Cobalt-Activated Transfer-Free Synthesis of the Graphene on Si(100) by Anode Layer Ion Source
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Single Step Synthesis
3.1.1. Temperature
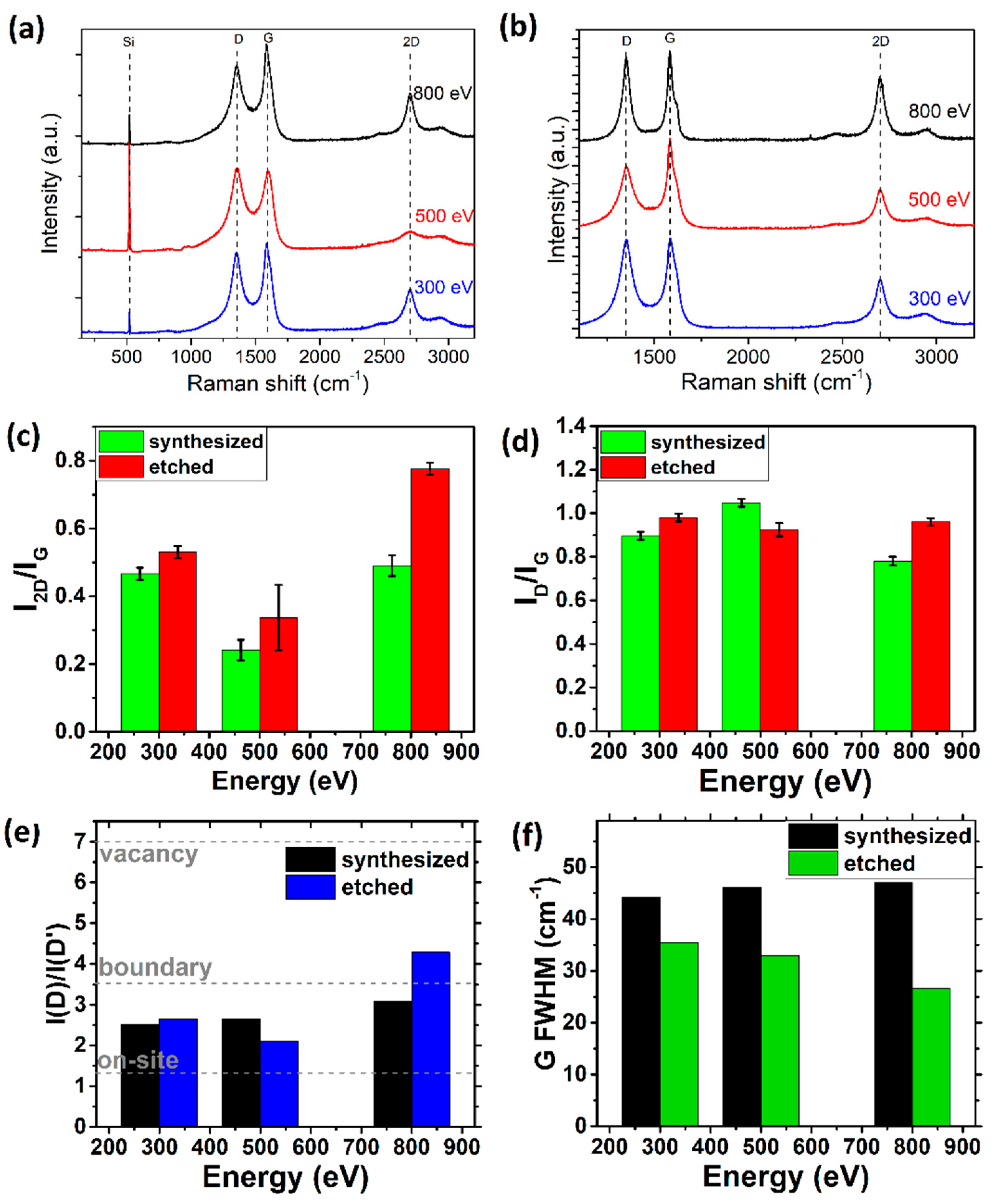
3.1.2. Ion Beam Energy
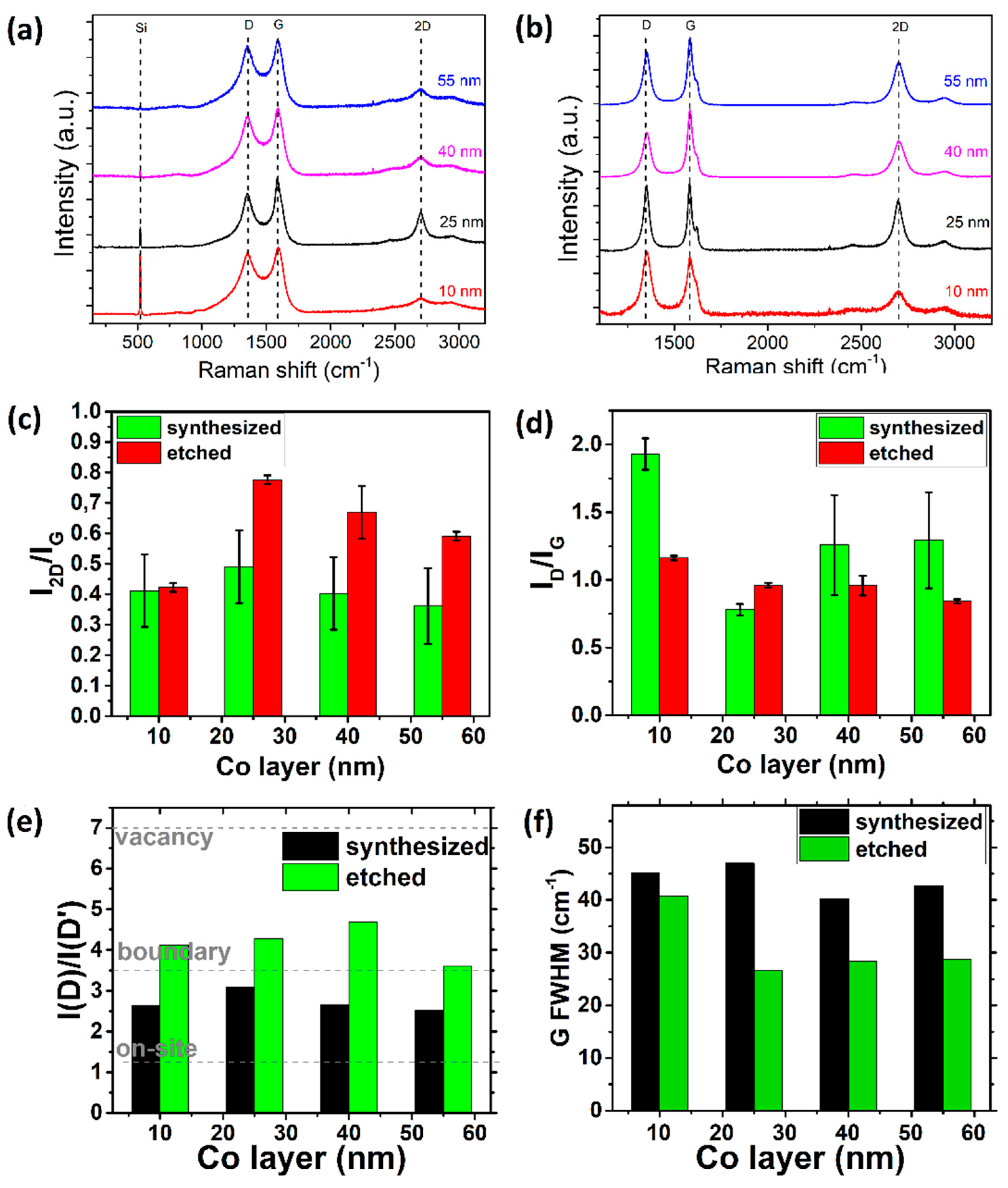
3.1.3. Thickness of Cobalt Layer
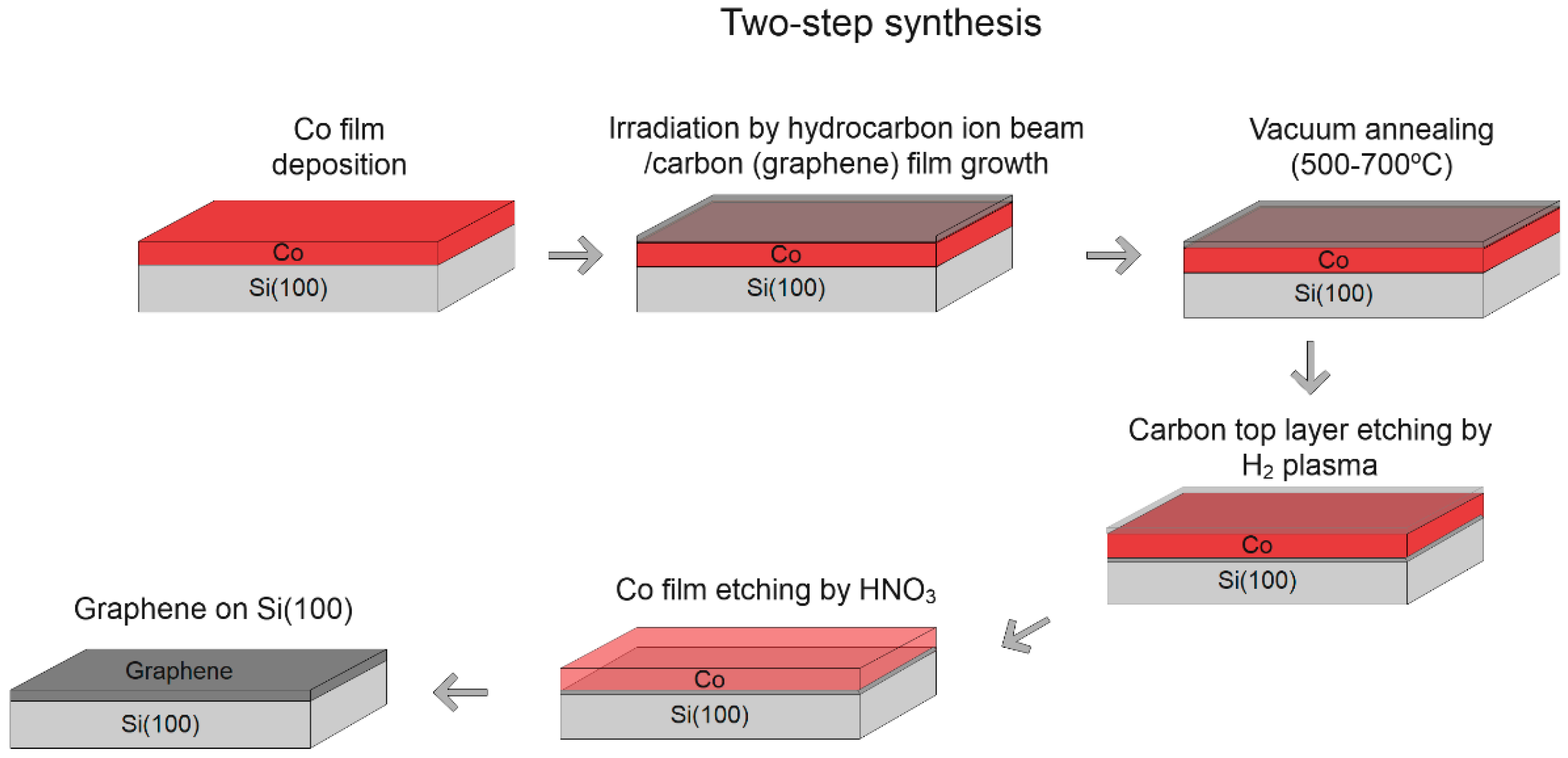
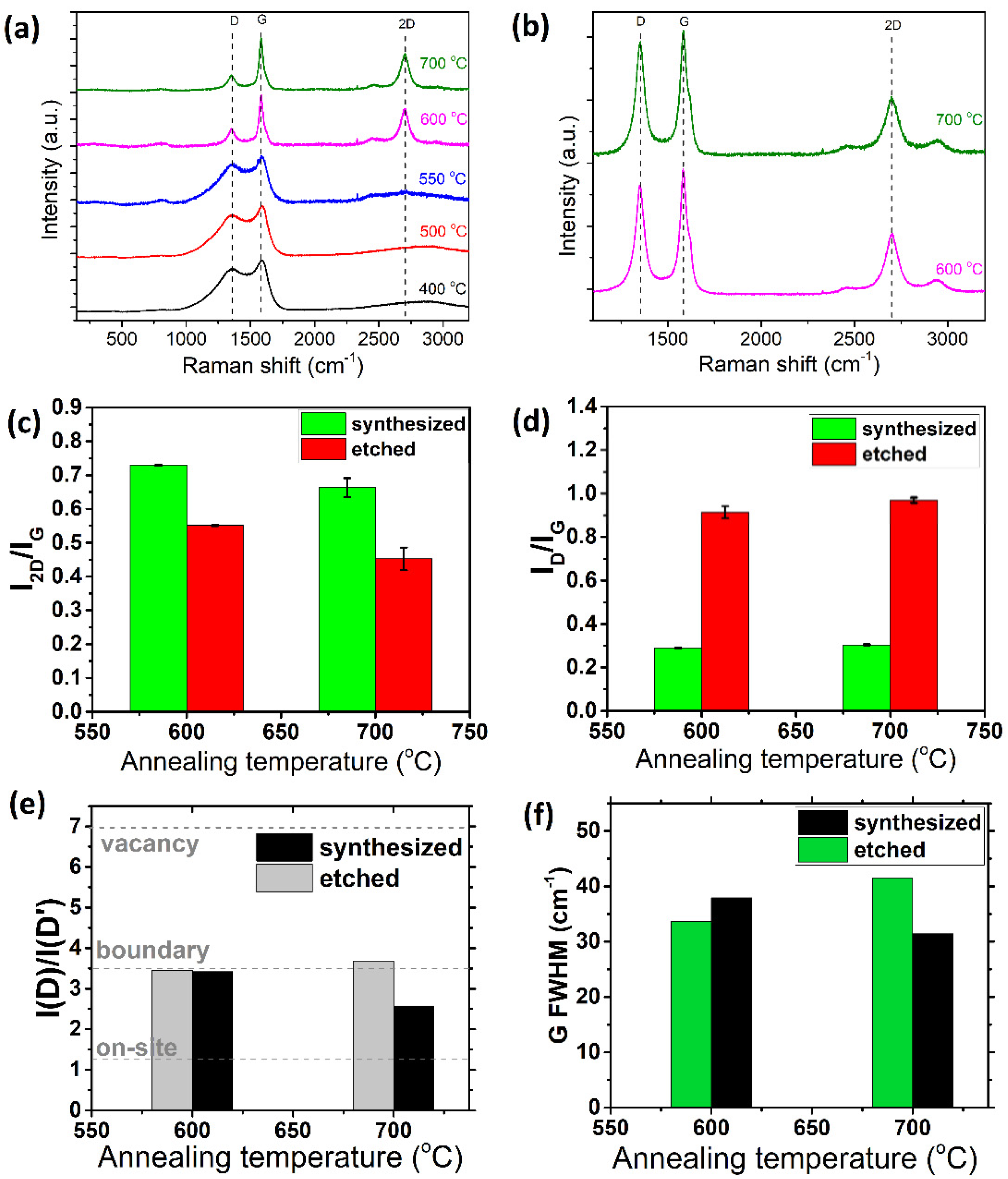
3.2. Two-Step Synthesis
3.2.1. Thickness of Co Layer
3.2.2. Temperature of Annealing
3.3. Single-Step versus Two-Step Graphene Synthesis
3.4. AFM and SEM Results
3.5. XPS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dhinakaran, V.; Lavanya, M.; Vigneswari, K.; Ravichandran, M.; Vijayakumar, M.D. Review on exploration of graphene in diverse applications and its future horizon. Mater. Today Proc. 2020, 27, 824–828. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Sahoo, S.; Wang, N.; Huczko, A. Graphene research and their outputs: Status and prospect. J. Sci. Adv. Mater. Devices 2020, 5, 10–29. [Google Scholar] [CrossRef]
- Losurdo, M.; Giangregorio, M.M.; Capezzuto, P.; Bruno, G. Graphene CVD growth on copper and nickel: Role of hydrogen in kinetics and structure. Phys. Chem. Chem. Phys. 2011, 13, 20836–20843. [Google Scholar] [CrossRef]
- Zou, Z.; Carnevali, V.; Patera, L.L.; Jugovac, M.; Cepek, C.; Peressi, M.; Comelli, G.; Africh, C. Operando atomic-scale study of graphene CVD growth at steps of polycrystalline nickel. Carbon 2020, 161, 528–534. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Zhang, G.; Prakash, J.; Chen, Z.; Gauthier, M.; Sun, S. Chemical vapour deposition of graphene: Layer control, the transfer process, characterisation, and related applications. Int. Rev. Phys. Chem. 2019, 38, 149–199. [Google Scholar] [CrossRef]
- Narula, U.; Tan, C.M.; Lai, C.S. Growth mechanism for low temperature PVD graphene synthesis on copper using amorphous carbon. Sci. Rep. 2017, 7, 44112. [Google Scholar] [CrossRef] [Green Version]
- Sato, M.; Takahashi, M.; Nakano, H.; Muro, T.; Takakuwa, Y.; Sato, S.; Nihei, M.; Yokoyama, N. High-current reliability and growth conditions of multilayer graphene wire obtained by annealing sputtered amorphous carbon. Jpn. J. Appl. Phys. 2013, 52, 04CB07. [Google Scholar] [CrossRef]
