Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS) and Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections (PANDAS): Immunological Features Underpinning Controversial Entities
Abstract
:1. Introduction
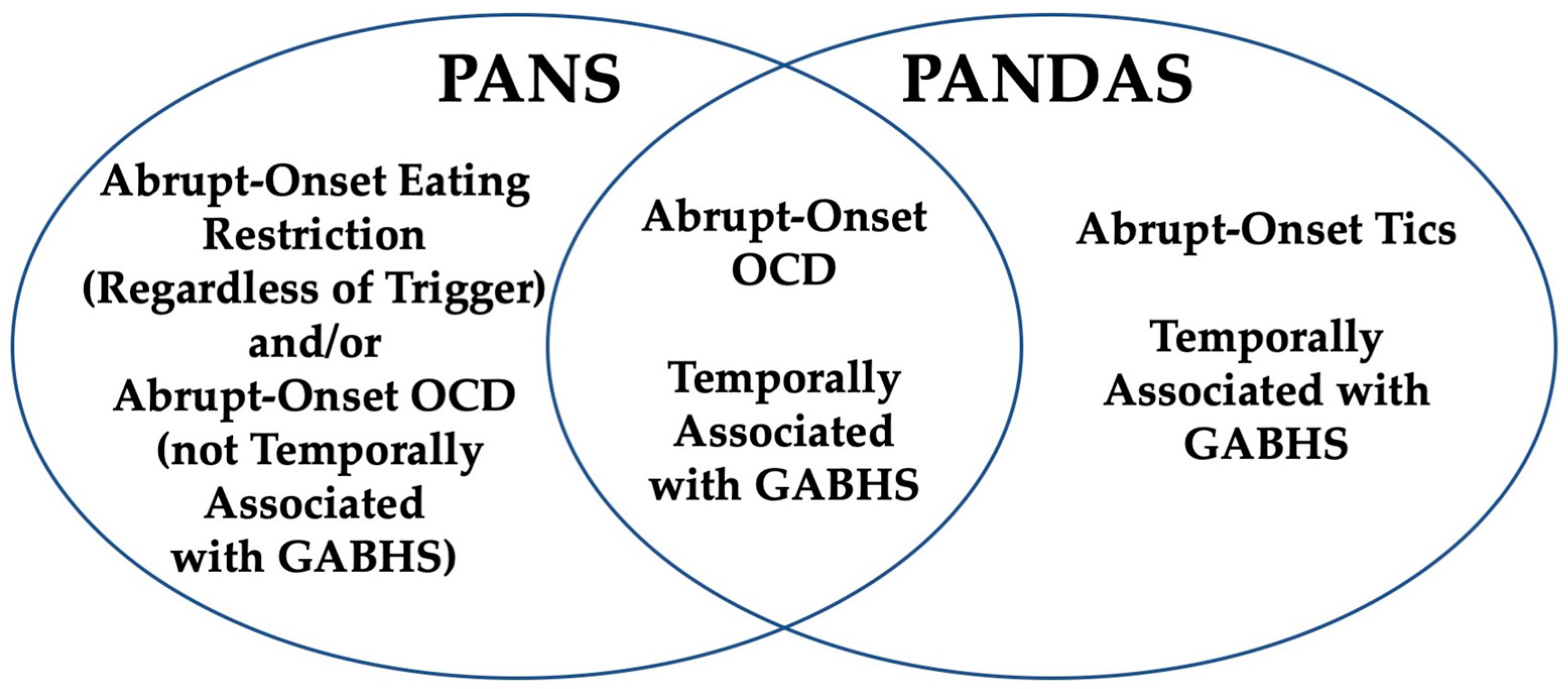
| 1998 PANDAS Criteria [5] | 2012 PANS Criteria [7] |
|---|---|
| (1) Presence of diagnosis of OCD and/or tic disorder | (1) Abrupt, dramatic onset of obsessive compulsive disorder or severely restricted food intake. |
| (2) Pediatric onset (symptoms first evident between age 3 and the beginning of puberty) | (2) Concurrent presence of additional neuropsychiatric symptoms, with a similarly severe and acute onset, from at least two of the following categories:
|
| (3) Episodic course (characterized by abrupt onset of symptoms or dramatic symptom exacerbations) | (3) Symptoms are not better explained by a known neurologic or medical disorder, such as Sydenham chorea, systemic lupus erythematosus, Tourette syndrome, or others. |
| (4) Association with group A beta-hemolytic Streptococcus infection. | |
| (5) Association with neurologic abnormalities |
2. Methods
3. PANS/PANDAS: Immunological Mechanisms
3.1. Th17 Lymphocytes and Cytokine Profiles Including IL-17
3.2. OCD and Cognitive Impairments: Possible Role of Monocytes and Cytokines Other than IL17 in the Neuroinflammatory Circuitry
3.3. Intestinal Inflammation and Immune Response: Possible Implication of Gut Brain Axis in PANDAS/PANS
3.4. Arthritis, Autoimmune Disease and Systemic Inflammatory Signs in Youth with PANS
3.5. Hypogammaglobulinemia in a Subset of PANS/PANDAS Patients
3.6. Autoantibodies in PANS/PANDAS
3.7. Immunomodulatory Treatment Trials Lacking in All Pediatric Neuroinflammatory and Many Pediatric Rheumatological Diseases
3.8. OCD in Neuroinflammatory Disorders
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Carapetis, J.R.; Steer, A.C.; Mulholland, E.K.; Weber, M. The global burden of group A streptococcal diseases. Lancet Infect. Dis. 2005, 5, 685–694. [Google Scholar] [CrossRef]
- Snider, L.A.; Swedo, S.E. Post-streptococcal autoimmune disorders of the central nervous system. Curr. Opin. Neurol. 2003, 16, 359–365. [Google Scholar] [CrossRef]
- Cunningham, M.W. Post-Streptococcal Autoimmune Sequelae: Rheumatic Fever and Beyond. In Streptococcus pyogenes: Basic Biology to Clinical Manifestations [Internet]; Ferretti, J.J., Stevens, D.L., Fischetti, V.A., Eds.; University of Oklahoma Health Sciences Center: Oklahoma City, OK, USA, 2016. Available online: https://www.ncbi.nlm.nih.gov/books/NBK333434/ (accessed on 26 August 2024).
