanov, A.I. Ways of Antioxidative Prevention of Complications in Immediate Postoperative Period in Children with Nephroblastoma. Vestn. Ross. Akad. Meditsinskikh Nauk 2000, 6, 41–43. [Google Scholar]
- Ekuk, E.; Odongo, C.N.; Tibaijuka, L.; Oyania, F.; Egesa, W.I.; Bongomin, F.; Atwiine, R.; Acan, M.; Situma, M. One Year Overall Survival of Wilms Tumor Cases and Its Predictors, among Children Diagnosed at a Teaching Hospital in South Western Uganda: A Retrospective Cohort Study. BMC Cancer 2023, 23, 196. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.; Hebra, A.; Jenrette, J.; Hudspeth, M. Successful Treatment of Late, Recurrent Wilms Tumor With High-Dose Chemotherapy and Autologous Stem Cell Rescue in Third Complete Response. J. Pediatr. Hematol. Oncol. 2010, 32, e241–e243. [Google Scholar] [CrossRef] [PubMed]
- Fish, J.D.; Lipton, J.M.; Lanzkowsky, P. Lanzkowsky’s Manual of Pediatric Hematology and Oncology; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 9780128216712. [Google Scholar]
- Shyirambere, C.; Xu, M.J.; Elmore, S.N.; Fadelu, T.; May, L.; Tapela, N.; Umuhizi, D.G.; Uwizeye, F.R.; Driscoll, C.; Muhayimana, C.; et al. Treating Nephroblastoma in Rwanda: Using International Society of Pediatric Oncology Guidelines in a Novel Oncologic Care Model. J. Glob. Oncol. 2016, 2, 105–113. [Google Scholar] [CrossRef]
- Atanda, A.; Anyanwu, L.-J.; Atanda, O.; Mohammad, A.; Abdullahi, L.; Farinyaro, A. Wilms′ Tumour: Determinants of Prognosis in an African Setting. Afr. J. Paediatr. Surg. 2015, 12, 171–176. [Google Scholar] [CrossRef]
- Hung, I.-J.; Chang, W.-H.; Yang, C.-P.; Jaing, T.-H.; Liang, D.-C.; Lin, K.-H.; Lin, D.-T.; Hsiao, C.-C.; Hsieh, Y.-L.; Chen, J.-S.; et al. Epidemiology, Clinical Features and Treatment Outcome of Wilms’ Tumor in Taiwan: A Report from Taiwan Pediatric Oncology Group. J. Formos. Med. Assoc. 2004, 103, 104–111. [Google Scholar]
- Faranoush, M.; Bahoush, G.; Mehrvar, A.; Hejazi, S.; Vossough, P.; Asl, A.A.H.; Rahiminejad, M.S.; Seighali, F.; Ghorbani, R.; Ehsani, M.A. Wilm’s Tumor: Epidemiology and Survival. Res. J. Biol. Sci. 2009, 4, 86–89. [Google Scholar]
- Chen, M.; Bie, L.; Ying, J. Cancer Cell-Intrinsic PD-1: Its Role in Malignant Progression and Immunotherapy. Biomed. Pharmacother. 2023, 167, 115514. [Google Scholar] [CrossRef]
- Liu, C.; Seeram, N.P.; Ma, H. Small Molecule Inhibitors against PD-1/PD-L1 Immune Checkpoints and Current Methodologies for Their Development: A Review. Cancer Cell Int. 2021, 21, 239. [Google Scholar] [CrossRef]
- Tang, Q.; Chen, Y.; Li, X.; Long, S.; Shi, Y.; Yu, Y.; Wu, W.; Han, L.; Wang, S. The Role of PD-1/PD-L1 and Application of Immune-Checkpoint Inhibitors in Human Cancers. Front. Immunol. 2022, 13, 964442. [Google Scholar] [CrossRef]
