Acetalization of Alkyl Alcohols with Benzaldehyde over Cesium Phosphomolybdovanadate Salts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of the Cs3PMo12O40 Acid
2.3. Synthesis of the Cs4PMo11VO40 Salt
2.4. Synthesis of the Cs5Pmo10V2O40 Salt
2.5. Synthesis of the Cs6PMo9V3O40 Salt
2.6. Catalysts Characterization
2.7. Identification of Main Reaction Products
2.8. Catalytic Tests
3. Results
3.1. Catalyst Characterization
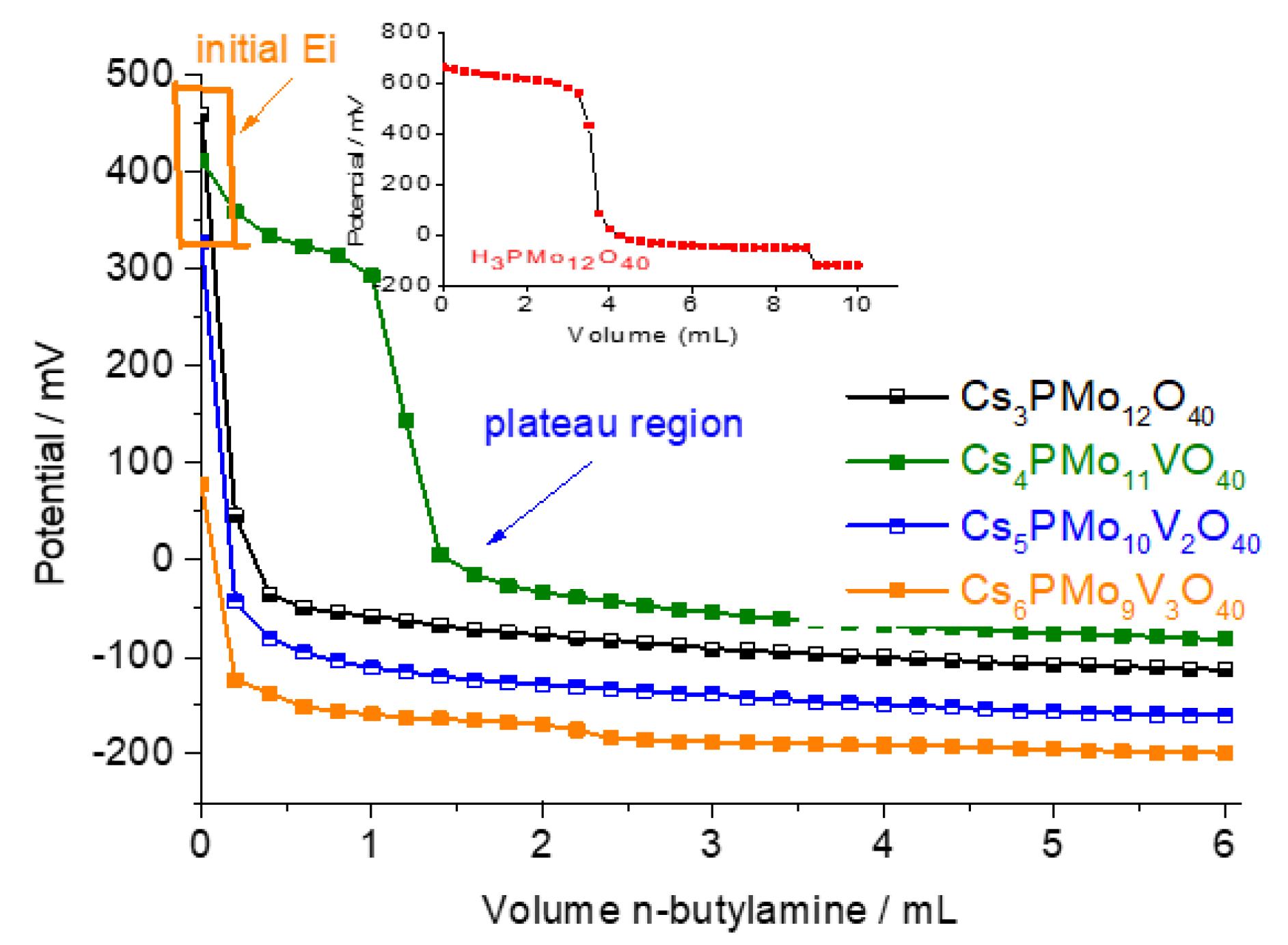
3.1.1. Measurements of Acid Site Strength
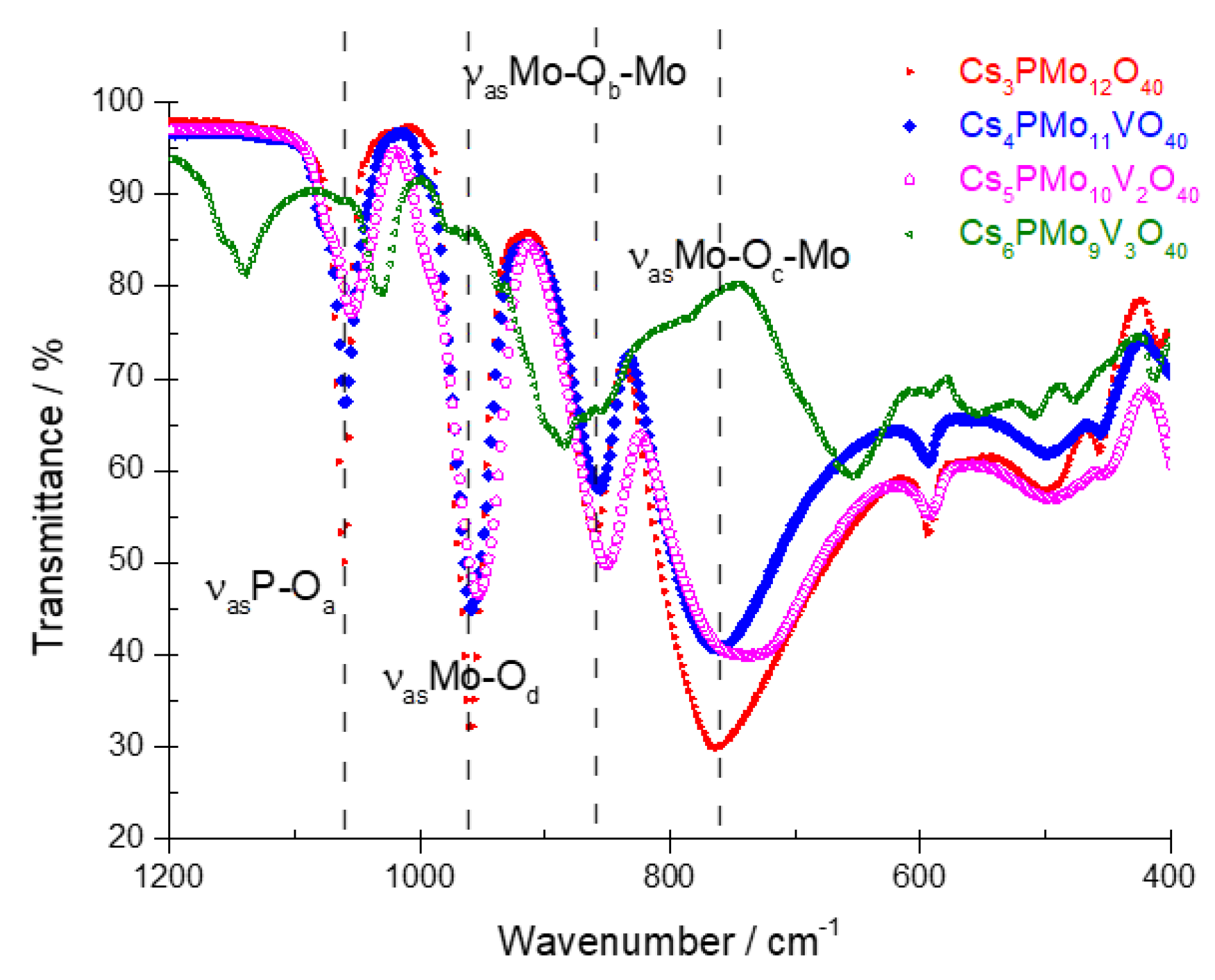
3.1.2. Infrared Spectroscopy
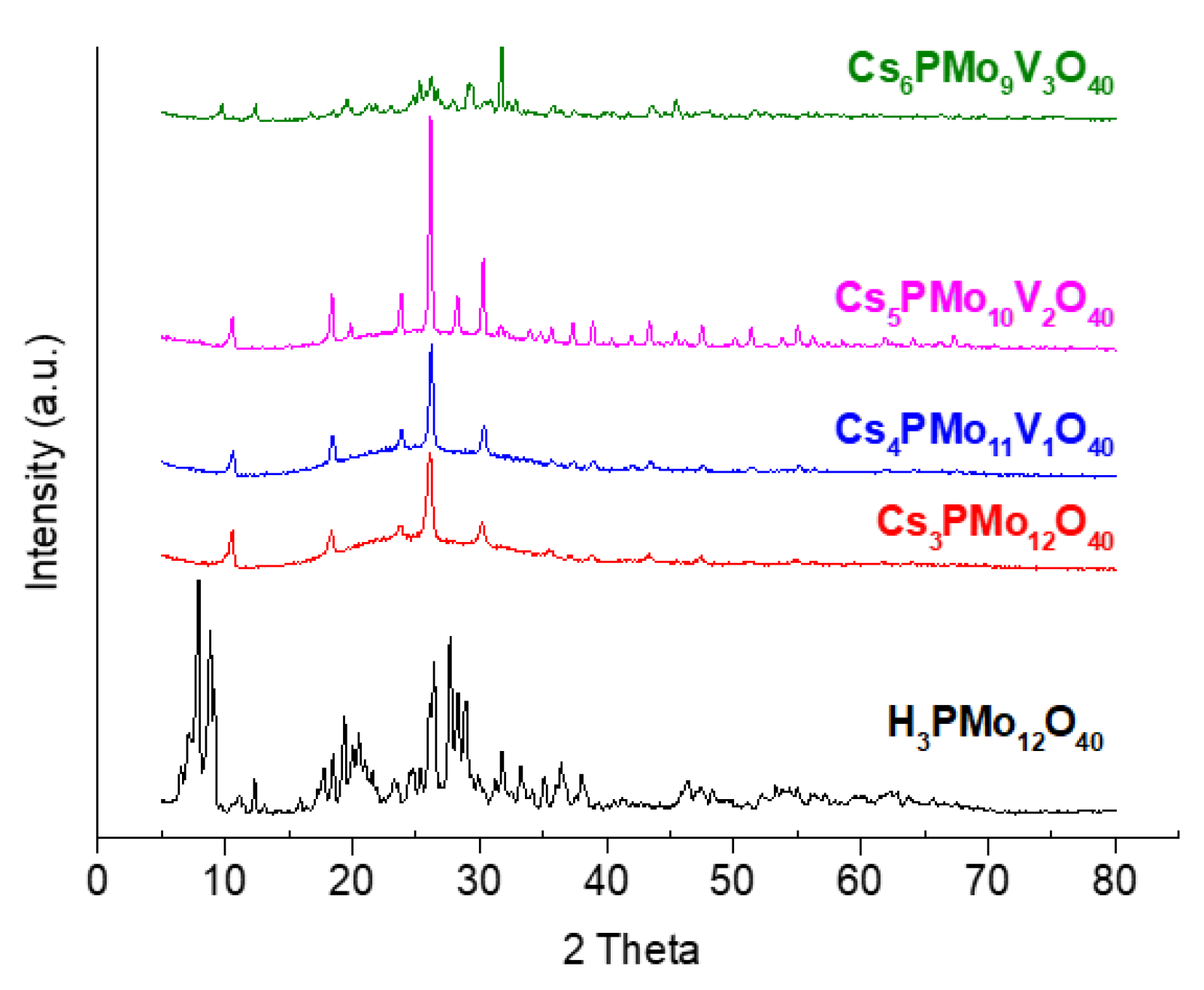
3.1.3. Powder XRD Patterns
3.1.4. Thermal Analyses
3.1.5. Surface Area and Porosimetry Properties
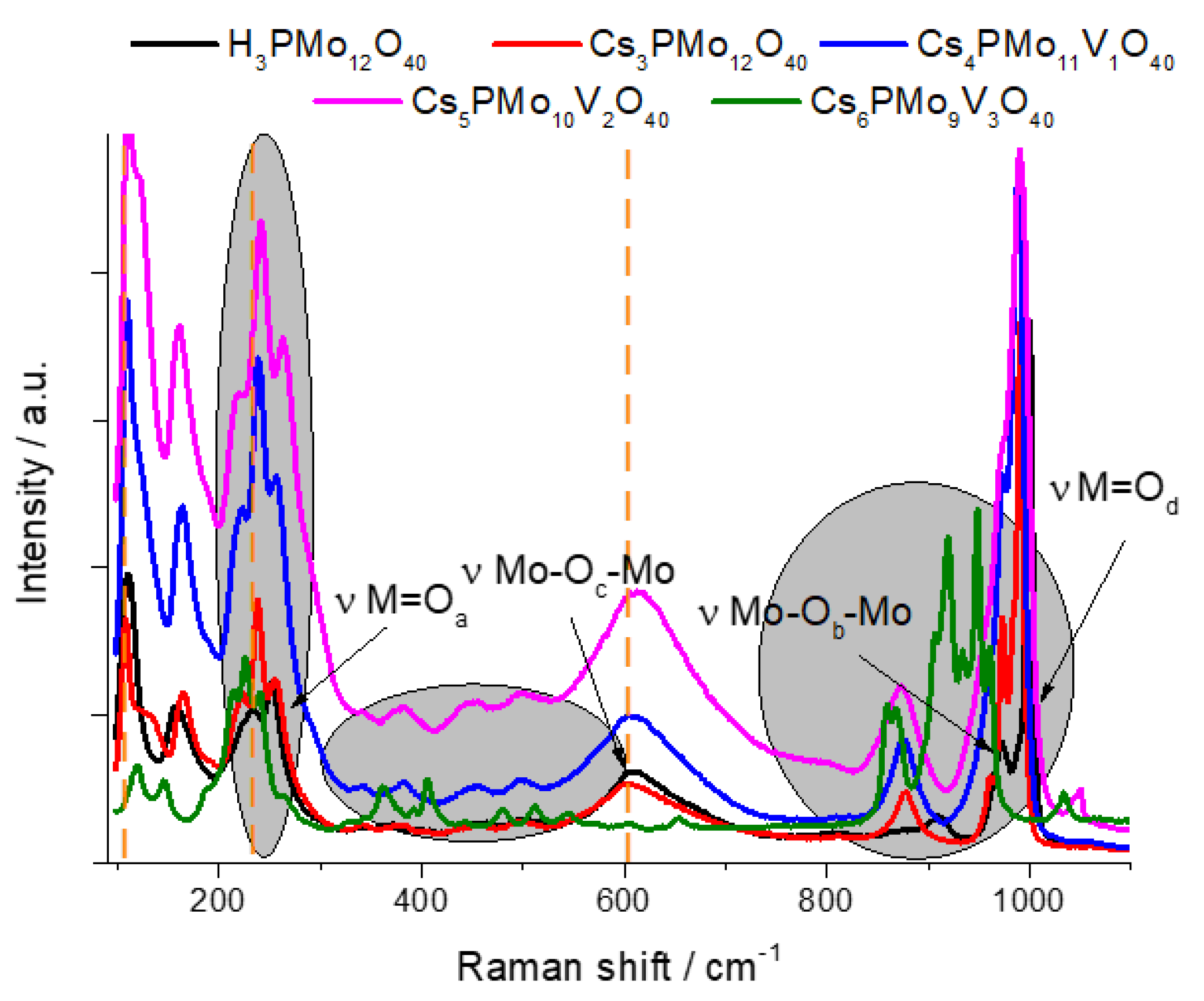
3.1.6. Raman Spectroscopy
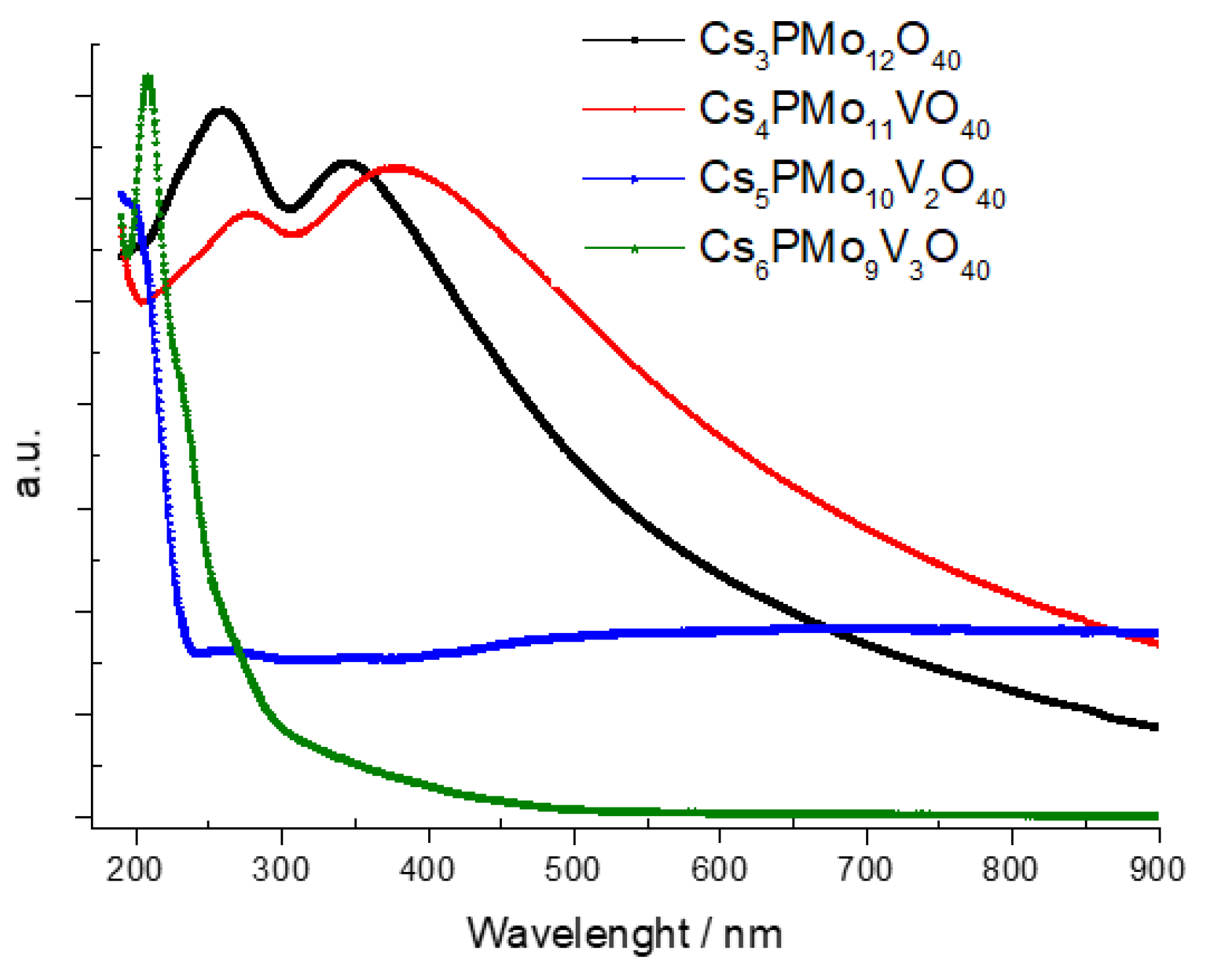
3.1.7. Ultraviolet–Visible Spectroscopy
3.2. Catalytic Tests
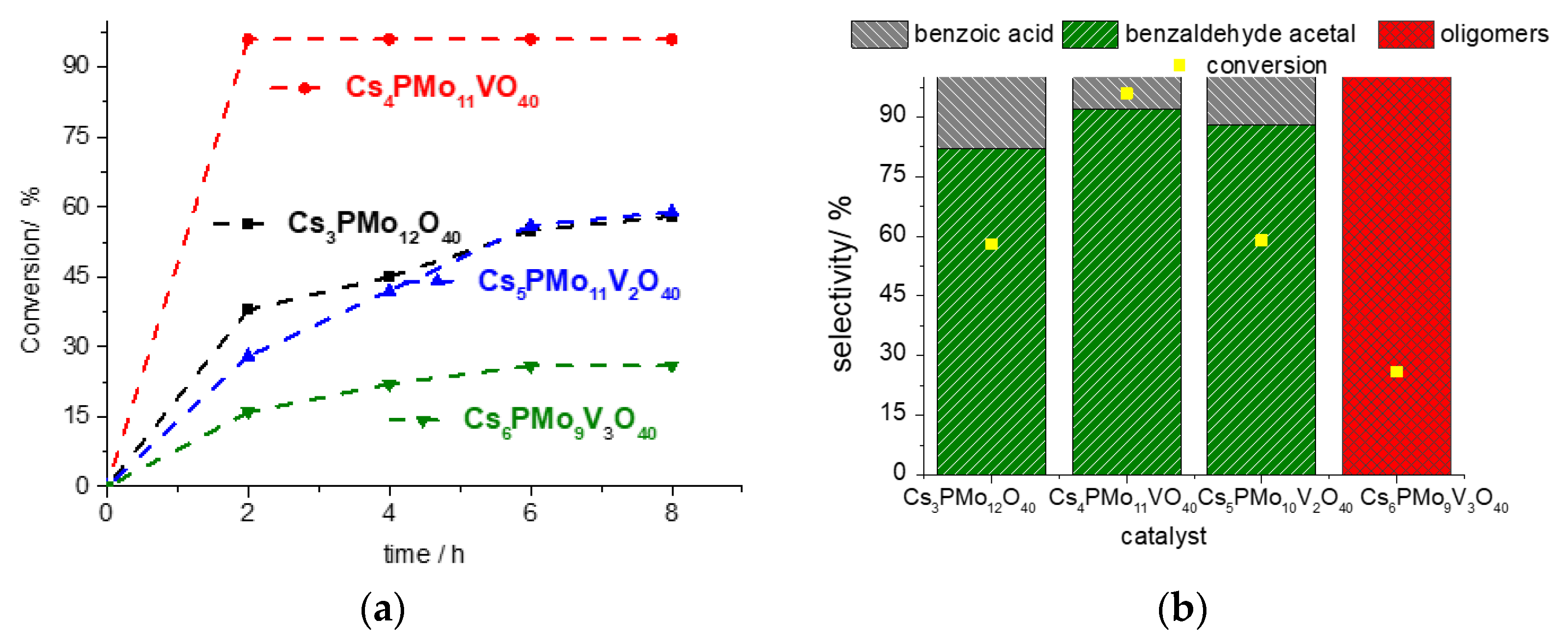
3.2.1. Effect of Vanadium Load on the Phosphomolybdic Acid Salt-Catalyzed Acetalization of Benzaldehyde
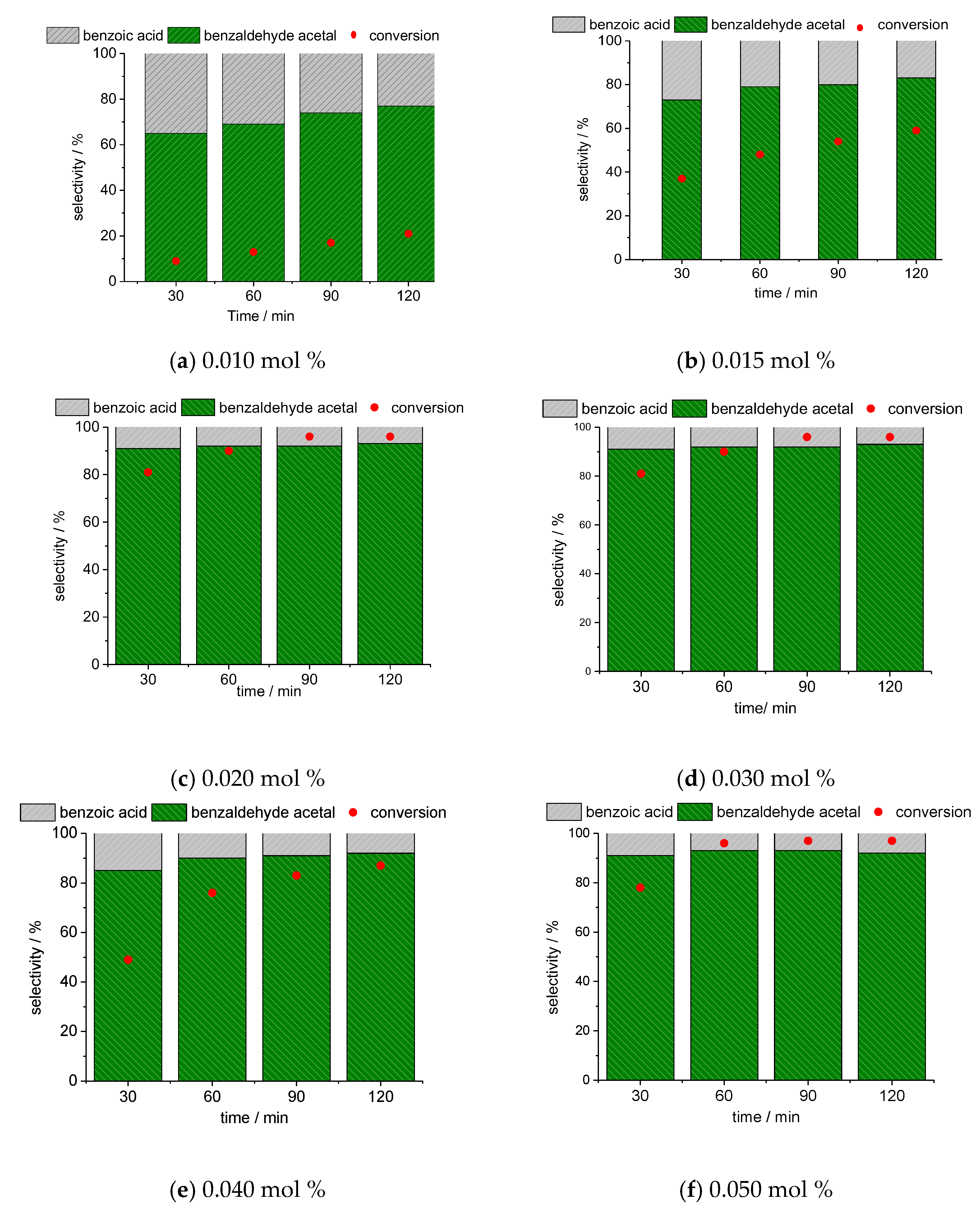
3.2.2. Impacts of Cs4PMo11VO40 Load on the Benzaldehyde Acetalization
3.2.3. Effects of Temperature on the Cs4PMo11VO40-Catalyzed Benzaldehyde Acetalization
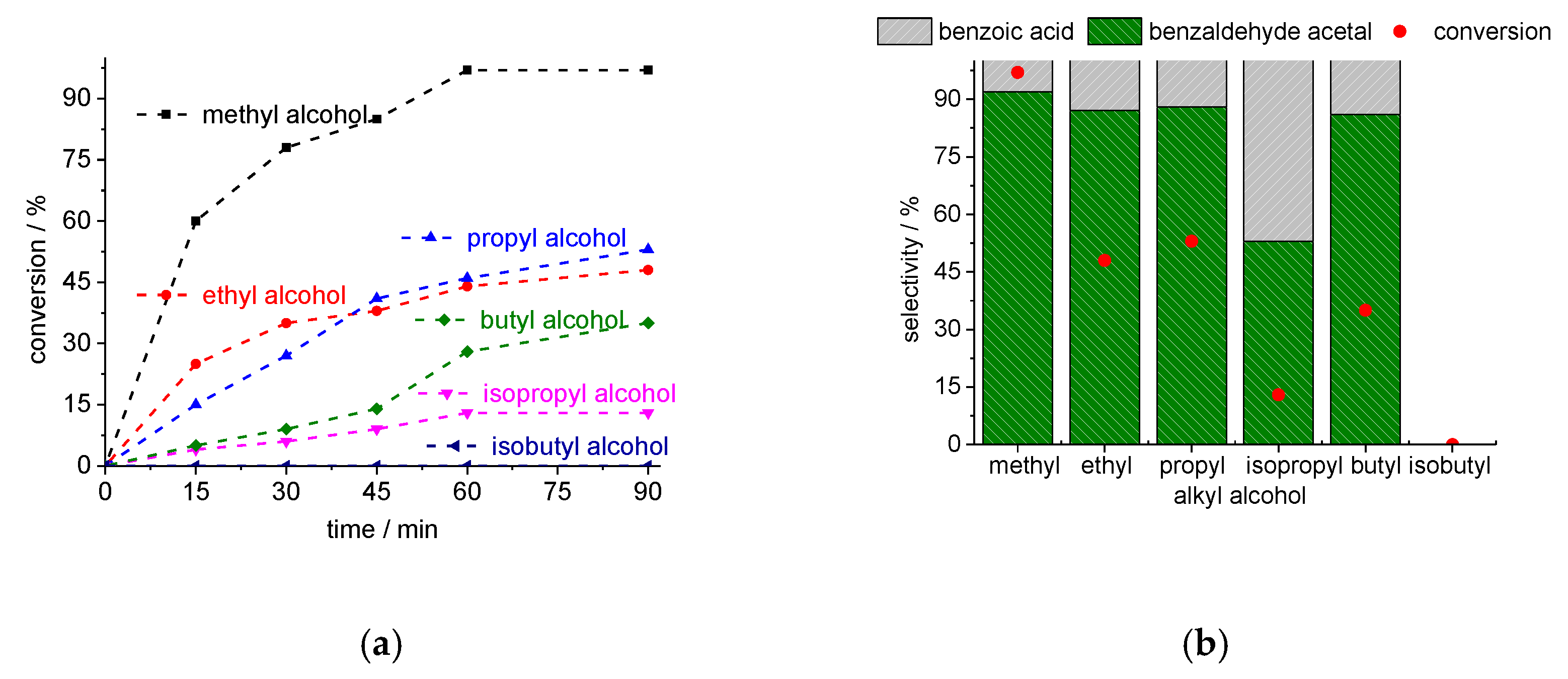
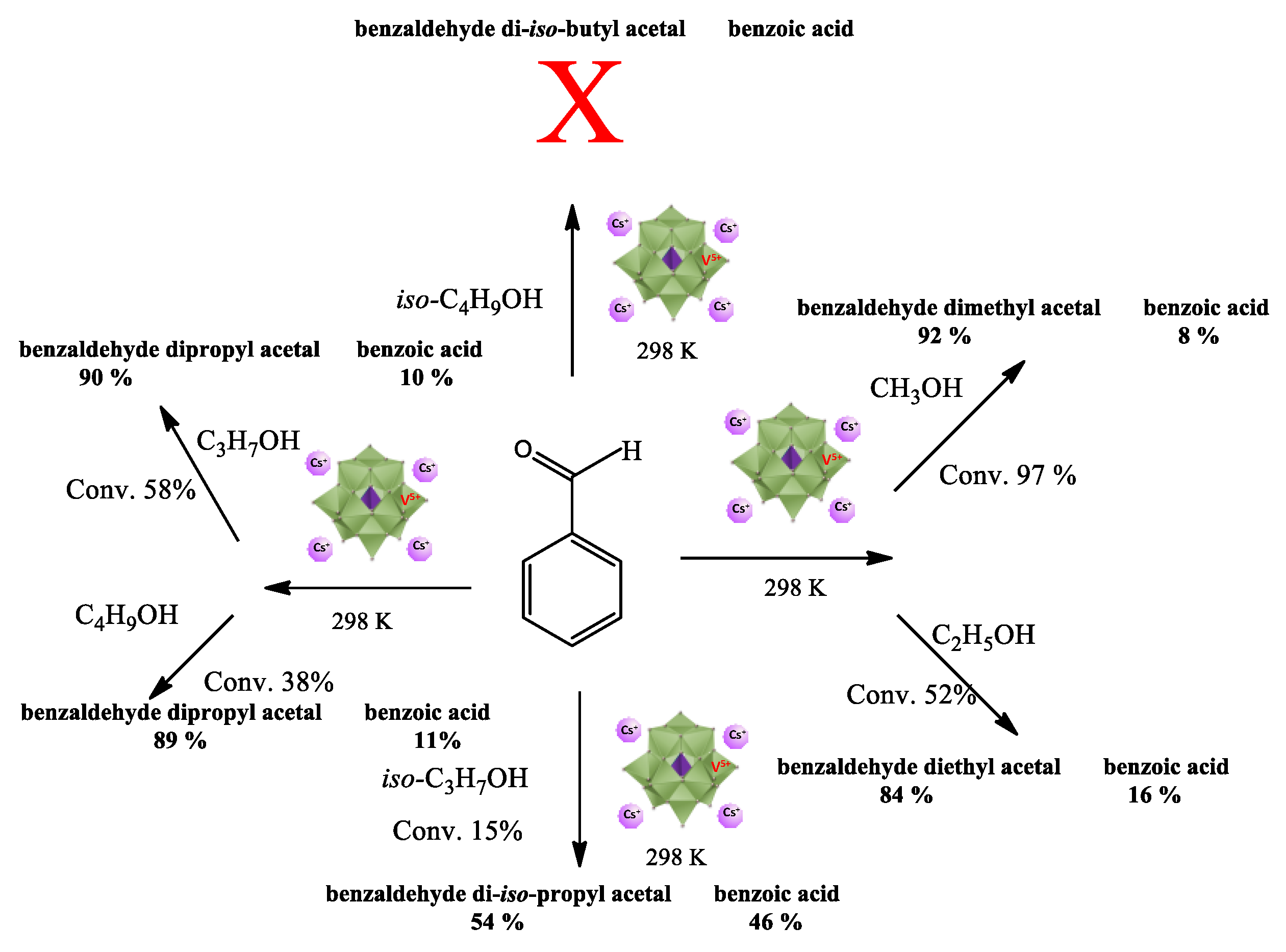
3.2.4. Impacts of Alcohol on the Cs4PMo11VO40-Catalyzed Benzaldehyde Acetalization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, J.L.; Yu, L.S.H.; Xie, J.W. A simple and versatile method for the formation of acetals/ketals using trace conventional acids. ACS Omega 2018, 3, 4974–4985. [Google Scholar] [CrossRef]
- Tsolakis, N.; Bam, W.; Srai, J.S.; Kumar, M. Renewable chemical feedstock supply network design: The case of terpenes. J. Clean. Prod. 2019, 222, 802–822. [Google Scholar] [CrossRef]
- Wu, L.; Moteki, T.; Gokhale, A.A.; Flaherty, D.W.; Toste, F.D. Production of fuels and chemicals from biomass: Condensation reactions and beyond. Chem 2016, 1, 32–58. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, L.M.; Thomas, H.J.; Climent, M.J.; Romanelli, G.P.; Iborra, S. Heteropolycompounds as catalysts for biomass product transformations. Catal. Rev. 2016, 58, 497–586. [Google Scholar] [CrossRef]