- Dharmaraj, P.; Venkatesh, P.S.; Kumar, P.; Asokan, K.; Jeganathan, K. Direct growth of few layer graphene on SiO2 substrate by low energy carbon ion implantation. RSC Adv. 2016, 6, 101347–101352. [Google Scholar] [CrossRef]
- Ueno, K.; Sano, S.; Matsumoto, Y. Direct deposition of multilayer graphene on dielectrics via solid-phase precipitation from carbon-doped cobalt with a copper capping layer. Jpn. J. Appl. Phys. 2019, 58, 026501. [Google Scholar] [CrossRef]
- Murata, H.; Toko, K.; Saitoh, N.; Yoshizawa, N.; Suemasu, T. Direct synthesis of multilayer graphene on an insulator by Ni-induced layer exchange growth of amorphous carbon. Appl. Phys. Lett. 2017, 110, 033108. [Google Scholar] [CrossRef]
- Murata, H.; Saitoh, N.; Yoshizawa, N.; Suemasu, T.; Toko, K. High-quality multilayer graphene on an insulator formed by diffusion controlled Ni-induced layer exchange. Appl. Phys. Lett. 2017, 111, 243104. [Google Scholar] [CrossRef]
- Bachmatiuk, A.; Boeckl, J.; Smith, H.; Ibrahim, I.; Gemming, T.; Oswald, S.; Kazmierczak, W.; Makarov, D.; Schmidt, O.G.; Eckert, J.; et al. Vertical graphene growth from amorphous carbon films using oxidizing gases. J. Phys. Chem. C 2015, 119, 17965–17970. [Google Scholar] [CrossRef]
- Ueda, Y.; Maruyama, T.; Naritsuka, S. Direct growth of multilayer graphene by precipitation using W capping layer Jumpei Yamada1. Jpn. J. Appl. Phys. 2016, 55, 100302. [Google Scholar] [CrossRef]
- Narula, U.; Tan, C.M. Engineering a PVD-based graphene synthesis method. IEEE Trans. Nanotechnol. 2017, 16, 784–789. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Lee, G. Wafer-scale synthesis of multi-layer graphene by high-temperature carbon ion implantation. Appl. Phys. Lett. 2015, 107, 033104. [Google Scholar] [CrossRef]
- Chen, D.; Guo, Q.; Yang, S.; Liu, Z.; Zheng, X.; Zhang, N.; Xu, A.; Wang, B.; Wang, G.; Ding, G. Interfacial monolayer graphene growth on arbitrary substrate by nickel-assisted ion implantation. J. Mater. Sci. 2018, 53, 2631–2637. [Google Scholar] [CrossRef]