- Pavone, P.; Parano, E.; Rizzo, R.; Trifiletti, R.R. Autoimmune neuropsychiatric disorders associated with streptococcal infection: Sydenham chorea, PANDAS, and PANDAS variants. J. Child Neurol. 2006, 21, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Swedo, S.E.; Leonard, H.L.; Garvey, M.; Mittleman, B.; Allen, A.J.; Perlmutter, S.; Lougee, L.; Dow, S.; Zamkoff, J.; Dubbert, B.K. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: Clinical description of the first 50 cases. Am. J. Psychiatry 1998, 155, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Vreeland, A.; Thienemann, M.; Cunningham, M.; Muscal, E.; Pittenger, C.; Frankovich, J. Neuroinflammation in Obsessive-Compulsive Disorder: Sydenham Chorea, Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections, and Pediatric Acute Onset Neuropsychiatric Syndrome. Psychiatr. Clin. N. Am. 2023, 46, 69–88. [Google Scholar] [CrossRef] [PubMed]
- Swedo, S.; Leckman, J.; Rose, N. From Research Subgroup to Clinical Syndrome: Modifying the PANDAS Criteria to Describe PANS (Pediatric Acute-onset Neuropsychiatric Syndrome). Pediatr. Ther. 2012, 2, 1000113. [Google Scholar] [CrossRef]
- Elamin, I.; Edwards, M.J.; Martino, D. Immune dysfunction in Tourette syndrome. Behav. Neurol. 2013, 27, 23–32. [Google Scholar] [CrossRef]
- Johnson, M.; Fernell, E.; Preda, I.; Wallin, L.; Fasth, A.; Gillberg, C.; Gillberg, C. Paediatric acute-onset neuropsychiatric syndrome in children and adolescents: An observational cohort study. Lancet Child Adolesc. Health 2019, 3, 175–180. [Google Scholar] [CrossRef]
- Frankovich, J.; Thienemann, M.; Pearlstein, J.; Crable, A.; Brown, K.; Chang, K. Multidisciplinary clinic dedicated to treating youth with pediatric acute-onset neuropsychiatric syndrome: Presenting characteristics of the first 47 consecutive patients. J. Child Adolesc. Psychopharmacol. 2015, 25, 38–47. [Google Scholar] [CrossRef]
- Gromark, C.; Harris, R.A.; Wickström, R.; Horne, A.C.; Silverberg-Mörse, M.; Serlachius, E.; Mataix-Cols, D. Establishing a Pediatric Acute-Onset Neuropsychiatric Syndrome Clinic: Baseline Clinical Features of the Pediatric Acute-Onset Neuropsychiatric Syndrome Cohort at Karolinska Institutet. J. Child Adolesc. Psychopharmacol. 2019, 29, 625–633. [Google Scholar] [CrossRef]
- Calaprice, D.; Tona, J.; Parker-Athill, E.C.; Murphy, T.K. A Survey of Pediatric Acute-Onset Neuropsychiatric Syndrome Characteristics and Course. J. Child Adolesc. Psychopharmacol. 2017, 27, 607–618. [Google Scholar] [CrossRef]
- Ferraguti, G.; Terracina, S.; Micangeli, G.; Lucarelli, M.; Tarani, L.; Ceccanti, M.; Spaziani, M.; D’orazi, V.; Petrella, C.; Fiore, M. NGF and BDNF in pediatrics syndromes. Neurosci. Biobehav. Rev. 2023, 145, 105015. [Google Scholar] [CrossRef]
- Micangeli, G.; Menghi, M.; Profeta, G.; Tarani, F.; Mariani, A.; Petrella, C.; Barbato, C.; Ferraguti, G.; Ceccanti, M.; Tarani, L.; et al. The Impact of Oxidative Stress on Pediatrics Syndromes. Antioxidants 2022, 11, 1983. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.A.; Swedo, S.E.; Farmer, C.A.; Grantz, H.; Grant, P.J.; D’souza, P.; Hommer, R.; Katsovich, L.; King, R.A.; Leckman, J.F. Randomized, Controlled Trial of Intravenous Immunoglobulin for Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections. J. Am. Acad. Child Adolesc. Psychiatry 2016, 55, 860–867.e2. [Google Scholar] [CrossRef] [PubMed]
- Gagliano, A.; Carta, A.; Tanca, M.G.; Sotgiu, S. Pediatric Acute-Onset Neuropsychiatric Syndrome: Current Perspectives. Neuropsychiatr. Dis. Treat. 2023, 19, 1221–1250. [Google Scholar] [CrossRef] [PubMed]
- Giedd, J.N.; Rapoport, J.L.; Garvey, M.A.; Perlmutter, S.; Swedo, S.E. MRI assessment of children with obsessive-compulsive disorder or tics associated with streptococcal infection. Am. J. Psychiatry 2000, 157, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Williams, M.T.; Chugani, H.T. Evaluation of basal ganglia and thalamic inflammation in children with pediatric autoimmune neuropsy-chiatric disorders associated with streptococcal infection and tourette syndrome: A positron emission tomo-graphic (PET) study using 11C-[R]-PK11195. J. Child Neurol. 2015, 30, 749–756. [Google Scholar] [CrossRef]