- Clark, J.H. Solid acids for green chemistry. Acc. Chem. Res. 2002, 35, 791–797. [Google Scholar] [CrossRef]
- Li, J.; Liang, X. Magnetic solid acid catalyst for biodiesel synthesis from waste oil. Energ. Conv. Manag. 2017, 141, 126–132. [Google Scholar] [CrossRef]
- Lotero, E.; Liu, Y.; Lopes, D.E.; Suwannakarn, K.; Bruce, D.A.; Goodwin, J.G. Synthesis of biodiesel via acid catalysis. Ind. Eng. Chem. Res. 2005, 44, 5353–5363. [Google Scholar] [CrossRef]
- Hamada, N.; Kazahaya, K.; Shimizu, H.; Sato, T. An efficient and versatile procedure for the synthesis of acetals from aldehydes and ketones catalyzed by lithium tetrafluoroborate. Synlett 2004, 6, 1074–1076. [Google Scholar] [CrossRef]
- Corma, A.; Garcia, H. Lewis Acids: From conventional homogeneous to green homogeneous and heterogeneous catalysis. Chem. Rev. 2003, 103, 4307–4366. [Google Scholar] [CrossRef]
- Wegenhart, B.L.; Liu, S.; Thom, M.; Stanley, D.; Abu-Omar, M.M. Solvent-free methods for making acetals derived from glycerol and furfural and their use as a biodiesel fuel component. ACS Catal. 2012, 2, 2524–2530. [Google Scholar] [CrossRef]
- Umbarkar, S.B.; Kotbagi, T.V.; Biradar, A.V.; Parsicha, R.; Chanale, J.; Dongare, M.K.; Mamede, A.S.; Lancelot, C.; Payen, E. Acetalization of glycerol using mesoporous MoO3/SiO2 solid acid catalyst. J. Mol. Catal. A 2009, 310, 150–158. [Google Scholar] [CrossRef]
- Liu, T.; Fu, W.; Zheng, X.; Jiang, J.; Hu, M.; Tang, T. Zeolite Nanofiber Assemblies as Acid Catalysts with High Activity for the Acetalization of Carbonyl Compounds with Alcohols. RSC Adv. 2014, 4, 18217–18221. [Google Scholar] [CrossRef]
- Poyraz, A.S.; Kuo, C.-H.; Kim, E.; Meng, Y.; Seraji, M.S.; Suib, S.L. Tungsten-Promoted Mesoporous Group 4 (Ti, Zr, and Hf) Transition-Metal Oxides for Room-Temperature Solvent-Free Acetalization and Ketalization Reactions. Chem. Mater. 2014, 26, 2803–2813. [Google Scholar] [CrossRef]