- Garaj, S.; Hubbard, W.; Golovchenko, J.A. Graphene synthesis by ion implantation. Appl. Phys. Lett. 2010, 97, 2–4. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Zhang, Z.; Sun, H.; Dai, D.; Cui, J.; Li, M.; Xu, Y.; Xu, M.; Du, Y.; Jiang, N.; et al. Direct formation of wafer-scale single-layer graphene films on the rough surface substrate by PECVD. Carbon 2018, 129, 456–461. [Google Scholar] [CrossRef]
- Murata, H.; Toko, K.; Suemasu, T. Multilayer graphene on insulator formed by Co-induced layer exchange. Jpn. J. Appl. Phys. 2017, 56, 05DE03. [Google Scholar] [CrossRef] [Green Version]
- Saenger, K.L.; Tsang, J.C.; Bol, A.A.; Chu, J.O.; Grill, A.; Lavoie, C. In situ X-ray diffraction study of graphitic carbon formed during heating and cooling of amorphous-C/Ni bilayers. Appl. Phys. Lett. 2010, 96, 153105. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Xiong, W.; Zhou, Y.S.; Lu, Y.F.; Zeng, X.C. An ab initio study of the nickel-catalyzed transformation of amorphous carbon into graphene in rapid thermal processing. Nanoscale 2016, 8, 9746–9755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Shi, D.; Guo, P.; Wei, J.; Cui, P.; Du, S.; Wang, A. Amorphous carbon to graphene: Carbon diffusion via nickel catalyst. Mater. Lett. 2020, 278, 128468. [Google Scholar] [CrossRef]
- Anders, A. Plasma and ion sources in large area coating: A review. Surf Coat. Technol. 2005, 200, 1893–1906. [Google Scholar] [CrossRef]
- Davis, C.A.; Amaratunga, G.A.J.; Knowles, K.M. Growth mechanism and cross-sectional structure of tetrahedral amorphous carbon thin films. Phys. Rev. Lett. 1998, 80, 13–16. [Google Scholar] [CrossRef]
- Zhurin, V.V.; Kaufman, H.R.; Robinson, R.S. Physics of closed drift thrusters. Plasma Sources Sci. Technol. 1999, 8, R1–R20. [Google Scholar] [CrossRef]
- Hwang, J.-S.; Lin, Y.-H.; Hwang, J.-Y.; Chang, R.; Chattopadhyay, S.; Chen, C.-J.; Chen, P.; Chiang, H.-P.; Tsai, T.-R.; Chen, L.-C. Imaging layer number and stacking order through formulating Raman fingerprints obtained from hexagonal single crystals of few layer graphene. Nanotechnology 2012, 24, 015702. [Google Scholar] [CrossRef]
- Eckmann, A.; Felten, A.; Mischenko, A.; Britnell, L.; Krupke, R.; Novoselov, K.S.; Casiraghi, C. Probing the nature of defects in graphene by Raman spectroscopy. Nano Lett. 2012, 12, 3925–3930. [Google Scholar] [CrossRef] [Green Version]
