Bioactivities of Waste Cork and Phloem Fractions of Quercus cerris Bark
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Preparation of Cork and Phloem Samples
2.3. Preparation of Hydroethanolic Extracts
2.4. UHPLC-DAD-ESI-IT-MS Characterization of Phenolic Compounds
2.5. Antioxidant Activity
2.5.1. Thiobarbituric Acid-Reactive Substances (TBARSs) Assay
2.5.2. Oxidative Hemolysis (OxHLIA) Assay
2.6. Antibacterial Activity
2.7. Antifungal Activity
2.8. Antiproliferative Activity
2.9. Nitric Oxide Production Inhibition
2.10. Statistical Analysis
3. Results
3.1. UHPLC-DAD-ESI-IT-MS Analysis of Phenolic Compounds
3.2. Ex Vivo Antioxidant Activity
3.3. Antibacterial and Antifungal Activity
3.4. Antiproliferative, Hepatotoxicity, and Inhibition of NO Production Activities
4. Discussion
5. Conclusions
- The hydroethanolic maceration of waste cork had higher extract yield than that of phloem.
- Gallic acid glucosides, phenolic acids, and ellagic acid were identified in waste cork and phloem fractions.
- The antioxidant activity of waste cork exceeded that of phloem and was comparable to pure cork.
- Waste cork and phloem extracts may be associated with antiproliferation of gastric, colon, and breast tumor cell lines.
- Phloem extracts did not exhibit hepatotoxicity.
- The bioactivities of waste cork and phloem extracts vary considerably, with distinct antioxidant, antimicrobial, and antiproliferative activities.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sherwood, J.; Clark, J.H.; Farmer, T.J.; Herrero-Davila, L.; Moity, L. Recirculation: A New Concept to Drive Innovation in Sustainable Product Design for Bio-Based Products. Molecules 2016, 22, 48. [Google Scholar] [CrossRef]
- FitzPatrick, M.; Champagne, P.; Cunningham, M.F.; Whitney, R.A. A Biorefinery Processing Perspective: Treatment of Lignocellulosic Materials for the Production of Value-Added Products. Bioresour. Technol. 2010, 101, 8915–8922. [Google Scholar] [CrossRef] [PubMed]
- Şen, U.; Esteves, B.; Pereira, H. Pyrolysis and Extraction of Bark in a Biorefineries Context: A Critical Review. Energies 2023, 16, 4848. [Google Scholar] [CrossRef]
- Trockenbrodt, M. Survey and Discussion of the Terminology Used in Bark Anatomy. IAWA J. 1990, 11, 141–166. [Google Scholar] [CrossRef]
- Angyalossy, V.; Pace, M.R.; Evert, R.F.; Marcati, C.R.; Oskolski, A.A.; Terrazas, T.; Kotina, E.; Lens, F.P.; Mazzoni-Viveiros, S.C.; Angeles, G. IAWA List of Microscopic Bark Features. IAWA J. 2016, 37, 517–615. [Google Scholar] [CrossRef]