- Myles, L.; Gore, R.G.; Gathergood, N.; Connon, S.J. A New Generation of Aprotic Yet Brønsted Acidic Imidazolium Salts: Low Toxicity, High Recyclability and Greatly Improved Activity. Green Chem. 2013, 15, 2740–2746. [Google Scholar] [CrossRef] [Green Version]
- da Silva, M.J.; Liberto, N.A. Soluble and solid supported Keggin heteropolyacids as catalysts in reactions for biodiesel production: Challenges and recent advances. Curr. Org. Chem. 2016, 20, 1263–1283. [Google Scholar] [CrossRef]
- da Silva, M.J.; de Oliveira, C.M. Catalysis by Keggin heteropolyacid salts. Curr. Catal. 2018, 7, 26–34. [Google Scholar] [CrossRef]
- Coronel, N.C.; da Silva, M.J. Lacunar Keggin heteropolyacid salts: Soluble, solid and solid-supported catalysts. J. Clust. Sci. 2018, 29, 195–205. [Google Scholar] [CrossRef]
- da Silva, M.J.; Liberto, N.A.; Leles, L.C.A.; Pereira, U.A. Fe4(SiW12O40)3-catalyzed glycerol acetylation: Synthesis of bioadditives by using highly active Lewis acid catalyst. J. Mol. Catal. A 2019, 422, 69–83. [Google Scholar] [CrossRef]
- Vilanculo, C.B.; da Silva, M.J. Unraveling the role of the lacunar Na7PW11O39 catalyst in the oxidation of terpene alcohols with hydrogen peroxide at room temperature. N. J. Chem. 2020, 44, 2813–2820. [Google Scholar] [CrossRef]
- da Silva, M.J.; Andrade, P.H.S.; Sampaio, V.F.C. Transition metal-substituted potassium silicotungstate salts as catalysts for oxidation of terpene alcohols with hydrogen peroxide. Catal. Lett. 2021, 151, 2094–2106. [Google Scholar] [CrossRef]
- Vilanculo, C.B.; da Silva, M.J. Na4PMo11VO40-catalyzed one-pot oxidative esterification of benzaldehyde with hydrogen peroxide. RSC Adv. 2021, 11, 34979–34987. [Google Scholar] [CrossRef]
- Diaz, J.; Pizzio, L.R.; Pecchi, G.; Campos, C.H.; Azócar, L.; Briones, R.; Romero, R.; Henríquez, A.; Gaigneaux, E.M.; Contreras, D. Tetrabutyl ammonium salts of Keggin-type vanadium-substituted phosphomolybdates and phosphotungstates for selective aerobic catalytic oxidation of benzyl alcohol. Catalysts 2022, 12, 507. [Google Scholar] [CrossRef]
- Patel, A.; Patel, J. Nickel salt of phosphomolybdic acid as a bi-functional homogeneous recyclable catalyst for base free transformation of an aldehyde into ester. RSC Adv. 2020, 10, 22146–22155. [Google Scholar] [CrossRef]