- Merlen, A.; Buijnsters, J.; Pardanaud, C. A guide to and review of the use of multiwavelength raman spectroscopy for characterizing defective aromatic carbon solids: From graphene to amorphous carbons. Coatings 2017, 7, 153. [Google Scholar] [CrossRef]
- Thompson, C.V. Solid-state dewetting of thin films. Annu. Rev. 2012, 42, 399–434. [Google Scholar] [CrossRef]
- Simrick, N.J.; Kilner, J.; Atkinson, A. Thermal stability of silver thin films on zirconia substrates. Thin Solid Film. 2012, 520, 2855–2867. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, K.; Yuan, S.; Li, G.; Ma, Y.; Wang, Q. Solid-state dewetting in polycrystalline co films on native oxide Si(100) by kirkendall effects. J. Phys. Chem. C 2019, 123, 19572–19578. [Google Scholar] [CrossRef]
- Dzhumaliev, A.S.; Nikulin, Y.V.; Filimonov, Y.A. Influence of annealing and argon pressure on the microcrystalline structure of magnetron-sputtered textured cobalt films. Tech. Phys. 2018, 6311, 1678–1686. [Google Scholar] [CrossRef]
- Jeong, K.; Lee, J.; Byun, I.; Seong, M.J.; Park, J.; Kim, H.W.; Kim, M.J.; Kim, J.H.; Lee, J. Synthesis of highly conductive cobalt thin films by, L.C.;VD at atmospheric pressure. Mater. Sci. Semicond. Process. 2017, 68, 245–251. [Google Scholar] [CrossRef]
- Kumar, D.; Gupta, A. Evolution of structural and magnetic properties of sputtered nanocrystalline Co thin films with thermal annealing. J. Magn. Magn. Mater. 2007, 308, 318–324. [Google Scholar] [CrossRef]
- Li, M.; Tian, Z.; Yu, X.; Yu, D.; Ren, L.; Fu, Y. Influence of thermal annealing on the morphology and magnetic domain structure of Co thin films. Mater. Res. Express. 2021, 8, 56103. [Google Scholar] [CrossRef]
- Janke, D.; Hulman, M.; Wenisch, R.; Gemming, S.; Rafaja, D.; Krause, M. Influence of nickel catalyst morphology on layer-exchange-based carbon crystallisation of Ni/a-C bilayers. Phys. Status Solidi 2017, 254, 1700234. [Google Scholar] [CrossRef]
- Chaitoglou, S.E. Effect of temperature on graphene grown by chemical vapor deposition. J. Mater. Sci. 2017, 52, 8348–8356. [Google Scholar] [CrossRef]
- Sato, M.; Inukai, M.; Ikenaga, E.; Muro, T.; Ogawa, S.; Takakuwa, Y.; Nakano, H.; Kawabata, A.; Nihei, M.; Yokoyama, N. Fabrication of graphene directly on SiO2 without transfer processes by annealing sputtered amorphous carbon. Jpn. J. Appl. Phys. 2012, 51, 04DB01. [Google Scholar] [CrossRef]
