Quaternary Treatment of Urban Wastewater for Its Reuse
Abstract
:1. Introduction
2. Filtration
2.1. Sand Filtration
2.2. Membrane Filtration
2.3. Applications of Filtration for Micropollutant Removals
- Microplastics
- Microbial pollution
- Pharmaceuticals and endocrine disruptors
- Pesticides
- Heavy metals
3. Coagulation
3.1. Mechanisms of Coagulation
- Simple charge neutralization
- Charge patching
- Bridging
- Sweeping
3.2. Influencing Factors of Coagulation
- Mixing
- Coagulant type
- Coagulant dosage
- pH
3.3. Applications of Coagulation for Micropollutant Removals
- Microplastics
- Disinfection by-products
- Turbidity
- Pharmaceuticals
- Heavy metals
4. Adsorption
- (a).
- macroporous materials with pores structure > 50 nm and d > 50 nm,
- (b).
- mesoporous materials with pores structure 2–50 nm and d ≈ 2–50 nm,
- (c).
- microporous materials with pores structure < 2 nm and d < 2 nm.
4.1. Physisorption and Chemisorption
4.2. Characterization of Adsorbents and the Adsorbent Process
- (a).
- high selectivity for specific pollutants,
- (b).
- possibility of regeneration,
- (c).
- non-toxicity to humans and the environment,
- (d).
- non-corrosiveness to construction materials of the system,
- (e).
- low cost,
- (f).
- mechanical stability,
- (g).
- market availability.
4.3. Adsorption as a Method for Removal of Organic Micropollutants