- Zheng, J.; Frankovich, J.; McKenna, E.S.; Rowe, N.C.; MacEachern, S.J.; Ng, N.N.; Tam, L.T.; Moon, P.K.; Gao, J.; Thienemann, M.; et al. Association of Pediatric Acute-Onset Neuropsychiatric Syndrome with Microstructural Differences in Brain Regions Detected via Diffusion-Weighted Magnetic Resonance Imaging. JAMA Netw. Open 2020, 3, e204063. [Google Scholar] [CrossRef]
- Cabrera, B.; Romero-Rebollar, C.; Jiménez-Ángeles, L.; Genis-Mendoza, A.D.; Flores, J.; Lanzagorta, N.; Arroyo, M.; de la Fuente-Sandoval, C.; Santana, D.; Medina-Bañuelos, V.; et al. Neuroanatomical features and its usefulness in classification of patients with PANDAS. CNS Spectr. 2019, 24, 533–543. [Google Scholar] [CrossRef]
- Giedd, J.N.; Rapoport, J.L.; Kruesi, M.J.P.; Parker, C.; Schapiro, M.B.; Allen, A.J.; Leonard, H.; Kaysen, D.; Dickstein, D.; Marsh, W.; et al. Sydenham’s chorea: Magnetic resonance imaging of the basal ganglia. Neurology 1995, 45, 2199–2202. [Google Scholar] [CrossRef]
- Santoro, J.D.; Frankovich, J.; Bhargava, S. Continued Presence of Period Limb Movements During REM Sleep in Patients with Chronic Static Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS). J. Clin. Sleep Med. 2018, 14, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Gaughan, T.; Buckley, A.; Hommer, R.; Grant, P.; Williams, K.; Leckman, J.F.; Swedo, S.E. Rapid Eye Movement Sleep Abnormalities in Children with Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS). J. Clin. Sleep Med. 2016, 12, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Gagliano, A.; Puligheddu, M.; Ronzano, N.; Congiu, P.; Tanca, M.G.; Cursio, I.; Carucci, S.; Sotgiu, S.; Grossi, E.; Zuddas, A. Artificial Neural Networks Analysis of polysomnographic and clinical features in Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS): From sleep alteration to “Brain Fog”. Nat. Sci. Sleep 2021, 13, 1209–1224. [Google Scholar] [CrossRef] [PubMed]
- Zebrack, J.E.; Gao, J.; Verhey, B.; Tian, L.; Stave, C.; Farhadian, B.; Ma, M.; Silverman, M.; Xie, Y.; Tran, P.; et al. Prevalence of Neurological Soft Signs at Presentation in Pediatric Acute-Onset Neuropsychiatric Syndrome. MedRxiv 2024. [Google Scholar] [CrossRef] [PubMed]
- Trifiletti, R.; Lachman, H.M.; Manusama, O.; Zheng, D.; Spalice, A.; Chiurazzi, P.; Schornagel, A.; Serban, A.M.; van Wijck, R.; Cunningham, J.L.; et al. Identification of ultra-rare genetic variants in pediatric acute onset neuropsychiatric syndrome (PANS) by exome and whole genome sequencing. Sci. Rep. 2022, 12, 11106. [Google Scholar] [CrossRef]
- Dileepan, T.; Smith, E.D.; Knowland, D.; Hsu, M.; Platt, M.; Bittner-Eddy, P.; Cohen, B.; Southern, P.; Latimer, E.; Harley, E.; et al. Group A Streptococcus intranasal infection promotes CNS infiltration by streptococcal-specific Th17 cells. J. Clin. Investig. 2016, 126, 303–317. [Google Scholar] [CrossRef]
- Liebner, S.; Dijkhuizen, R.M.; Reiss, Y.; Plate, K.H.; Agalliu, D.; Constantin, G. Functional morphology of the blood–brain barrier in health and disease. Acta Neuropathol. 2018, 135, 311–336. [Google Scholar] [CrossRef]
- Xu, J.; Liu, R.-J.; Fahey, S.; Frick, L.; Leckman, J.; Vaccarino, F.; Duman, R.S.; Williams, K.; Swedo, S.; Pittenger, C. Antibodies from Children with PANDAS Bind Specifically to Striatal Cholinergic Interneurons and Alter Their Activity. Am. J. Psychiatry 2021, 178, 48–64. [Google Scholar] [CrossRef]
- Frick, L.R.; Rapanelli, M.; Jindachomthong, K.; Grant, P.; Leckman, J.F.; Swedo, S.; Williams, K.; Pittenger, C. Differential binding of antibodies in PANDAS patients to cholinergic interneurons in the striatum. Brain, Behav. Immun. 2018, 69, 304–311. [Google Scholar] [CrossRef]