- Ramón, M.E.; Gupta, A.; Corbet, C.; Ferrer, D.A.; Movva, H.C.P.; Carpenter, G.; Colombo, L.; Bourianoff, G.; Doczy, M.; Akinwande, D.; et al. CMOS-compatible synthesis of large-area, high-mobility graphene by chemical vapor deposition of acetylene on cobalt thin films. ACS Nano 2011, 5, 7198–7204. [Google Scholar] [CrossRef]
- Pan, G.; Li, B.; Heath, M.; Horsell, D.; Wears, M.L.; Al Taan, L.; Awan, S. Transfer-free growth of graphene on SiO2 insulator substrate from sputtered carbon and nickel films. Carbon 2013, 65, 349–358. [Google Scholar] [CrossRef] [Green Version]
- Meškinis, Š.; Andrulevičius, M.; Šlapikas, K.; Iljinas, A.; Gudaitis, R.; Puišo, J.; Tamulevičius, S. Growth and properties of the ion beam deposited SiOx containing DLC films. Vacuum 2009, 83 (Suppl. 1), S121–S123. [Google Scholar] [CrossRef]
- Xu, W.; Lin, S.; Dai, M.; Shi, Q.; Wei, C.; Zhang, X.; Zhou, K. Effects of bias voltage on the microstructure and properties of Al-doped hydrogenated amorphous carbon films synthesized by a hybrid deposition technique. Vacuum 2018, 154, 159–166. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, J.; Su, H.; Li, J.; Sun, L.; Wang, Z.; Xu, F.; Liu, C.; Lopatin, S.; Zhu, Y.; et al. Towards super-clean graphene. Nat. Commun. 2019, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jia, K.; Zhang, J.; Lin, L.; Li, Z.; Gao, J.; Sun, L.; Xue, R.; Li, J.; Kang, N.; Luo, Z.; et al. Copper-containing carbon feedstock for growing superclean graphene. J. Am. Chem. Soc. 2019, 141, 7670–7674. [Google Scholar] [CrossRef]
- Venezuela, P.; Lazzeri, M.; Mauri, F. Theory of double-resonant Raman spectra in graphene: Intensity and line shape of defect-induced and two-phonon bands. Phys. Rev. B Condens Mater. Mater. Phys. 2011, 84, 035433. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Joo, K.; Jerng, S.K.; Lee, J.H.; Yoon, E.; Chun, S.H. Direct growth of patterned graphene on SiO2 substrates without the use of catalysts or lithography. Nanoscale 2014, 6, 10100–10105. [Google Scholar] [CrossRef]