- Pereira, H. Cork: Biology, Production and Uses; Elsevier: Amsterdam, Netherlands, 2007; ISBN 978-0-444-52967-1. [Google Scholar]
- Şen, A.; Miranda, I.; Santos, S.; Graça, J.; Pereira, H. The Chemical Composition of Cork and Phloem in the Rhytidome of Quercus cerris Bark. Ind. Crops Prod. 2010, 31, 417–422. [Google Scholar] [CrossRef]
- Giannotas, G.; Kamperidou, V.; Barboutis, I. Tree bark utilization in insulating bio-aggregates: A review. Biofuels Bioprod. Biorefining 2021, 15, 1989–1999. [Google Scholar] [CrossRef]
- Le Normand, M.; Edlund, U.; Holmbom, B.; Ek, M. Hot-Water Extraction and Characterization of Spruce Bark Non-Cellulosic Polysaccharides. Nord. Pulp. Pap. Res. J. 2012, 27, 18–23. [Google Scholar] [CrossRef]
- Le Normand, M.; Moriana, R.; Ek, M. Isolation and Characterization of Cellulose Nanocrystals from Spruce Bark in a Biorefinery Perspective. Carbohydr. Polym. 2014, 111, 979–987. [Google Scholar] [CrossRef]
- Şen, A.; Leite, C.; Lima, L.; Lopes, P.; Pereira, H. Industrial Valorization of Quercus Cerris Bark: Pilot Scale Fractionation. Ind. Crops Prod. 2016, 92, 42–49. [Google Scholar] [CrossRef]
- Vieira, P.G.; de Melo, M.M.R.; Şen, A.; Simões, M.M.Q.; Portugal, I.; Pereira, H.; Silva, C.M. Quercus Cerris Extracts Obtained by Distinct Separation Methods and Solvents: Total and Friedelin Extraction Yields, and Chemical Similarity Analysis by Multidimensional Scaling. Sep. Purif. Technol. 2020, 232, 115924. [Google Scholar] [CrossRef]
- Şen, A.; Miranda, I.; Esteves, B.; Pereira, H. Chemical Characterization, Bioactive and Fuel Properties of Waste Cork and Phloem Fractions from Quercus Cerris L. Bark. Ind. Crops Prod. 2020, 157, 112909. [Google Scholar] [CrossRef]
- Şen, U.; Viegas, C.; Duarte, M.P.; Maurício, E.M.; Nobre, C.; Correia, R.; Pereira, H.; Gonçalves, M. Maceration of Waste Cork in Binary Hydrophilic Solvents for the Production of Functional Extracts. Environments 2023, 10, 142. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Finimundy, T.C.; Polyzos, N.; Pinela, J.; Ivanov, M.; Soković, M.; Ferreira, I.C.F.R.; Barros, L. The Bioactivities and Chemical Profile of Turnip-Rooted Parsley Germplasm. Horticulturae 2022, 8, 639. [Google Scholar] [CrossRef]
- Lockowandt, L.; Pinela, J.; Roriz, C.L.; Pereira, C.; Abreu, R.M.V.; Calhelha, R.C.; Alves, M.J.; Barros, L.; Bredol, M.; Ferreira, I.C.F.R. Chemical Features and Bioactivities of Cornflower (Centaurea Cyanus L.) Capitula: The Blue Flowers and the Unexplored Non-Edible Part. Ind. Crops Prod. 2019, 128, 496–503. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Edible Flowers as Sources of Phenolic Compounds with Bioactive Potential. Food Res. Int. 2018, 105, 580–588. [Google Scholar] [CrossRef]