- Chain, J.L.; Alvarez, K.; Mascaro-Blanco, A.; Reim, S.; Bentley, R.; Hommer, R.; Grant, P.; Leckman, J.F.; Kawikova, I.; Williams, K.; et al. Autoantibody Biomarkers for Basal Ganglia Encephalitis in Sydenham Chorea and Pediatric Autoimmune Neuropsychiatric Disorder Associated with Streptococcal Infections. Front. Psychiatry 2020, 11, 564. [Google Scholar] [CrossRef]
- Kirvan, C.A.; Swedo, S.E.; Heuser, J.S.; Cunningham, M.W. Mimicry and autoantibody-mediated neuronal cell signaling in Sydenham chorea. Nat. Med. 2003, 9, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Kirvan, C.A.; Cox, C.J.; Swedo, S.E.; Cunningham, M.W. Tubulin is a neuronal target of autoantibodies in Sydenham’s chorea. J. Immunol. 2007, 178, 7412–7421. [Google Scholar] [CrossRef] [PubMed]
- Kirvan, C.A.; Swedo, S.E.; Kurahara, D.; Cunningham, M.W. Streptococcal mimicry and antibody-mediated cell signaling in the pathogenesis of Sydenham’s chorea. Autoimmunity 2006, 39, 21–29. [Google Scholar] [CrossRef]
- Kirvan, C.A.; Swedo, S.E.; Snider, L.A.; Cunningham, M.W. Antibody-mediated neuronal cell signaling in behavior and movement disorders. J. Neuroimmunol. 2006, 179, 173–179. [Google Scholar] [CrossRef]
- Cox, C.J.; Zuccolo, A.J.; Edwards, E.V.; Mascaro-Blanco, A.; Alvarez, K.; Stoner, J.; Chang, K.; Cunningham, M.W. Antineuronal antibodies in a heterogeneous group of youth and young adults with tics and obsessive-compulsive disorder. J. Child Adolesc. Psychopharmacol. 2015, 25, 76–85. [Google Scholar] [CrossRef]
- Swedo, S.E.; Seidlitz, J.; Kovacevic, M.; Latimer, M.E.; Hommer, R.; Lougee, L.; Grant, P. Clinical presentation of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections in research and community settings. J. Child Adolesc. Psychopharmacol. 2015, 25, 26–30. [Google Scholar] [CrossRef]
- Swedo, S.E.; Frankovich, J.; Murphy, T.K. Overview of Treatment of Pediatric Acute-Onset Neuropsychiatric Syndrome. J. Child Adolesc. Psychopharmacol. 2017, 27, 562–565. [Google Scholar] [CrossRef]
- Cooperstock, M.S.; Swedo, S.E.; Pasternack, M.S.; Murphy, T.K. Clinical Management of Pediatric Acute-Onset Neuropsychiatric Syndrome: Part III-Treatment and Preven-tion of Infections. J. Child Adolesc. Psychopharmacol. 2017, 27, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Farmer, C.; Farhadian, B.; Hernandez, J.; Thienemann, M.; Frankovich, J. Pediatric Acute-Onset Neuropsychiatric Syndrome Response to Oral Corticosteroid Bursts: An Observational Study of Patients in an Academic Community-Based PANS Clinic. J. Child Adolesc. Psychopharmacol. 2017, 27, 629–639. [Google Scholar] [CrossRef]
- Brown, K.D.; Farmer, C.; Freeman, G.M.; Spartz, E.J.; Farhadian, B.; Thienemann, M.; Frankovich, J. Effect of Early and Prophylactic Nonsteroidal Anti-Inflammatory Drugs on Flare Duration in Pediatric Acute-Onset Neuropsychiatric Syndrome: An Observational Study of Patients Followed by an Academic Community-Based Pediatric Acute-Onset Neuropsychiatric Syndrome Clinic. J. Child Adolesc. Psychopharmacol. 2017, 27, 619–628. [Google Scholar] [CrossRef]
- Spartz, E.J.; Freeman, G.M.; Brown, K.; Farhadian, B.; Thienemann, M.; Frankovich, J. Course of Neuropsychiatric Symptoms after Introduction and Removal of Nonsteroidal Anti-Inflammatory Drugs: A Pediatric Observational Study. J. Child Adolesc. Psychopharmacol. 2017, 27, 652–659. [Google Scholar] [CrossRef]
- Frankovich, J.; Swedo, S.; Murphy, T.; Dale, R.C.; Agalliu, D.; Williams, K.; Daines, M.; Hornig, M.; Chugani, H.; Sanger, T.; et al. Clinical Management of Pediatric Acute-Onset Neuropsychiatric Syndrome: Part II—Use of Immunomodulatory Therapies. J. Child Adolesc. Psychopharmacol. 2017, 27, 574–593. [Google Scholar] [CrossRef] [PubMed]