- Chen, C.Y.; Dai, D.; Chen, G.; Yu, J.H.; Nishimura, K.; Lin, C.T.; Jiang, N.; Zhan, Z.L. Rapid growth of single-layer graphene on the insulating substrates by thermal CVD. Appl. Surf. Sci. 2015, 346, 41–45. [Google Scholar] [CrossRef]
- Kozłowski, W.; Balcerski, J.; Kowalczyk, P.J.; Kowalczyk, P.J.; Cichomski, M.; Szmaja, W. Investigation of thermally evaporated nanocrystalline thin cobalt films. Appl. Phys. A 2017, 123, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Nast, O.; Hartmann, A.J. Influence of interface and Al structure on layer exchange during aluminum-induced crystallization of amorphous silicon. J. Appl. Phys. 2000, 88, 716. [Google Scholar] [CrossRef]
- Peng, K.J.; Wu, C.L.; Lin, Y.H.; Liu, Y.J.; Tsai, D.P.; Pai, Y.H.; Lin, G.R. Hydrogen-free P.ECVD growth of few-layer graphene on an ultra-thin nickel film at the threshold dissolution temperature. J. Mater. Chem. C 2013, 1, 3862–3870. [Google Scholar] [CrossRef]
- Tallant, D.R.; Friedmann, T.A.; Missert, N.A.; Siegal, M.P.; Sullivan, J.P. Raman spectroscopy of amorphous carbon. Mater. Res. Soc. Symp. Proc. 1997, 498, 37–48. [Google Scholar] [CrossRef] [Green Version]
- Viana, G.A.; Marques, F.C. Raman and thermal desorption spectroscopy analyses of amorphous graphite-like carbon films with incorporated xenon. Vacuum 2015, 112, 17–24. [Google Scholar] [CrossRef]
- Schuepfer, D.B.; Badaczewski, F.; Guerra-Castro, J.M.; Hofmann, D.M.; Heiliger, C.; Smarsly, B.; Klar, P.J. Assessing the structural properties of graphitic and non-graphitic carbons by Raman spectroscopy. Carbon 2020, 161, 359–372. [Google Scholar] [CrossRef]
- Tamura, T.; Ueno, K. Low-Temperature synthesis of multilayer graphene directly on SiO2 by current-enhanced solid-phase deposition using Ni catalyst. Jpn. J. Appl. Phys. 2020, 59, 066501. [Google Scholar] [CrossRef]
- Zheng, M.; Takei, K.; Hsia, B.; Fang, H.; Zhang, X.; Ferralis, N.; Ko, H.; Chueh, Y.L.; Zhang, Y.; Maboudian, R.; et al. Metal-catalyzed crystallization of amorphous carbon to graphene. Appl. Phys. Lett. 2010, 96, 063110. [Google Scholar] [CrossRef]
- Dai, C.Y.; Wang, W.C.; Tseng, C.A.; Ding, F.C.; Chen, Y.T.; Chen, C.C. spatial confinement approach using ni to modulate local carbon supply for the growth of uniform transfer-free graphene monolayers. J. Phys. Chem. C 2020, 124, 23094–23105. [Google Scholar] [CrossRef]