- Xu, W.; Atkins, M.B.; McDermott, D.F. Checkpoint Inhibitor Immunotherapy in Kidney Cancer. Nat. Rev. Urol. 2020, 17, 137–150. [Google Scholar] [CrossRef]
- Shigemori, T.; Toiyama, Y.; Okugawa, Y.; Yamamoto, A.; Yin, C.; Narumi, A.; Ichikawa, T.; Ide, S.; Shimura, T.; Fujikawa, H.; et al. Soluble PD-L1 Expression in Circulation as a Predictive Marker for Recurrence and Prognosis in Gastric Cancer: Direct Comparison of the Clinical Burden Between Tissue and Serum PD-L1 Expression. Ann. Surg. Oncol. 2019, 26, 876–883. [Google Scholar] [CrossRef]
- Holl, E.K.; Routh, J.C.; Johnston, A.W.; Frazier, V.; Rice, H.E.; Tracy, E.T.; Nair, S.K. Immune Expression in Children with Wilms Tumor: A Pilot Study. J. Pediatr. Urol. 2019, 15, 441.e1–441.e8. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Z.; Hou, Y.; Jiao, H.; Ren, J.; Wang, G. EV PD-L1 Contributes to Immunosuppressive CD8 + T Cells in Peripheral Blood of Pediatric Wilms Tumor. Technol. Cancer Res. Treat. 2021, 20, 15330338211041264. [Google Scholar] [CrossRef]
- Pinto, N.R.; Park, J.R.; Murphy, E.; Yearley, J.; McClanahan, T.; Annamalai, L.; Hawkins, D.S.; Rudzinski, E.R. Patterns of PD-1, PD-L1 and PD-L2 Expression in Pediatric Solid Tumors. J. Clin. Oncol. 2016, 34, 10561. [Google Scholar] [CrossRef]
- Monti, P.; Menichini, P.; Speciale, A.; Cutrona, G.; Fais, F.; Taiana, E.; Neri, A.; Bomben, R.; Gentile, M.; Gattei, V.; et al. Heterogeneity of TP53 Mutations and P53 Protein Residual Function in Cancer: Does It Matter? Front. Oncol. 2020, 10, 593383. [Google Scholar] [CrossRef]
- Liu, S.; Liu, T.; Jiang, J.; Guo, H.; Yang, R. P53 Mutation and Deletion Contribute to Tumor Immune Evasion. Front. Genet. 2023, 14, 1088455. [Google Scholar] [CrossRef] [PubMed]
- Jadali, F.; Sayadpour, D.; Rakhshan, M.; Karimi, A.; Rouzrokh, M.; Shamsian, B.S.; Shamshiri, A.R. Immunohistochemical Detection of P53 Protein Expression as a Prognostic Factor in Wilms Tumor. Iran. J. Kidney Dis. 2011, 5, 149–153. [Google Scholar]
- He, C.; Qin, H.; Tang, H.; Yang, D.; Li, Y.; Huang, Z.; Zhang, D.; Lv, C. Comprehensive Bioinformatics Analysis of the TP53 Signaling Pathway in Wilms’ Tumor. Ann. Transl. Med. 2020, 8, 1228. [Google Scholar] [CrossRef]
- Iwahashi, N.; Ikezaki, M.; Komohara, Y.; Fujiwara, Y.; Noguchi, T.; Nishioka, K.; Sakai, K.; Nishio, K.; Ueda, M.; Ihara, Y.; et al. Cytoplasmic P53 Aggregates Accumulated in P53-Mutated Cancer Correlate with Poor Prognosis. PNAS Nexus 2022, 1, pgac128. [Google Scholar] [CrossRef] [PubMed]
- Hannan, K.M.; Soo, P.; Wong, M.S.; Lee, J.K.; Hein, N.; Poh, P.; Wysoke, K.D.; Williams, T.D.; Montellese, C.; Smith, L.K.; et al. Nuclear Stabilization of P53 Requires a Functional Nucleolar Surveillance Pathway. Cell Rep. 2022, 41, 111571. [Google Scholar] [CrossRef] [PubMed]