- Okuhara, T. Water-tolerant solid acid catalysts. Chem. Rev. 2002, 102, 3641–3666. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.S.; Lima, S.; Pillinger, M.; Valente, A.A. Acidic cesium salts of 12-tungstophosphoric acid as catalysts for the dehydration of xylose into furfural. Carbohydr. Res. 2006, 341, 2946–2953. [Google Scholar] [CrossRef]
- Barteau, K.P.; Lyons, J.E.; Song, I.K.; Barteau, M.A. UV–visible spectroscopy as a probe of heteropolyacid redox properties: Application to liquid phase oxidations. Top. Catal. 2006, 41, 55–62. [Google Scholar] [CrossRef]
- Da Silva, M.J.; Lopes, N.P.G.; Ferreira, S.O.; Da Silva, R.C.; Natalino, R.; Chaves, D.M.; Texeira, M.G. Monoterpenes etherification reactions with alkyl alcohols over cesium partially exchanged Keggin heteropoly salts: Effects of the catalyst composition. Chem. Pap. 2021, 75, 153–168. [Google Scholar] [CrossRef]
- Batalha, D.C.; Ferreira, S.O.; Da Silva, R.C.; Da Silva, M.J. Cesium-exchanged lacunar Keggin heteropolyacid salts: Efficient solid catalysts for the green oxidation of terpenic alcohols with hydrogen peroxide. Chem. Select 2020, 5, 1976–1986. [Google Scholar] [CrossRef]
- Chaves, D.M.; Ferreira, S.O.; Chagas da Silva, R.; Natalino, R.; da Silva, M.J. Glycerol esterification over Sn(II)-exchanged Keggin heteropoly salt catalysts: Effect of thermal treatment temperature. Energ. Fuels 2019, 33, 7705–7716. [Google Scholar] [CrossRef]
- da Silva, M.J.; Chaves, D.M.; Ferreira, S.O.; da Silva, R.C.; Filho, J.B.G.; Bruziquesi, C.G.O.; Al-Rabiah, A.A. Impacts of Sn(II) doping on the Keggin heteropolyacid-catalyzed etherification of glycerol with tert-butyl alcohol. Chem. Eng. Sci. 2022, 247, 116913. [Google Scholar] [CrossRef]
- da Silva, M.J.; Chaves, D.M.; Julio, A.A.; Rodrigues, F.A.; Bruziques, C.G.; da Silva, M.J. Sn(II)-exchanged Keggin silicotungstic acid-catalyzed etherification of glycerol and ethylene glycol with alkyl alcohols. Ind. Eng. Chem. Res. 2020, 59, 9858–9868. [Google Scholar] [CrossRef]