- Storch, E.A.; Murphy, T.K.; Geffken, G.R.; Mann, G.; Adkins, J.; Merlo, L.J.; Duke, D.; Munson, M.; Swaine, Z.; Goodman, W.K. Cognitive-behavioral therapy for PANDAS-related obsessive-compulsive disorder: Findings from a prelimi-nary waitlist controlled open trial. J. Am. Acad. Child Adolesc. Psychiatry 2006, 45, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Thienemann, M.; Park, M.; Chan, A.; Frankovich, J. Patients with abrupt early-onset OCD due to PANS tolerate lower doses of antidepressants and antipsychotics. J. Psychiatr. Res. 2021, 135, 270–278. [Google Scholar] [CrossRef]
- Ramanathan, S.; Brilot, F.; Irani, S.R.; Dale, R.C. Origins and immunopathogenesis of autoimmune central nervous system disorders. Nat. Rev. Neurol. 2023, 19, 172–190. [Google Scholar] [CrossRef] [PubMed]
- Annunziato, F.; Cosmi, L.; Santarlasci, V.; Maggi, L.; Liotta, F.; Mazzinghi, B.; Parente, E.; Filì, L.; Ferri, S.; Frosali, F.; et al. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 2007, 204, 1849–1861. [Google Scholar] [CrossRef]
- Gaffen, S.L.; Jain, R.; Garg, A.V.; Cua, D.J. The IL-23–IL-17 immune axis: From mechanisms to therapeutic testing. Nat. Rev. Immunol. 2014, 14, 585–600. [Google Scholar] [CrossRef]
- Cipollini, V.; Anrather, J.; Orzi, F.; Iadecola, C. Th17 and Cognitive Impairment: Possible Mechanisms of Action. Front. Neuroanat. 2019, 13, 95. [Google Scholar] [CrossRef]
- Rahman, M.T.; Ghosh, C.; Hossain, M.; Linfield, D.; Rezaee, F.; Janigro, D.; Marchi, N.; van Boxel-Dezaire, A.H. IFN-γ, IL-17A, or zonulin rapidly increase the permeability of the blood-brain and small intestinal epithelial barriers: Relevance for neuro-inflammatory diseases. Biochem. Biophys. Res. Commun. 2018, 507, 274–279. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Wimmer, I.; Tietz, S.; Nishihara, H.; Deutsch, U.; Sallusto, F.; Gosselet, F.; Lyck, R.; Muller, W.A.; Lassmann, H.; Engelhardt, B. PECAM-1 stabilizes blood-brain barrier integrity and favors paracellular T-cell diapedesis across the blood-brain barrier during neuroinflammation. Front. Immunol. 2019, 10, 711. [Google Scholar] [CrossRef] [PubMed]
- Sie, C.; Korn, T.; Mitsdoerffer, M. Th17 cells in central nervous system autoimmunity. Exp. Neurol. 2014, 262 Pt A, 18–27. [Google Scholar] [CrossRef]
- Kang, Z.; Wang, C.; Zepp, J.; Wu, L.; Sun, K.; Zhao, J.; Chandrasekharan, U.; E DiCorleto, P.; Trapp, B.D.; Ransohoff, R.M.; et al. Act1 mediates IL-17-induced EAE pathogenesis selectively in NG2+ glial cells. Nat. Neurosci. 2013, 16, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Huppert, J.; Closhen, D.; Croxford, A.; White, R.; Kulig, P.; Pietrowski, E.; Bechmann, I.; Becher, B.; Luhmann, H.J.; Waisman, A.; et al. Cellular mechanisms of IL-17-induced blood-brain barrier disruption. FASEB J. 2010, 24, 1023–1034. [Google Scholar] [CrossRef]
- Kebir, H.; Kreymborg, K.; Ifergan, I.; Dodelet-Devillers, A.; Cayrol, R.; Bernard, M.; Giuliani, F.; Arbour, N.; Becher, B.; Prat, A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat. Med. 2007, 13, 1173–1175. [Google Scholar] [CrossRef]
- Cooney, S.J.; Bermudez-Sabogal, S.L.; Byrnes, K.R. Cellular and temporal expression of NADPH oxidase (NOX) isotypes after brain injury. J. Neuroinflammation 2013, 10, 155. [Google Scholar] [CrossRef]
- Loffredo, L.; Spalice, A.; Salvatori, F.; De Castro, G.; Guido, C.A.; Zicari, A.M.; Ciacci, P.; Battaglia, S.; Brindisi, G.; Ettorre, E.; et al. Oxidative stress and gut-derived lipopolysaccharides in children affected by paediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. BMC Pediatr. 2020, 20, 127. [Google Scholar] [CrossRef] [PubMed]
- Schofield, C.; Fischer, S.K.; Townsend, M.J.; Mosesova, S.; Peng, K.; Setiadi, A.F.; Song, A.; Baruch, A. Characterization of IL-17AA and IL-17FF in rheumatoid arthritis and multiple sclerosis. Bioanalysis 2016, 8, 2317–2327. [Google Scholar] [CrossRef]
- Setiadi, A.F.; Abbas, A.R.; Jeet, S.; Wong, K.; Bischof, A.; Peng, I.; Lee, J.; Bremer, M.; Eggers, E.L.; DeVoss, J.; et al. IL-17A is associated with the breakdown of the blood-brain barrier in relapsing-remitting multiple sclerosis. J. Neuroimmunol. 2019, 332, 147–154. [Google Scholar] [CrossRef]