- Pires, T.C.; Dias, M.I.; Barros, L.; Alves, M.J.; Oliveira, M.B.P.; Santos-Buelga, C.; Ferreira, I.C. Antioxidant and antimicrobial properties of dried Portuguese apple variety (Malus domestica Borkh. cv Bravo de Esmolfe). Food Chem. 2018, 240, 701–706. [Google Scholar] [CrossRef]
- Heleno, S.A.; Ferreira, I.C.F.R.; Esteves, A.P.; Ćirić, A.; Glamočlija, J.; Martins, A.; Soković, M.; Queiroz, M.J.R.P. Antimicrobial and demelanizing activity of Ganoderma lucidum extract, p-hydroxybenzoic and cinnamic acids and their synthetic acetylated glucuronide methyl esters. Food Chem. Toxicol. 2013, 58, 95–100. [Google Scholar] [CrossRef]
- De la Fuente, B.; Pinela, J.; Mandim, F.; Heleno, S.A.; Ferreira, I.C.; Barba, F.J.; Berrada, H.; Caleja, C.; Barros, L. Nutritional and bioactive oils from salmon (Salmo salar) side streams obtained by Soxhlet and optimized microwave-assisted extraction. Food Chem. 2022, 386, 132778. [Google Scholar] [CrossRef]
- Mandim, F.; Graça, V.C.; Calhelha, R.C.; Machado, I.L.F.; Ferreira, L.F.V.; Ferreira, I.C.F.R.; Santos, P.F. Synthesis, Photochemical and in Vitro Cytotoxic Evaluation of New Iodinated Aminosquaraines as Potential Sensitizers for Photodynamic Therapy. Molecules 2019, 24, 863. [Google Scholar] [CrossRef]
- Taofiq, O.; Calhelha, R.C.; Heleno, S.; Barros, L.; Martins, A.; Santos-Buelga, C.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. The Contribution of Phenolic Acids to the Anti-Inflammatory Activity of Mushrooms: Screening in Phenolic Extracts, Individual Parent Molecules and Synthesized Glucuronated and Methylated Derivatives. Food Res. Int. 2015, 76, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Báidez, A.G.; Gómez, P.; Del Río, J.A.; Ortuño, A. Dysfunctionality of the Xylem in Olea europaea L. Plants Associated with the Infection Process by Verticillium dahliae Kleb. Role of Phenolic Compounds in Plant Defense Mechanism. J. Agric. Food Chem. 2007, 55, 3373–3377. [Google Scholar] [CrossRef]
- Ferreira, J.P.A.; Miranda, I.; Gominho, J.; Pereira, H. Chemical Characterization of Cork and Phloem from Douglas Fir Outer Bark. Holzforschung 2016, 70, 475–483. [Google Scholar] [CrossRef]
- Zhang, D.; Hamauzu, Y. Phenolic Compounds and Their Antioxidant Properties in Different Tissues of Carrots (Daucus Carota L.). J. Food Agric. Env. 2004, 2, 95–100. [Google Scholar]