- Komissarov, I.V.; Kovalchuk, N.G.; Labunov, V.A.; Girel, K.V.; Korolik, O.V.; Tivanov, M.S.; Lazauskas, A.; Andrulevičius, M.; Tamulevičius, T.; Grigaliūnas, V.; et al. Nitrogen-doped twisted graphene grown on copper by atmospheric pressure CVD from a decane precursor. Beilstein J. Nanotechnol. 2017, 8, 145–158. [Google Scholar] [CrossRef] [Green Version]
- Parambath Vinayan, B.; Diemant, T.; Lin, X.-M.; Cambaz, M.A.; Golla-Schindler, U.; Kaiser, U.; Jürgen Behm, R.; Fichtner, M. Nitrogen rich hierarchically organized porous carbon/sulfur composite cathode electrode for high performance Li/S battery: A Mechanistic Investigation by operando spectroscopic studies. Adv. Mater. Interfaces 2016, 3, 1600372. [Google Scholar] [CrossRef] [Green Version]
- Egiza, M.; Naragino, H.; Tominaga, A.; Hanada, K.; Kamitani, K.; Sugiyama, T.; Ikenaga, E.; Murasawa, K.; Gonda, H.; Sakurai, M.; et al. Effects of air exposure on hard and soft X-ray photoemission spectra of ultrananocrystalline diamond/amorphous carbon composite films. Coatings 2018, 8, 359. [Google Scholar] [CrossRef] [Green Version]
- Egiza, M.; Murasawa, K.; Ali, A.M.; Fukui, Y.; Gonda, H.; Sakurai, M.; Yoshitake, T. Enhanced hardness of nanocarbon films deposited on cemented tungsten carbide substrates by coaxial arc plasma deposition owing to employing silicon-doped graphite targets. Jpn. J. Appl. Phys. 2019, 58, 075507. [Google Scholar] [CrossRef]
- Rajackaitė, E.; Peckus, D.; Gudaitis, R.; Andrulevičius, M.; Tamulevičius, T.; Volyniuk, D.; Meškinis, Š.; Tamulevičius, S. Transient absorption spectroscopy as a promising optical tool for the quality evaluation of graphene layers deposited by microwave plasma. Surf. Coat. Technol. 2020, 395, 125887. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Li, A.; Yang, Y.; Tang, Q.; Cao, H.; Qi, T.; Li, C. Electrocatalysis of carbon black- or poly(diallyldimethylammonium chloride)-functionalized activated carbon nanotubes-supported Pd-Tb towards methanol oxidation in alkaline media. J. Power Sources 2014, 257, 138–146. [Google Scholar] [CrossRef]