- Dileepan, T.; Linehan, J.L.; Moon, J.J.; Pepper, M.; Jenkins, M.K.; Cleary, P.P. Robust antigen specific th17 t cell response to group a streptococcus is dependent on il-6 and intranasal route of infection. PLOS Pathog. 2011, 7, e1002252. [Google Scholar] [CrossRef]
- Wang, B.; Dileepan, T.; Briscoe, S.; Hyland, K.A.; Kang, J.; Khoruts, A.; Cleary, P.P. Induction of TGF-β1 and TGF-β1-dependent predominant Th17 differentiation by group A streptococcal infection. Proc. Natl. Acad. Sci. USA 2010, 107, 5937–5942. [Google Scholar] [CrossRef]
- Alvarez, J.I.; Cayrol, R.; Prat, A. Disruption of central nervous system barriers in multiple sclerosis. Biochim Biophys Acta 2011, 1812, 252–264. [Google Scholar] [CrossRef]
- Platt, M.P.; Bolding, K.A.; Wayne, C.R.; Chaudhry, S.; Cutforth, T.; Franks, K.M.; Agalliu, D. Th17 lymphocytes drive vascular and neuronal deficits in a mouse model of postinfectious autoimmune encephalitis. Proc. Natl. Acad. Sci. USA 2020, 117, 6708–6716. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, L.; Lorenzetti, G.; Carsetti, R.; Mortari, E.P.; Guido, C.A.; Zicari, A.M.; Förster-Waldl, E.; Loffredo, L.; Duse, M.; Spalice, A. Immunological characterization of an Italian PANDAS cohort. Front. Pediatr. 2023, 11, 1216282. [Google Scholar] [CrossRef]
- Wohleb, E.S.; McKim, D.B.; Sheridan, J.F.; Godbout, J.P. Monocyte trafficking to the brain with stress and inflammation: A novel axis of immune-to-brain communication that influences mood and behavior. Front. Neurosci. 2015, 9, 447. [Google Scholar] [CrossRef]
- Felger, J.C.; Lotrich, F.E. Inflammatory Cytokines in Depression: Neurobiological Mechanisms and Therapeutic Implications. Neuroscience 2013, 246, 199–229. [Google Scholar] [CrossRef]
- Karagüzel, E.Ö.; Arslan, F.C.; Uysal, E.K.; Demir, S.; Aykut, D.S.; Tat, M.; Karahan, S.C. Blood levels of interleukin-1 beta, interleukin-6 and tumor necrosis factor-alpha and cognitive functions in patients with obsessive compulsive disorder. Compr. Psychiatry 2019, 89, 61–66. [Google Scholar] [CrossRef]
- Gönenir Erbay, L.; Akti Kavuran, N.; Taşkapan, Ç.; İnce, L.; Yoloğlu, S.; Temelli, H.; Nal, S. 157 Serum IL-1, IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ levels in drug-free, comorbidity-free obses-sive-compulsive disorder patients. Anatol. J. Psychiatry 2018, 19, 157–162. [Google Scholar] [CrossRef]
- Singer, H.S.; Gause, C.; Morris, C.; Lopez, P. Serial immune markers do not correlate with clinical exacerbations in pediatric autoimmune neuropsychiat-ric disorders associated with streptococcal infections. Pediatrics 2008, 121, 1198–1205. [Google Scholar] [CrossRef]
- Gromark, C.; Hesselmark, E.; Djupedal, I.G.; Silverberg, M.; Horne, A.; Harris, R.A.; Serlachius, E.; Mataix-Cols, D. A Two-to-Five Year Follow-Up of a Pediatric Acute-Onset Neuropsychiatric Syndrome Cohort. Child Psychiatry Hum. Dev. 2022, 53, 354–364. [Google Scholar] [CrossRef]
- Leckman, J.F.; Katsovich, L.; Kawikova, I.; Lin, H.; Zhang, H.; Krönig, H.; Morshed, S.; Parveen, S.; Grantz, H.; Lombroso, P.J.; et al. Increased serum levels of interleukin-12 and tumor necrosis factor-alpha in Tourette’s syndrome. Biol. Psychiatry 2005, 57, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Parker-Athill, E.C.; Ehrhart, J.; Tan, J.; Murphy, T.K. Cytokine correlations in youth with tic disorders. J. Child Adolesc. Psychopharmacol. 2015, 25, 86–92. [Google Scholar] [CrossRef]
- Chi, S.H.; Mok, Y.E.; Kang, J.; Gim, J.A.; Han, C.; Lee, M.S. Cytokine levels reflect tic symptoms more prominently during mild phases. BMC Neurosci. 2023, 24, 57. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M.M. Microbiota-Brain-Gut Axis and Neurodegenerative Diseases. Curr. Neurol. Neurosci. Rep. 2017, 17, 94. [Google Scholar] [CrossRef] [PubMed]