- Sen, U.; Almeida, D.; da Silveira, T.F.F.; Pires, T.S.P.; Añibarro-Ortega, M.; Mandim, F.; Barros, L.; Ferreira, I.C.F.R.; Pereira, H.; Fernandes, Â. Exploring the Bioactive Properties of Hydroethanolic Cork Extracts of Quercus Cerris and Quercus Suber. Processes 2024, 12, 1579. [Google Scholar] [CrossRef]
- Lefebvre, T.; Destandau, E.; Lesellier, E. Selective Extraction of Bioactive Compounds from Plants Using Recent Extraction Techniques: A Review. J. Chromatogr. A 2021, 1635, 461770. [Google Scholar] [CrossRef]
- Dobiáš, P.; Pavlíková, P.; Adam, M.; Eisner, A.; Beňová, B.; Ventura, K. Comparison of Pressurised Fluid and Ultrasonic Extraction Methods for Analysis of Plant Antioxidants and Their Antioxidant Capacity. Cent. Eur. J. Chem. 2010, 8, 87–95. [Google Scholar] [CrossRef]
- Liu, S.; Lu, H.; Hu, R.; Shupe, A.; Lin, L.; Liang, B. A Sustainable Woody Biomass Biorefinery. Biotechnol. Adv. 2012, 30, 785–810. [Google Scholar] [CrossRef]
- Le Normand, M.; Moriana, R.; Ek, M. The Bark Biorefinery: A Side-Stream of the Forest Industry Converted into Nanocomposites with High Oxygen-Barrier Properties. Cellulose 2014, 21, 4583–4594. [Google Scholar] [CrossRef]
- Singh, A.; Bajpai, V.; Kumar, S.; Sharma, K.R.; Kumar, B. Profiling of Gallic and Ellagic Acid Derivatives in Different Plant Parts of Terminalia Arjuna by HPLC-ESI-QTOF-MS/MS. Nat. Prod. Commun. 2016, 11, 1934578X1601100227. [Google Scholar] [CrossRef]
- Patyra, A.; Dudek, M.K.; Kiss, A.K. LC-DAD–ESI-MS/MS and NMR Analysis of Conifer Wood Specialized Metabolites. Cells 2022, 11, 3332. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Price, W.E.; Ashton, J.; Tapsell, L.C.; Johnson, S. Identification and Characterization of Phenolic Compounds in Hydromethanolic Extracts of Sorghum Wholegrains by LC-ESI-MSn. Food Chem. 2016, 211, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Yuan, Z.; Rong, L.; Zhang, Y.; Xiong, G.; Liu, Y.; Li, C. An Optimized Method for Extraction and Characterization of Phenolic Compounds in Dendranthema Indicum Var. Aromat. Flower. Sci. Rep. 2019, 9, 7745. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ. Antioxidant Activity of Caffeic Acid (3, 4-Dihydroxycinnamic Acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef]
- Yasuko, K.; Tomohiro, N.; Sei-Itsu, M.; Ai-Na, L.; Yasuo, F.; Takashi, T. Caffeic Acid Is a Selective Inhibitor for Leukotriene Biosynthesis. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1984, 792, 92–97. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Kim, S.-H.; Hagerman, A.E.; Lü, J. Anti-Cancer, Anti-Diabetic and Other Pharmacologic and Biological Activities of Penta-Galloyl-Glucose. Pharm. Res. 2009, 26, 2066–2080. [Google Scholar] [CrossRef]