- NIST X-ray Photoelectron Spectroscopy Database, NIST Standard Reference Database Number 20, National Institute of Standards and Technology, Gaithersburg MD. 2000; p. 20899. Available online: https://srdata.nist.gov/xps/citation.aspx (accessed on 13 December 2021).





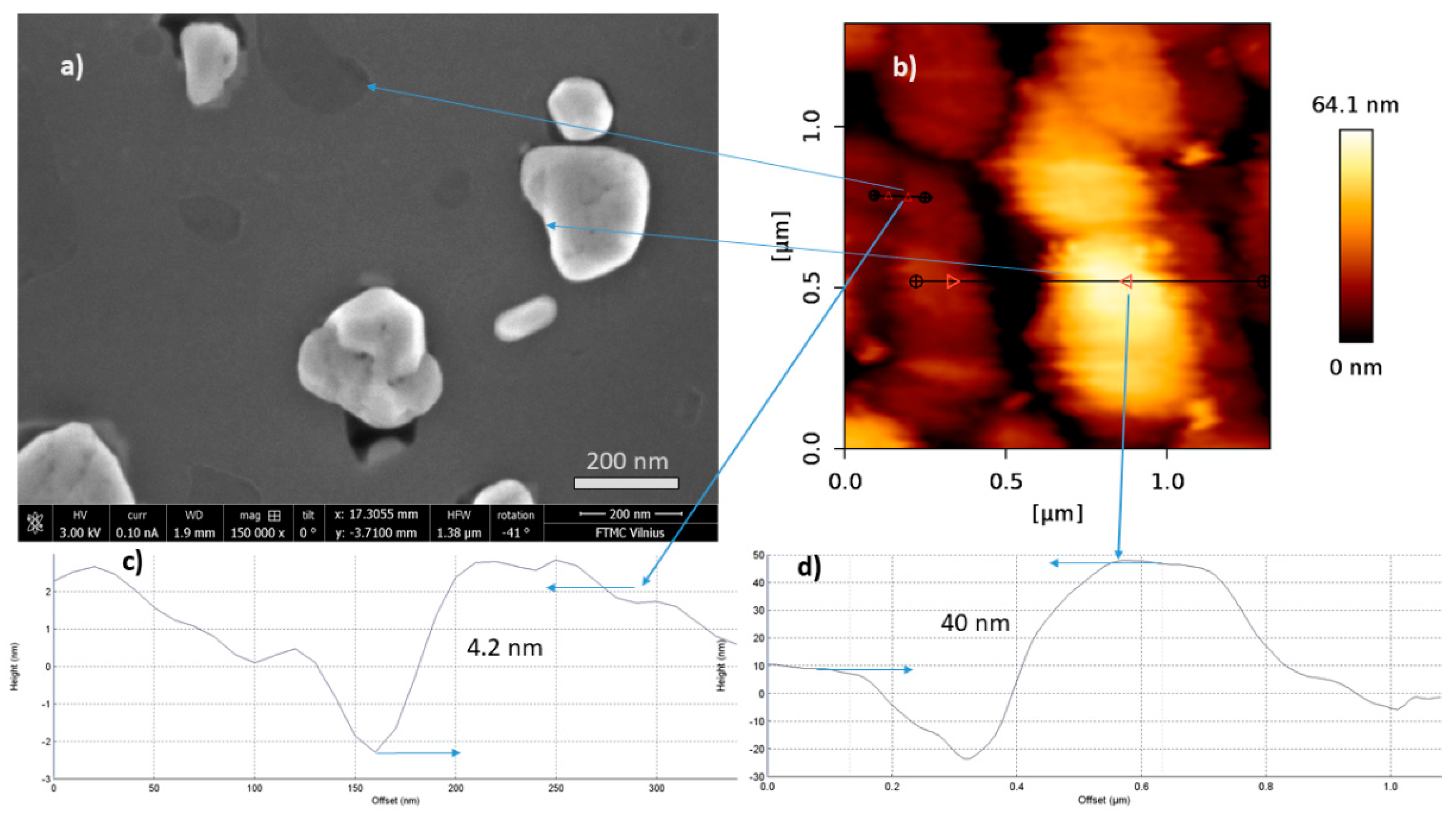
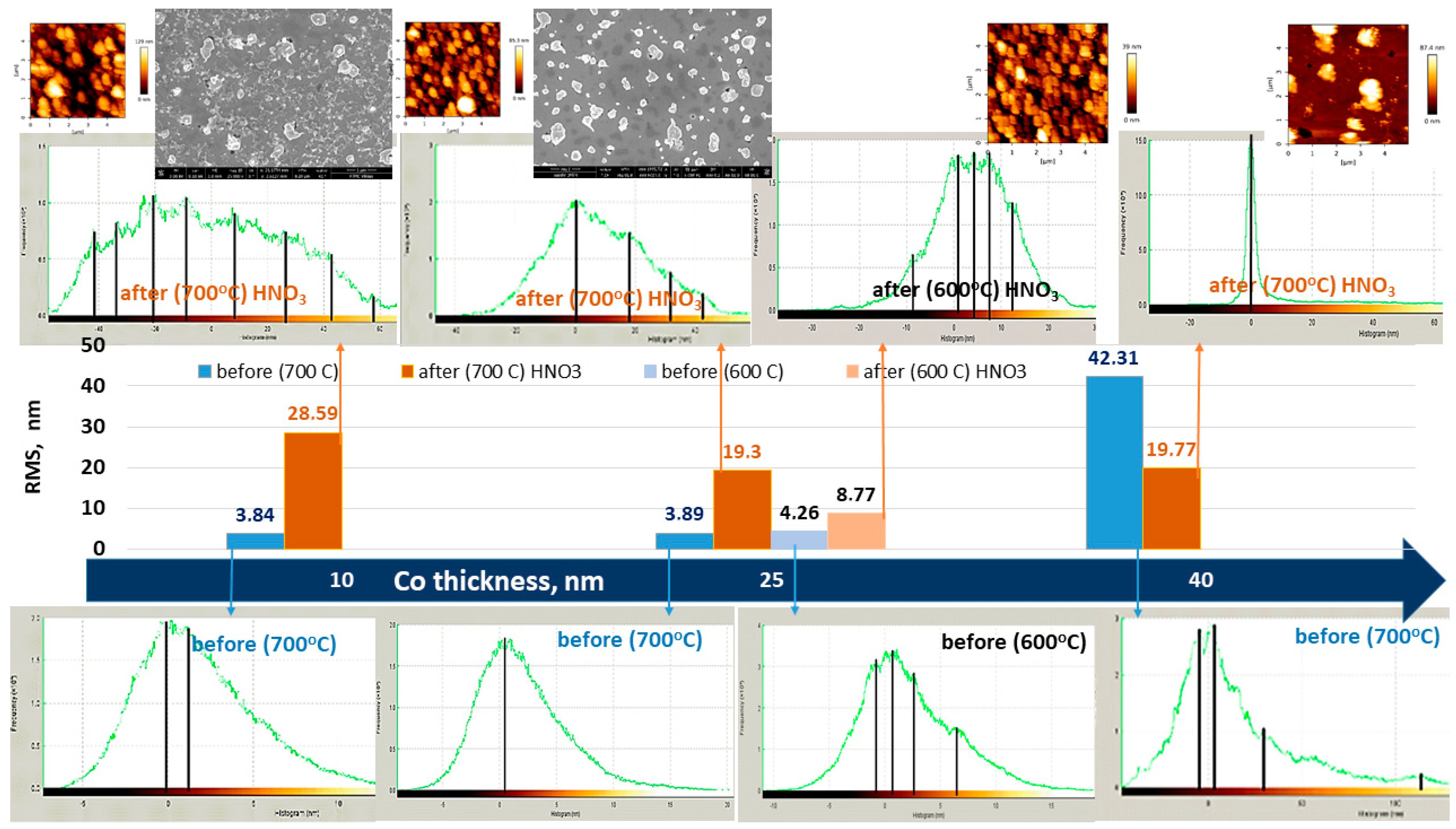
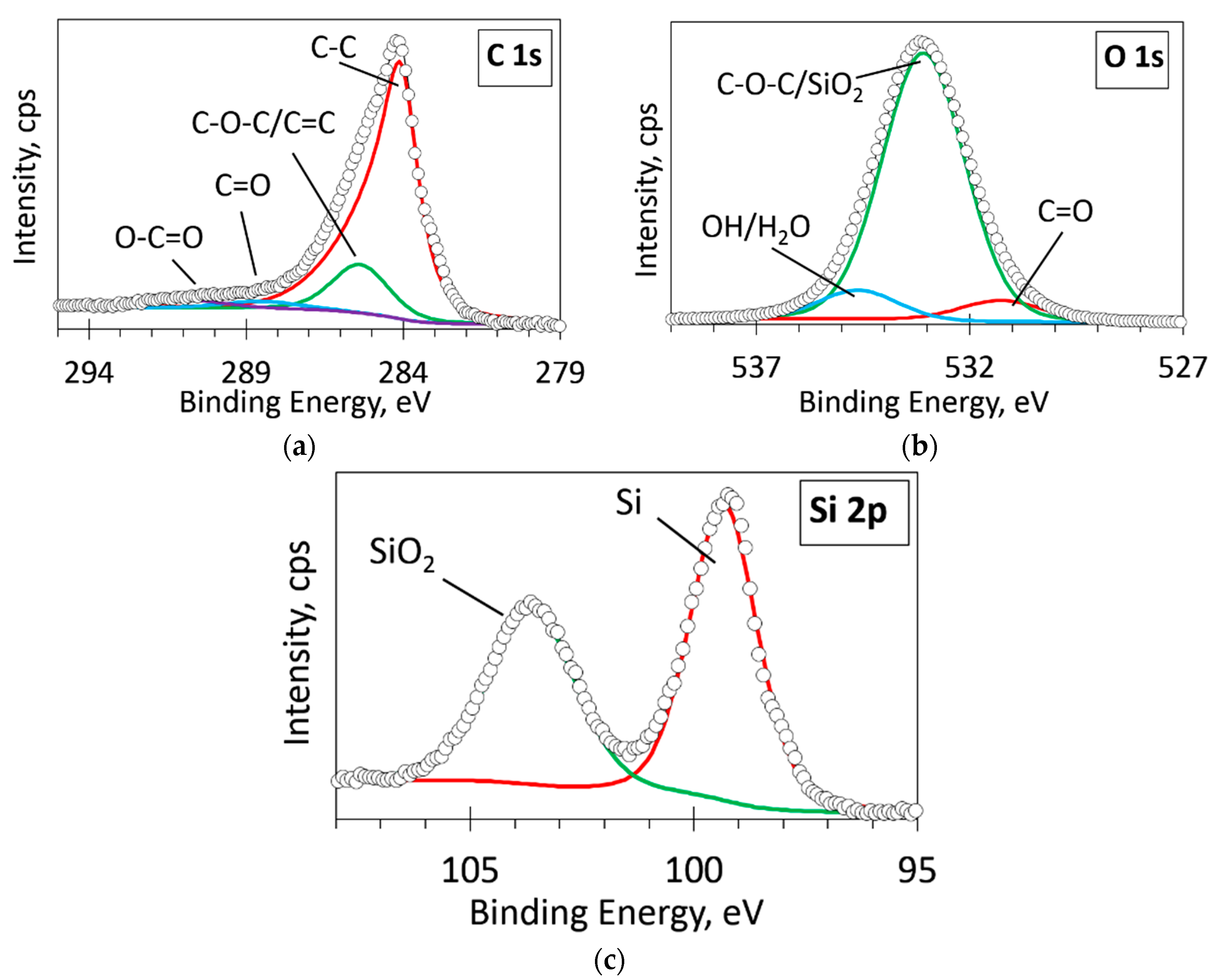
| Sample No. | Co, nm | Temperature during the Ion Beam Irradiation, °C | Annealing Temperature, °C | Ion Beam Energy, eV | t, min |
|---|---|---|---|---|---|
| 1 | 25 | 700 | - | 800 | 15 |
| 2 | 25 | 800 | - | 800 | 15 |
| 3 | 25 | 600 | - | 800 | 15 |
| 4 | 25 | 700 | - | 500 | 15 |
| 5 | 25 | 700 | - | 300 | 15 |
| 8 | 10 | 600 | - | 800 | 15 |
| 9 | 55 | 600 | - | 800 | 15 |
| 10 | 40 | 600 | - | 800 | 15 |
| 11 | 25 | 400 | 700 | 800 | 15 |
| 12 | 10 | 400 | 700 | 800 | 15 |
| 13 | 40 | 400 | 700 | 800 | 15 |
| 14 | 30 | 400 | 700 | 800 | 15 |
| 15 | 40 | 400 | 600 | 800 | 15 |
| 16 | 40 | 400 | 500 | 800 | 15 |
| 17 | 40 | 400 | - | 800 | 15 |
| 18 | 40 | 400 | 550 | 800 | 15 |
| Peak | Atomic Concentration (%) |
|---|---|
| O 1s | 36.71 |
| C 1s | 42.77 |
| Si 2p | 20.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bener, G.; Kopustinskas, V.; Guobienė, A.; Vasiliauskas, A.; Andrulevičius, M.; Meškinis, Š. Cobalt-Activated Transfer-Free Synthesis of the Graphene on Si(100) by Anode Layer Ion Source. Processes 2022, 10, 272. https://doi.org/10.3390/pr10020272
Bener G, Kopustinskas V, Guobienė A, Vasiliauskas A, Andrulevičius M, Meškinis Š. Cobalt-Activated Transfer-Free Synthesis of the Graphene on Si(100) by Anode Layer Ion Source. Processes. 2022; 10(2):272. https://doi.org/10.3390/pr10020272
Chicago/Turabian StyleBener, Greta, Vitoldas Kopustinskas, Asta Guobienė, Andrius Vasiliauskas, Mindaugas Andrulevičius, and Šarūnas Meškinis. 2022. "Cobalt-Activated Transfer-Free Synthesis of the Graphene on Si(100) by Anode Layer Ion Source" Processes 10, no. 2: 272. https://doi.org/10.3390/pr10020272
APA StyleBener, G., Kopustinskas, V., Guobienė, A., Vasiliauskas, A., Andrulevičius, M., & Meškinis, Š. (2022). Cobalt-Activated Transfer-Free Synthesis of the Graphene on Si(100) by Anode Layer Ion Source. Processes, 10(2), 272. https://doi.org/10.3390/pr10020272