- da Silva, M.J.; Julio, A.A.; Ferreira, S.O.; da Silva, R.C.; Chaves, D.M. Tin(II) phosphotungstate heteropoly salt: An efficient solid catalyst to synthesize bioadditives ethers from glycerol. Fuel 2019, 254, 115607. [Google Scholar] [CrossRef]
- Pinheiro, P.F.; Chaves, D.M.; da Silva, M.J. One-pot synthesis of alkyl levulinates from biomass derivative carbohydrates in tin(II) exchanged silicotungstates-catalyzed reactions. Cellulose 2019, 26, 7953–7969. [Google Scholar] [CrossRef]
- Vilanculo, C.B.; Da Silva, M.J.; Rodrigues, A.A.; Ferreira, S.O.; Da Silva, R.C. Vanadium-doped sodium phosphomolybdate salts as catalysts in the terpene alcohols oxidation with hydrogen peroxide. RSC Adv. 2021, 11, 24072–24085. [Google Scholar] [CrossRef] [PubMed]
- Vilanculo, C.B.; da Silva, M.J.; Torres, J.A.V. Vanadium-doped phosphomolybdic acids as catalysts for geraniol oxidation with hydrogen peroxide. RSC Adv. 2022, 12, 11796–11806. [Google Scholar] [CrossRef]
- da Silva, M.J.; Ribeiro, C.J.A.; Vilanculo, C.B. How the content of protons and vanadium affects the activity of H3+nPMo12-nVnO40 (n = 0, 1, 2, or 3) catalysts on the oxidative esterification of benzaldehyde with hydrogen peroxide. Catal. Lett. 2023, 153, 2045–2056. [Google Scholar] [CrossRef]
- Tsigdinos, G.A.; Hallada, C. Molybdovanadophosphoric acids and their salts. I. Investigation of methods of preparation and characterization. J. Inorg. Chem. 1968, 7, 437–441. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Li, H.-X.; Davis, M.E. Studies on mesoporous materials: I. Synthesis and characterization of MCM-41. Micropor. Mat. 1993, 2, 17–26. [Google Scholar] [CrossRef]
- Jing, F.; Katryniok, B.; Dumeignil, F.; Bordes-Richard, E. Catalytic selective oxidation of isobutane to methacrylic acid on supported (NH4)3HPMo11VO40 catalysts. J. Catal. 2014, 309, 121–135. [Google Scholar] [CrossRef]
- Pizzio, L.R.; Blanco, M.N. A contribution to the physicochemical characterization of nonstoichiometric salts of tungstosilicic acid. Micropor. Mesopor. Mat. 2007, 103, 40–47. [Google Scholar] [CrossRef]