- Xiang, Y.; Ju, H.; Li, S.; Zhang, Y.; Yang, C.; Wang, Y. Effects of 1, 2, 4, 6-Tetra-O-Galloyl-β-D-Glucose from P. Emblica on HBsAg and HBeAg Secretion in HepG2. 2.15 Cell Culture. Virol. Sin. 2010, 25, 375–380. [Google Scholar] [CrossRef]
- Vieira, V.; Pereira, C.; Abreu, R.M.V.; Calhelha, R.C.; Alves, M.J.; Coutinho, J.A.P.; Ferreira, O.; Barros, L.; Ferreira, I.C.F.R. Hydroethanolic Extract of Juglans Regia L. Green Husks: A Source of Bioactive Phytochemicals. Food Chem. Toxicol. 2020, 137, 111189. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; González-García, V.; Casanova-Gascón, J.; Barriuso-Vargas, J.J.; Balduque-Gil, J.; Lorenzo-Vidal, B.; Martín-Gil, J.; Martín-Ramos, P. Valorization of Quercus Suber L. Bark A Source Phytochem. Antimicrob. Act. Against Apple Tree Diseases. Plants 2022, 11, 3415. [Google Scholar] [CrossRef]
- Carriço, C.; Ribeiro, H.M.; Marto, J. Converting Cork By-Products to Ecofriendly Cork Bioactive Ingredients: Novel Pharmaceutical and Cosmetics Applications. Ind. Crops Prod. 2018, 125, 72–84. [Google Scholar] [CrossRef]
- Mota, S.; Pinto, C.; Cravo, S.; Rocha e Silva, J.; Afonso, C.; Sousa Lobo, J.M.; Tiritan, M.E.; Cidade, H.; Almeida, I.F. Quercus Suber: A Promising Sustainable Raw Material for Cosmetic Application. Appl. Sci. 2022, 12, 4604. [Google Scholar] [CrossRef]
- Gonçalves, F.; Correia, P.; Silva, S.P.; Almeida-Aguiar, C. Evaluation of Antimicrobial Properties of Cork. FEMS Microbiol. Lett. 2016, 363, fnv231. [Google Scholar] [CrossRef] [PubMed]
- Hassikou, R.; Oulladi, H.; Arahou, M. Antifungal Activity of Quercus Suber Extracts on Trichophyton Rubrum and Candida Albicans. Phytothérapie 2014, 12, 206–212. [Google Scholar] [CrossRef]
- Ita, B.N.; Eduok, S.I. In Vitro Antioxidant and Antifungal Activities of Rhizophora Racemosa GFW Mey. Stem Bark Extracts. Sci. Afr. 2022, 15, e01091. [Google Scholar] [CrossRef]
- Wang, H.; Qiu, C.; Chen, L.; Abbasi, A.M.; Guo, X.; Liu, R.H. Comparative Study of Phenolic Profiles, Antioxidant and Antiproliferative Activities in Different Vegetative Parts of Ramie (Boehmeria nivea L.). Molecules 2019, 24, 1551. [Google Scholar] [CrossRef]
- Srinivas, B.K.; Shivamadhu, M.C.; Devegowda, P.S.; Mathew, G.; Tamizhmani, T.; Prabhakaran, S.G.; Jayarama, S. Screening and Evaluation of Lectin and Anti-Cancer Activity from the Phloem Exudate/Sap of the Indian Dietary Ethnomedicinal Plants. Pharmacogn. J. 2019, 11, 570–578. [Google Scholar] [CrossRef]

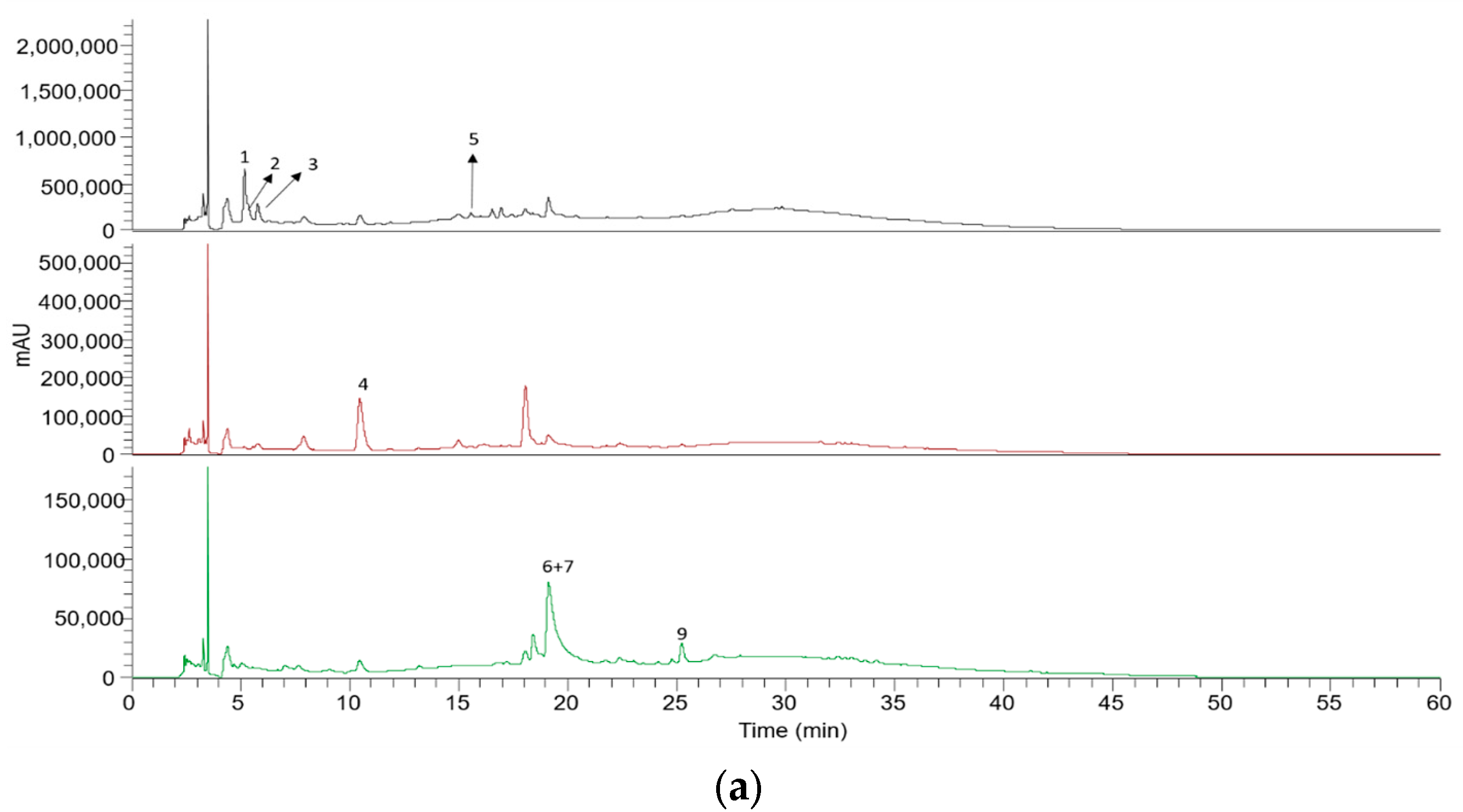