- Speidel, D. The Role of DNA Damage Responses in P53 Biology. Arch. Toxicol. 2015, 89, 501–517. [Google Scholar] [CrossRef] [PubMed]
- Marvalim, C.; Datta, A.; Lee, S.C. Role of P53 in Breast Cancer Progression: An Insight into P53 Targeted Therapy. Theranostics 2023, 13, 1421–1442. [Google Scholar] [CrossRef] [PubMed]
- Kessler, E.; Bryant, R.; Dillavou, J.; Hiremath, A.; Howard, A.; Masoud, K.; Rast, R.; Rosenberg, M.; Singh, A.; Smith, A.; et al. Function in, and Mutation of, the P53 Protein: Exploring Tumor Suppression and the Development of Cancer. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Blagih, J.; Buck, M.D.; Vousden, K.H. P53, Cancer and the Immune Response. J. Cell Sci. 2020, 133, jcs237453. [Google Scholar] [CrossRef]
- Hassin, O.; Oren, M. Drugging P53 in Cancer: One Protein, Many Targets. Nat. Rev. Drug Discov. 2023, 22, 127–144. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; Xu, D.; Zhang, T.; Hu, W.; Feng, Z. Gain-of-Function Mutant P53 in Cancer Progression and Therapy. J. Mol. Cell Biol. 2020, 12, 674–687. [Google Scholar] [CrossRef]
- Chan, G.; Jordaan, G.; Nishimura, R.N.; Weisbart, R.H. Combining Intracellular Antibodies to Restore Function of Mutated P53 in Cancer. Int. J. Cancer 2016, 138, 182–186. [Google Scholar] [CrossRef]
- Abuetabh, Y.; Wu, H.H.; Chai, C.; Al Yousef, H.; Persad, S.; Sergi, C.M.; Leng, R. DNA Damage Response Revisited: The P53 Family and Its Regulators Provide Endless Cancer Therapy Opportunities. Exp. Mol. Med. 2022, 54, 1658–1669. [Google Scholar] [CrossRef] [PubMed]
- Purohit, V.; Simeone, D.M.; Lyssiotis, C.A. Metabolic Regulation of Redox Balance in Cancer. Cancers 2019, 11, 955. [Google Scholar] [CrossRef] [PubMed]
- Ordukhanian, Z.S.; Gorozhanskaia, E.G.; Saltanov, A.I. Antioxidant Defense Status of Children in the Early Post-Operative Period in Wilms’ Tumor. Vopr. Onkol. 2000, 46, 407–409. [Google Scholar] [PubMed]
- Sharma, A.; Rajappa, M.; Satyam, A.; Sharma, M. Oxidant/Anti-Oxidant Dynamics in Patients with Advanced Cervical Cancer: Correlation with Treatment Response. Mol. Cell. Biochem. 2010, 341, 65–72. [Google Scholar] [CrossRef] [PubMed]


| WT (n = 25) | Hypernephroma (n = 17) | CCS (n = 8) | |
|---|---|---|---|
| Age (year) | |||
| Mean | 3.78 | 5.7 | 3.22 |
| Range | 0.67–9.2 | 0.33–13 | 0.75–8 |
| Sex | |||
| Male | 13 (52%) | 6 (35.3%) | 4 (50%) |
| Female | 12 (48%) | 11 (64.7%) | 4 (50%) |
| Site | |||
| Right | 8 (32%) | 10 (58.8%) | 2 (25%) |
| Left | 17 (68%) | 7 (41.18%) | 6 (75%) |
| Stages | |||
| I | 9 (36%) | 9 (52%) | 6 (75%) |
| II | 8 (32%) | 3 (17.6%) | 1 (12.5%) |