- Serwicka, E.M.; Bruckman, K.; Haber, J.; Paukshtis, E.A.; Yurchenko, E.A. Acid-base properties of H3+nPVnMo12-nO40 heteropolyacids, pure and supported on K3PMo12O40. Appl. Catal. 1991, 73, 153–163. [Google Scholar] [CrossRef]
- Villabrille, P.; Romanelli, G.; Vázquez, P.; Cáceres, C. Vanadium-substituted Keggin heteropolycompounds as catalysts for eco-friendly liquid phase oxidation of 2,6-dimethylphenol to 2,6-dimethyl-1,4-benzoquinone. Appl. Catal. A 2004, 270, 101–111. [Google Scholar] [CrossRef]
- da Silva, M.J.; Rodrigues, A.A.; Teixeira, M.G. Iron (III) Silicotungstate: An efficient and recyclable catalyst for converting glycerol to solketal. Energ. Fuels 2020, 34, 9664–9673. [Google Scholar] [CrossRef]
- Viswanadham, B.; Jhansi, P.; Chary, K.V.R.; Friedrich, H.B.; Singh, S. Efficient solvent-free Knoevenagel condensation over vanadium containing heteropolyacid catalysts. Catal. Lett. 2016, 146, 364–371. [Google Scholar] [CrossRef]
- Li, X.-K.; Zhao, J.; Ji, W.J.; Zhang, Z.-B.; Chen, Y.; Au, C.-T.; Han, S.; Hibst, H. Effect of vanadium substitution in the cesium salts of Keggin-type heteropolyacids on propane partial oxidation. J. Catal. 2006, 237, 58–66. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, T.; Ma, X.; Zhu, W. Composition, structural evolution and the related property variations in preparation of mixed cesium/ammonium acidic salts of heteropolyacids. Catalysts 2016, 6, 187. [Google Scholar] [CrossRef] [Green Version]
- Rocchiccioli-Deltcheff, C.; Fournier, M. Catalysis by polyoxometalates. Part 3.-Influence of vanadium(V) on the thermal stability of 12-metaphosphoric acids from in situ infrared studies. J. Chem. Soc. Faraday Trans. 1991, 87, 3913–3920. [Google Scholar] [CrossRef]
- Casarini, D.; Centi, G.; Jiru, P.; Lena, V.; Tvaruzkova, Z. Reactivity of molybdovanadophosphoric Acids: Influence of the presence of vanadium in the primary and secondary structure. J. Catal. 1993, 142, 325–344. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, J.; Cao, C.; Zhang, Q.; Wang, Y.; Wan, H. Significant effect of acidity on catalytic behaviours of Cs-substituted polyoxometalates for oxidative dehydrogenation of propane. Appl. Catal. A 2008, 349, 212–221. [Google Scholar] [CrossRef]