| Peak | Rt (min) | λ Max (nm) | [M − H]− | MS2 | MS3 | Compound | mg/g Extract | * Student’s t-Test p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| WCE | PE | ||||||||
| 1 | 5.17 | 264 | 477 | 183 | 169, 124 | Methyl gallate–pentosyl–hexoside | 4.57 ± 0.01 | 3.21 ± 0.01 | <0.001 |
| 2 | 5.39 | - | 345 | 183 | 169, 124 | Methyl gallate hexoside | 0.12 ± 0.03 | tr | - |
| 3 | 5.78 | 5.78 | 260, 292 | 153 | - | Protocatechuic acid | 1.62 ± 0.01 | 1.35 ± 0.01 | <0.001 |
| 4 | 10.45 | 323 | 179 | - | - | Caffeic acid | 0.72 ± 0.01 | - | - |
| 5 | 15.56 | 279 | 787 | 617, 635 | 573, 447, 403, 313, 279, 235 | Tetragalloylglucose | 0.49 ± 0.04 | - | - |
| 6 | 19.08 | 366 | 301 | - | - | Ellagic acid | 2.35 ± 0.07 | 1.62 ± 0.01 | <0.001 |
| 7 | 19.14 | - | 491 | 359 | 344, 313 | Isolariciresinol pentoside | 2.21 ± 0.01 | 1.38 ± 0.12 | <0.001 |
| 8 | 21.8 | - | 187 | 169, 125 | - | Gallic acid monohydrate | - | tr | - |
| 9 | 25.21 | - | 461 | 315 | 300 | Methyl ellagic acid rhamnoside | 1.34 ± 0.01 | 1.33 ± 0.01 | 0.001 |
| TBARSs EC50 (μg/mL) | OxHLIA IC50 (μg/mL) | |
|---|---|---|
| WCE | 79 ± 4 | 21 ± 1 |
| PE | 660 ± 80 | 13.9 ± 0.8 |
| Trolox | 5.4 ± 3.0 | 19.7 ± 0.4 |
| * Student’s t-Test p-Value | <0.001 | <0.001 |
| Antibacterial Activity | WCE | PE | Streptomycin * | Methicillin * | Ampicillin * | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Food Isolates | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC |
| Gram-negative bacteria | ||||||||||
| E. cloacae | 5 | >10 | 5 | >10 | 0.007 | 0.007 | n.t. | n.t | 0.15 | 0.15 |
| E. coli | 5 | >10 | 5 | >10 | 0.01 | 0.01 | n.t. | n.t. | 0.15 | 0.15 |
| P. aeruginosa | 10 | >10 | 10 | >10 | 0.06 | 0.06 | n.t. | n.t. | 0.63 | 0.63 |
| S. enterica | 5 | >10 | 5 | >10 | 0.007 | 0.007 | n.t. | n.t. | 0.15 | 0.15 |
| Y. enterocolitica | 2.5 | >10 | 2.5 | >10 | 0.007 | 0.007 | n.t. | n.t. | 0.15 | 0.15 |
| Gram-positive bacteria | ||||||||||
| B. cereus | 10 | >10 | 10 | >10 | 0.007 | 0.007 | n.t. | n.t. | n.t. | n.t. |
| L. monocytogenes | 10 | >10 | 10 | >10 | 0.007 | 0.007 | n.t. | n.t. | 0.15 | 0.15 |
| S. aureus | 2.5 | >10 | 5 | >10 | 0.007 | 0.007 | 0.007 | 0.007 | 0.15 | 0.15 |
| Clinical isolates | ||||||||||
| Gram-negative bacteria | ||||||||||
| E. coli | 2.5 | >10 | 2.5 | >10 | <0.15 | <0.15 | <0.0078 | <0.0078 | n.t. | n.t. |
| K. pneumoniae | 5 | >10 | 5 | >10 | 10 | >10 | <0.0078 | <0.0078 | n.t. | n.t. |
| M. morganii | 5 | >10 | 5 | >10 | >10 | >10 | <0.0078 | <0.0078 | n.t. | n.t. |
| P. mirabilis | 5 | >10 | 5 | >10 | <015 | <0.15 | <0.0078 | <0.0078 | n.t. | n.t. |
| P. aeruginosa | 10 | >10 | 10 | >10 | >10 | >10 | 0.5 | 1 | n.t. | n.t. |