- Clapp, M.; Aurora, N.; Herrera, L.; Bhatia, M.; Wilen, E.; Wakefield, S. Gut microbiota’s effect on mental health: The gut-brain axis. Clin. Pract 2017, 7, 987. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. Regulation of the stress response by the gut microbiota: Implications for psychoneuroendocrinology. Psychoneuroendocrinology 2012, 37, 1369–1378. [Google Scholar] [CrossRef]
- Quagliariello, A.; Del Chierico, F.; Russo, A.; Reddel, S.; Conte, G.; Lopetuso, L.R.; Ianiro, G.; Dallapiccola, B.; Cardona, F.; Gasbarrini, A.; et al. Gut microbiota profiling and gut-brain crosstalk in children affected by pediatric acute-onset neuropsychi-atric syndrome and pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. Front. Microbiol. 2018, 9, 334456. [Google Scholar] [CrossRef]
- Rees, J.C. Obsessive–compulsive disorder and gut microbiota dysregulation. Med. Hypotheses 2014, 82, 163–166. [Google Scholar] [CrossRef]
- Ma, M.; Sandberg, J.; Farhadian, B.; Silverman, M.; Xie, Y.; Thienemann, M.; Frankovich, J. Arthritis in Children with Psychiatric Deteriorations: A Case Series. Dev. Neurosci. 2023, 45, 325–334. [Google Scholar] [CrossRef]
- Kawikova, I.; Grady, B.P.X.; Tobiasova, Z.; Zhang, Y.; Vojdani, A.; Katsovich, L.; Richmand, B.J.; Park, T.W.; Bothwell, A.L.; Leckman, J.F. Children with Tourette’s Syndrome May Suffer Immunoglobulin A Dysgammaglobulinemia: Preliminary Report. Biol. Psychiatry 2010, 67, 679–683. [Google Scholar] [CrossRef]
- Sigra, S.; Hesselmark, E.; Bejerot, S. Treatment of PANDAS and PANS: A systematic review. Neurosci. Biobehav. Rev. 2018, 86, 51–65. [Google Scholar] [CrossRef]
- Rapanelli, M.; Frick, L.R.; Pittenger, C. The Role of Interneurons in Autism and Tourette Syndrome. Trends Neurosci. 2017, 40, 397–407. [Google Scholar] [CrossRef]
- Xu, M.; Kobets, A.; Du, J.C.; Lennington, J.; Li, L.; Banasr, M.; Duman, R.S.; Vaccarino, F.M.; DiLeone, R.J.; Pittenger, C. Targeted ablation of cholinergic interneurons in the dorsolateral striatum produces behavioral manifestations of Tourette syndrome. Proc. Natl. Acad. Sci. USA 2015, 112, 893–898. [Google Scholar] [CrossRef]
- Pavone, P.; Bianchini, R.; Parano, E.; Incorpora, G.; Rizzo, R.; Mazzone, L.; Trifiletti, R.R. Anti-brain antibodies in PANDAS versus uncomplicated streptococcal infection. Pediatr. Neurol. 2004, 30, 107–110. [Google Scholar] [CrossRef]
- Dalmau, J.; Geis, C.; Graus, F. Autoantibodies to synaptic receptors and neuronal cell surface proteins in autoimmune diseases of the central nervous system. Physiol. Rev. 2017, 97, 839–887. [Google Scholar] [CrossRef]
- Shimasaki, C.; Frye, R.E.; Trifiletti, R.; Cooperstock, M.; Kaplan, G.; Melamed, I.; Greenberg, R.; Katz, A.; Fier, E.; Kem, D.; et al. Evaluation of the Cunningham Panel™ in pediatric autoimmune neuropsychiatric disorder associated with streptococcal infection (PANDAS) and pediatric acute-onset neuropsychiatric syndrome (PANS): Changes in antineuronal antibody titers parallel changes in patient symptoms. J. Neuroimmunol. 2020, 339, 577138. [Google Scholar] [CrossRef]
- Hesselmark, E.; Bejerot, S. Biomarkers for diagnosis of Pediatric Acute Neuropsychiatric Syndrome (PANS)—Sensitivity and specificity of the Cunningham Panel. J. Neuroimmunol. 2017, 312, 31–37. [Google Scholar] [CrossRef]
- Bejerot, S.; Hesselmark, E. The Cunningham Panel is an unreliable biological measure. Transl. Psychiatry 2019, 9, 49. [Google Scholar] [CrossRef]
- Perlmutter, S.J.; Leitman, S.F.; Garvey, M.A.; Hamburger, S.; Feldman, E.; Leonard, H.L.; E Swedo, S. Therapeutic plasma exchange and intravenous immunoglobulin for obsessive-compulsive disorder and tic disorders in childhood. Lancet 1999, 354, 1153–1158. [Google Scholar] [CrossRef]
- Asbahr, F.R.; Negrão, A.B.; Gentil, V.; Zanetta, D.M.T.; Da Paz, J.A.; Marques-Dias, M.J.; Kiss, M.H. Obsessive-compulsive and related symptoms in children and adolescents with rheumatic fever with and without chorea: A prospective 6-month study. Am. J. Psychiatry 1998, 155, 1122–1124. [Google Scholar] [CrossRef]