- Khillare, K.R.; Aher, D.S.; Chavan, L.D.; Shankarwar, S.G. Cesium salt of 2-molybdo-10-tungstophosphoric acid as an efficient and reusable catalyst for the synthesis of uracil derivatives via a green route. RSC Adv. 2021, 11, 33980–33989. [Google Scholar] [CrossRef] [PubMed]
- Sen, R.; Bera, R.; Bhattacharjee, A.; Gütlich, P.; Ghosh, S.; Mukherjee, A.K.; Koner, S. Oxo-Vanadium(IV) dihydrogen phosphate: Preparation, magnetic study, and heterogeneous catalytic epoxidation. Langmuir 2008, 24, 5970–5975. [Google Scholar] [CrossRef] [PubMed]
- Balaraju, M.; Rekha, V.; Davi, B.L.A.P.; Prasad, R.B.N.; Prasad, P.S.S.; Lingaiah, N. Surface and structural properties of titania-supported Ru catalysts for hydrogenolysis of glycerol. Appl. Catal. A 2010, 384, 107–114. [Google Scholar] [CrossRef]
- Tan, Z.; Dong, L.; Huang, Z.; Du, L.; Wang, X. A theoretical study on the selective adsorption of NH4+ and Cs+ on the phosphomolybdate ion. Colloids surf. A: Physicochem. Engin. Asp. 2016, 502, 74–80. [Google Scholar] [CrossRef]
- Okuhara, T.; Watanabe, H.; Nishimura, T.; Inumarau, K.; Misono, M. Microstructure of cesium hydrogen salts of 12-tungstophosphoric acid relevant to novel acid catalysis. Chem. Mater. 2000, 12, 2230–2238. [Google Scholar] [CrossRef]
- Narasimharao, K.; Brown, D.R.; Lee, A.F.; Newman, A.D.; Siril, P.F.; Tavener, S.J.; Wilson, K. Structure–activity relations in Cs-doped heteropolyacid catalysts for biodiesel production. J. Catal. 2007, 248, 226–234. [Google Scholar] [CrossRef]
- Soares, J.C.S.; Golçalves, A.H.A.; Zotin, F.M.Z.; Araújo, L.R.R.; Gaspar, A.B. Cyclohexene to adipic acid synthesis using heterogeneous polyoxometalate catalysts. Mol. Catal. 2018, 458, 223–229. [Google Scholar] [CrossRef]
- Alcañiz-Monge, J.; Trautwein, G.; Garcia-Garcia, A. Influence of peroxometallic intermediaries present on polyoxometalates nanoparticles surface on the adipic acid synthesis. J. Mol. Catal. A 2014, 394, 211–216. [Google Scholar] [CrossRef] [Green Version]