| Gram-positive bacteria | ||||||||||
| E. faecalis | 5 | >10 | 5 | >10 | <0.15 | <0.15 | n.t. | n.t. | <0.0078 | <0.0078 |
| L. monocytogenes | 10 | >10 | 5 | >10 | <0.15 | <0.15 | <0.0078 | <0.0078 | n.t. | n.t. |
| MRSA | 5 | >10 | 5 | >10 | <0.15 | <0.15 | n.t. | n.t. | 0.25 | 0.5 |
| WCE | PE | Ketoconazole * | ||||
|---|---|---|---|---|---|---|
| MIC | MFC | MIC | MFC | MIC | MFC | |
| A. brasiliensis | 5 | >10 | 5 | >10 | 0.06 | 0.125 |
| A. fumigatus | 10 | >10 | 5 | >10 | 0.5 | 1 |
| WCE | PE | Positive Control | Student’s t-Test p-Value | |
|---|---|---|---|---|
| Antiproliferative Activity (GI50 μg/mL) a against Tumor Cell Lines | Ellipticine | |||
| AGS | 173 ± 6 | 230 ± 8 | 1.23 ± 0.03 | <0.001 |
| CaCO2 | 238 ± 10 | 167 ± 10 | 1.21 ± 0.02 | <0.001 |
| NCI-H460 | 247 ± 19 | >400 | 1.03 ± 0.09 | - |
| MCF-7 | 230 ± 10 | 138 ± 17 | 1.02 ± 0.02 | <0.001 |
| Hepatotoxicity (GI50 µg/mL) a | ||||
| PLP2 | 226 ± 15 | >400 | 1.4 ± 0.1 | - |
| Inhibition of NO production (EC50 μg/mL) b | Dexamethasone | |||
| RAW 264.7 | >400 | >400 | 6.3 ± 0.4 | - |
| Sample | Extraction Time (h) | Extraction Temperature (°C) | Extraction Method | Extraction Agent | Yield (%) | Reference |
|---|---|---|---|---|---|---|
| Waste cork | 2 | 25 | Maceration | EtOH/Water (80/20, v/v) | 4.4 | This work |
| Phloem | 2 | 25 | Maceration | 2.4 | This work | |
| Pure cork | 2 | 25 | Maceration | 3.0 | [26] | |
| Waste cork | 18 * | 75–100 ** | Soxhlet | EtOH (100%) followed by H2O (100%) | 7.4 | [12] |
| Phloem | 18 * | 75–100 ** | Soxhlet | 5.9 | [12] | |
| Pure cork | 1.5 * | 75–100 ** | Soxtec | 5.8 | [7] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Șen, A.U.; Almeida, D.; da Silveira, T.F.F.; Pires, T.S.P.; Añibarro-Ortega, M.; Mandim, F.; Barros, L.; Ferreira, I.C.F.R.; Pereira, H.; Fernandes, Â. Bioactivities of Waste Cork and Phloem Fractions of Quercus cerris Bark. Processes 2024, 12, 2081. https://doi.org/10.3390/pr12102081
Șen AU, Almeida D, da Silveira TFF, Pires TSP, Añibarro-Ortega M, Mandim F, Barros L, Ferreira ICFR, Pereira H, Fernandes Â. Bioactivities of Waste Cork and Phloem Fractions of Quercus cerris Bark. Processes. 2024; 12(10):2081. https://doi.org/10.3390/pr12102081
Chicago/Turabian StyleȘen, Ali Umut, Daiana Almeida, Tayse F. F. da Silveira, Tânia S. P. Pires, Mikel Añibarro-Ortega, Filipa Mandim, Lillian Barros, Isabel C. F. R. Ferreira, Helena Pereira, and Ângela Fernandes. 2024. "Bioactivities of Waste Cork and Phloem Fractions of Quercus cerris Bark" Processes 12, no. 10: 2081. https://doi.org/10.3390/pr12102081