| III | 8 (32%) | 5 (29.41%) | 1 (12.5%) |
| Anaplasia | |||
| With | 3 (12%) | 2 (11.76%) | |
| Without | 22 (88%) | 15 (88.24%) | 8 (100%) |
| Location | |||
| Rural | 21 (84%) | 12 (70.6%) | 5 (62.5%) |
| Urban | 4 (16%) | 5 (29.4%) | 3 (37.5%) |
| Parameters | Wilms Tumor | Hypernephroma | CCS In Remission (n = 8) | |||
|---|---|---|---|---|---|---|
| Relapsed (n = 8) | In Remission (n = 17) | Relapsed (n = 7) | In Remission (n = 10) | |||
| cPD-1 | Before | 22.85 ± 5.37 | 18.59 ± 4.33 | 24.07 ± 5.28 | 19.72 ±1.24 | 32.3 ± 1.3 |
| After | 27.58 ± 2.85 * | 15.73 ± 2.02 * | 29.32 ± 3.17 * | 19.38 ± 0.9 | 31.14 ± 0.77 | |
| cPD-L1 | Before | 69.76 ± 4.21 | 53.58 ± 6.27 | 76.68 ± 7.32 | 70.17 ± 6.45 | 69. 6 ± 6.25 |
| After | 79.3 ± 3.57 * | 33.74 ± 3.18 * | 82.53 ± 0.37 * | 51.12 ± 1.68 * | 59.82 ± 1.8 * | |
| P53 | Before | 48.5 ± 1.08 | 46.21 ± 1.33 | 51.87 ± 1.16 | 32.88 ± 3.6 | 28.86 ± 2.73 |
| After | 38.55 ± 3.3 * | 50.9 ± 3.10 * | 48.64 ± 3.04 * | 47.21± 2.43 * | 46.65 ± 1.0 * | |
| Parameters | Wilms Tumor | Hypernephroma | CCS (n = 8) | |||
|---|---|---|---|---|---|---|
| Relapsed (n = 8) | In Remission (n = 17) | Relapsed (n = 7) | In Remission (n = 10) | |||
| MDA nmol/mL | Before | 198.9 ± 20 | 185.11 ± 38.78 | 401.28 ± 10.5 | 397.10 ± 39.1 | 159 ± 7.9 |
| After | 198.37 ± 17.5 | 144.35 ± 20.50 *# | 397.7 ± 13.1 | 174.5 ± 33.5 *# | 141.25 ± 10.29 * | |
| Catalase mM | Before | 6.23 ± 0.15 | 5.52 ± 2.7 | 2.48 ± 0.27 | 2.84 ± 1.0 | 11.2 ± 1.3 |
| After | 5.9 ± 0.5 * | 7.43 ± 1.92 *# | 2.09 ± 0.25 | 11.67 ± 1.6 *# | 13.38 ± 1.2 * | |
| SOD U/mL | Before | 160.26 ± 6.1 | 159.67 ± 14.9 | 46.32 ± 16.3 | 39.72 ± 2.6 | 70.9 ± 6.7 |
| After | 152.95 ± 11.3 * | 166.68 ± 11.2 *# | 36.73 ± 2.58 * | 43.7 ± 8.69 *# | 53.07 ± 9.8 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahyon, H.A.; Alharbi, N.S.; Asad, Z.; El Shishtawy, M.A.; Derbala, S.A. Assessment of the Circulating PD-1 and PD-L1 Levels and P53 Expression as a Predictor of Relapse in Pediatric Patients with Wilms Tumor and Hypernephroma. Children 2024, 11, 1035. https://doi.org/10.3390/children11091035
Sahyon HA, Alharbi NS, Asad Z, El Shishtawy MA, Derbala SA. Assessment of the Circulating PD-1 and PD-L1 Levels and P53 Expression as a Predictor of Relapse in Pediatric Patients with Wilms Tumor and Hypernephroma. Children. 2024; 11(9):1035. https://doi.org/10.3390/children11091035
Chicago/Turabian StyleSahyon, Heba A., Nadaa S. Alharbi, Zummar Asad, Mohamed A. El Shishtawy, and Safaa A. Derbala. 2024. "Assessment of the Circulating PD-1 and PD-L1 Levels and P53 Expression as a Predictor of Relapse in Pediatric Patients with Wilms Tumor and Hypernephroma" Children 11, no. 9: 1035. https://doi.org/10.3390/children11091035