- Predoeva, A.; Damyanova, S.; Gaigneaux, E.M.; Petrov, L. The surface and catalytic properties of titania-supported mixed PMoV heteropoly compounds for total oxidation of chlorobenzene. Appl. Catal. A 2007, 319, 14–24. [Google Scholar] [CrossRef]
- Maestre, J.M.; Lopes, X.; Bo, C.; Poblet, J.-M.; Casoñ-Pastor, N. Electronic and magnetic properties of α-Keggin anions: A DFT study of [XM12O40]n−, (M = W, Mo; X = AlIII, SiIV, PV, FeIII, CoII, CoIII) and [SiM11VO40]m− (M = Mo and W). J. Am. Chem. Soc. 2001, 123, 3749–3758. [Google Scholar] [CrossRef]
- Ali, B.E.; El-Ghanam, A.M.; Fettouhi, M. H3+nPMo12−nVnO40-catalyzed selective oxidation of benzoins to benzils or aldehydes and esters by dioxygen. J. Mol. Catal. A 2001, 165, 283–290. [Google Scholar] [CrossRef]
- Ballarini, N.; Casagrandi, L.; Cavani, F.; D’Alessandro, T.; Frattini, A.; Accorinti, P.; Alini, S.; Babini, P. The liquid-phase oxidation of cyclohexanone with oxygen, catalysed by Keggin-type polyoxometalates. A cleaner alternative to the current industrial process for adipic acid synthesis. In Book of Abstracts Convegno Nazionale Chimica Verde Chimica Sicura; Università degli Studi di Pavia e GI Green Chemist: Pavia, Italy, 2008; pp. 225–232. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, M.J.; Ribeiro, C.J.A.; de Araújo, E.N.; Torteloti, I.M. Acetalization of Alkyl Alcohols with Benzaldehyde over Cesium Phosphomolybdovanadate Salts. Processes 2023, 11, 2220. https://doi.org/10.3390/pr11072220
da Silva MJ, Ribeiro CJA, de Araújo EN, Torteloti IM. Acetalization of Alkyl Alcohols with Benzaldehyde over Cesium Phosphomolybdovanadate Salts. Processes. 2023; 11(7):2220. https://doi.org/10.3390/pr11072220
Chicago/Turabian Styleda Silva, Márcio José, Cláudio Júnior Andrade Ribeiro, Eduardo Nery de Araújo, and Isadora Merighi Torteloti. 2023. "Acetalization of Alkyl Alcohols with Benzaldehyde over Cesium Phosphomolybdovanadate Salts" Processes 11, no. 7: 2220. https://doi.org/10.3390/pr11072220
APA Styleda Silva, M. J., Ribeiro, C. J. A., de Araújo, E. N., & Torteloti, I. M. (2023). Acetalization of Alkyl Alcohols with Benzaldehyde over Cesium Phosphomolybdovanadate Salts. Processes, 11(7), 2220. https://doi.org/10.3390/pr11072220