- Faustino, P.C.; Terreri, M.T.R.A.; da Rocha, A.; Zappitelli, M.C.; Lederman, H.M.; Hilário, M.O.E. Clinical, laboratory, psychiatric and magnetic resonance findings in patients with Sydenham chorea. Neuroradiology 2003, 45, 456–462. [Google Scholar] [CrossRef]
- Mell, L.K.; Davis, R.L.; Owens, D. Association between streptococcal infection and obsessive-compulsive disorder, Tourette’s syndrome, and tic disorder. Pediatrics 2005, 116, 56–60. [Google Scholar] [CrossRef]
- Martino, D.; Chiarotti, F.; Buttiglione, M.; Cardona, F.; Creti, R.; Nardocci, N.; Orefici, G.; Veneselli, E.; Rizzo, R.; On Behalf of The Italian Tourette Syndrome Study Group. The relationship between group A streptococcal infections and Tourette syndrome: A study on a large service-based cohort. Dev. Med. Child Neurol. 2011, 53, 951–957. [Google Scholar] [CrossRef]
- Hsu, C.-J.; Wong, L.-C.; Lee, W.-T. Immunological Dysfunction in Tourette Syndrome and Related Disorders. Int. J. Mol. Sci. 2021, 22, 853. [Google Scholar] [CrossRef]
- Renna, M.E.; O’Toole, M.S.; Spaeth, P.E.; Lekander, M.; Mennin, D.S. The association between anxiety, traumatic stress, and obsessive-compulsive disorders and chronic inflammation: A systematic review and meta-analysis. Depress. Anxiety 2018, 35, 1081–1094. [Google Scholar] [CrossRef]
- Church, A.J.; Dale, R.C.; Giovannoni, G. Anti-basal ganglia antibodies: A possible diagnostic utility in idiopathic movement disorders? Arch. Dis. Child. 2004, 89, 611–614. [Google Scholar] [CrossRef]
- Connery, K.; Tippett, M.; Delhey, L.M.; Rose, S.; Slattery, J.C.; Kahler, S.G.; Hahn, J.; Kruger, U.; Cunningham, M.W.; Shimasaki, C.; et al. Intravenous immunoglobulin for the treatment of autoimmune encephalopathy in children with autism. Transl. Psychiatry 2018, 8, 148. [Google Scholar] [CrossRef]
- Chiarello, F.; Spitoni, S.; Hollander, E.; Matucci Cerinic, M.; Pallanti, S. An expert opinion on PANDAS/PANS: Highlights and controversies. Int. J. Psychiatry Clin. Pract 2017, 21, 91–98. [Google Scholar] [CrossRef]
- Sørensen, C.B.; Skov, L.; Lundby, L.; Grejsen, J.; Aaslet, L.; Debes, N.M. PANDAS and PANS in children and adolescents are still controversial diagnoses. Ugeskr Laeger 2018, 180, V01180045. [Google Scholar]
- Cocuzza, S.; Maniaci, A.; La Mantia, I.; Nocera, F.; Caruso, D.; Caruso, S.; Iannella, G.; Vicini, C.; Privitera, E.; Lechien, J.R.; et al. Obsessive-Compulsive Disorder in PANS/PANDAS in Children: In Search of a Qualified Treatment—A Systematic Review and Metanalysis. Children 2022, 9, 155. [Google Scholar] [CrossRef]
- Wilbur, C.; Bitnun, A.; Kronenberg, S.; Laxer, R.M.; Levy, D.M.; Logan, W.J.; Shouldice, M.; Yeh, E.A. PANDAS/PANS in childhood: Controversies and evidence. Paediatr. Child Health 2019, 24, 85–91. [Google Scholar] [CrossRef]
- Blackburn, J.S. Tic Disorders and PANDAS. Semin. Pediatr. Neurol. 2018, 25, 25–33. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonardi, L.; Perna, C.; Bernabei, I.; Fiore, M.; Ma, M.; Frankovich, J.; Tarani, L.; Spalice, A. Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS) and Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections (PANDAS): Immunological Features Underpinning Controversial Entities. Children 2024, 11, 1043. https://doi.org/10.3390/children11091043
Leonardi L, Perna C, Bernabei I, Fiore M, Ma M, Frankovich J, Tarani L, Spalice A. Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS) and Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections (PANDAS): Immunological Features Underpinning Controversial Entities. Children. 2024; 11(9):1043. https://doi.org/10.3390/children11091043
Chicago/Turabian StyleLeonardi, Lucia, Camilla Perna, Irene Bernabei, Marco Fiore, Meiqian Ma, Jennifer Frankovich, Luigi Tarani, and Alberto Spalice. 2024. "Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS) and Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections (PANDAS): Immunological Features Underpinning Controversial Entities" Children 11, no. 9: 1043. https://doi.org/10.3390/children11091043