Adsorption Mechanism of Organic Pollutants
4.4. Heavy Metal Removal from Wastewaters by Adsorption
4.4.1. Effect of pH
4.4.2. Effect of Temperature
4.4.3. Effect of Contact Time
4.4.4. Adsorption Mechanisms of Heavy Metals
4.5. Control of Disinfection Byproducts Contained in Wastewaters with Adsorption
4.6. Removal of Micro/Nanoplastics by Adsorption
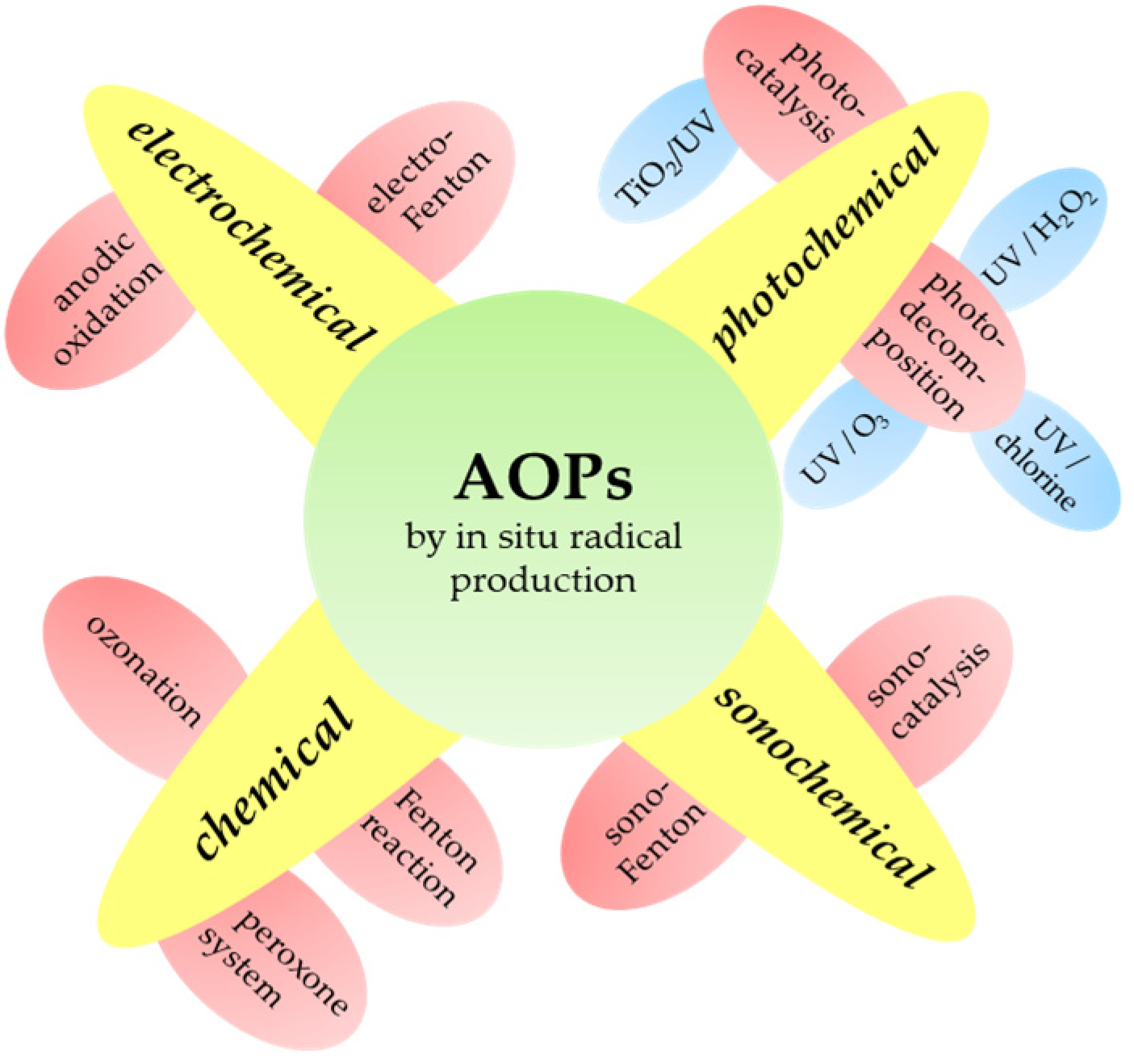
5. Advanced Oxidation Processes (AOPs)
5.1. Chemical Types of AOPs
5.1.1. Fenton’s Reaction Technique
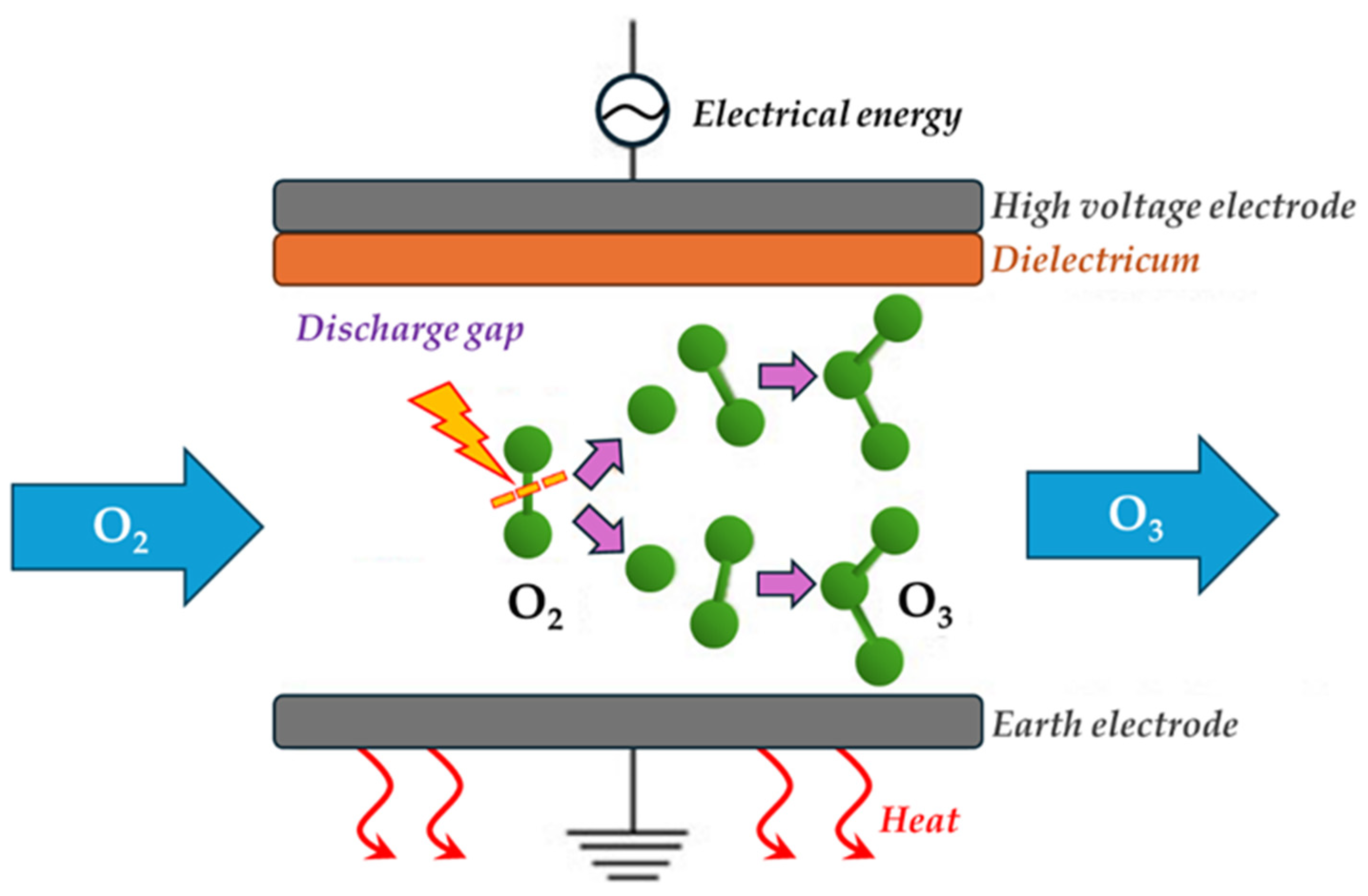
5.1.2. Ozone-Based Processes
5.2. Photochemical Types of AOPs
5.2.1. Photodecomposition Technique
5.2.2. Photocatalysis Technique
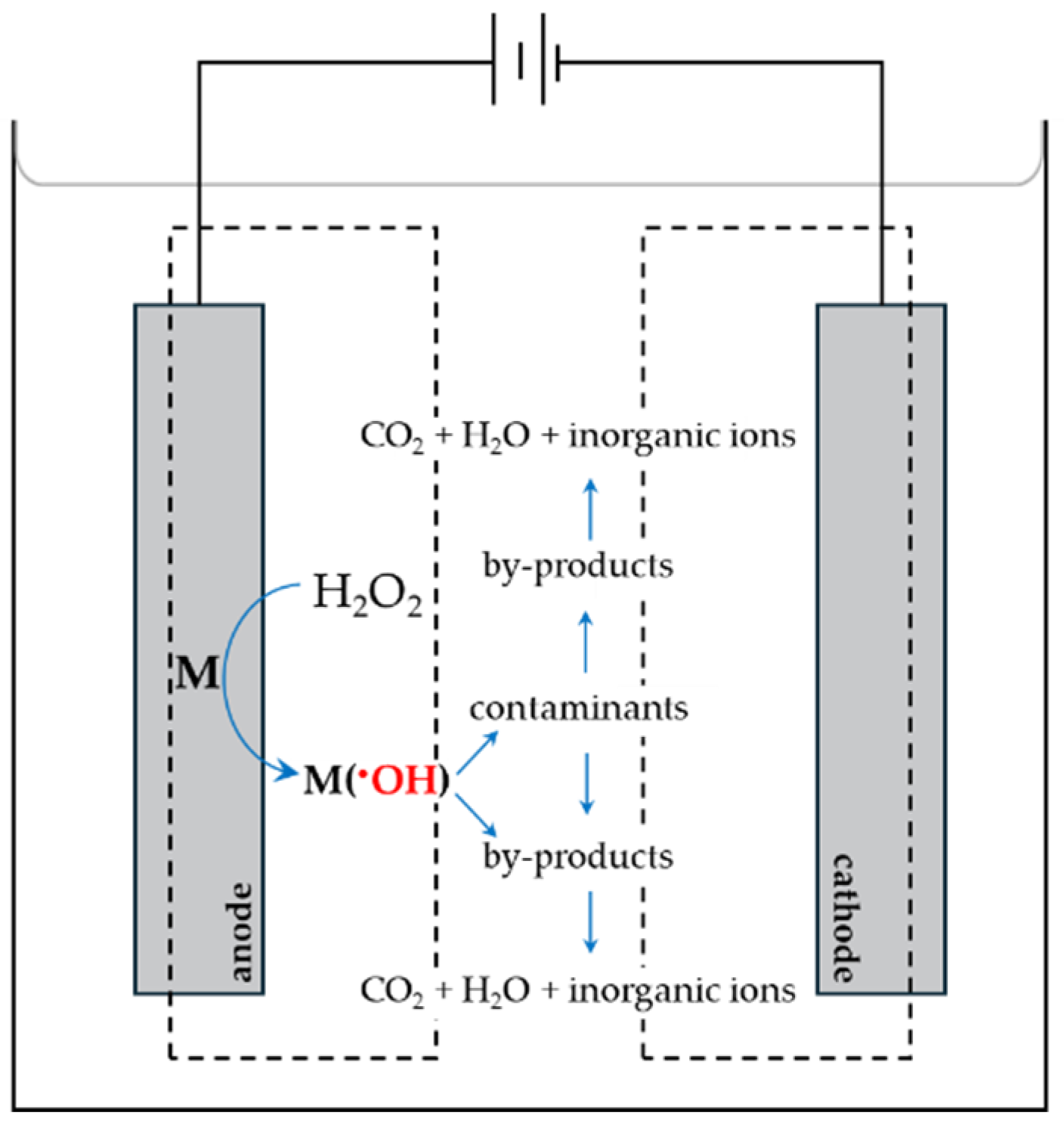
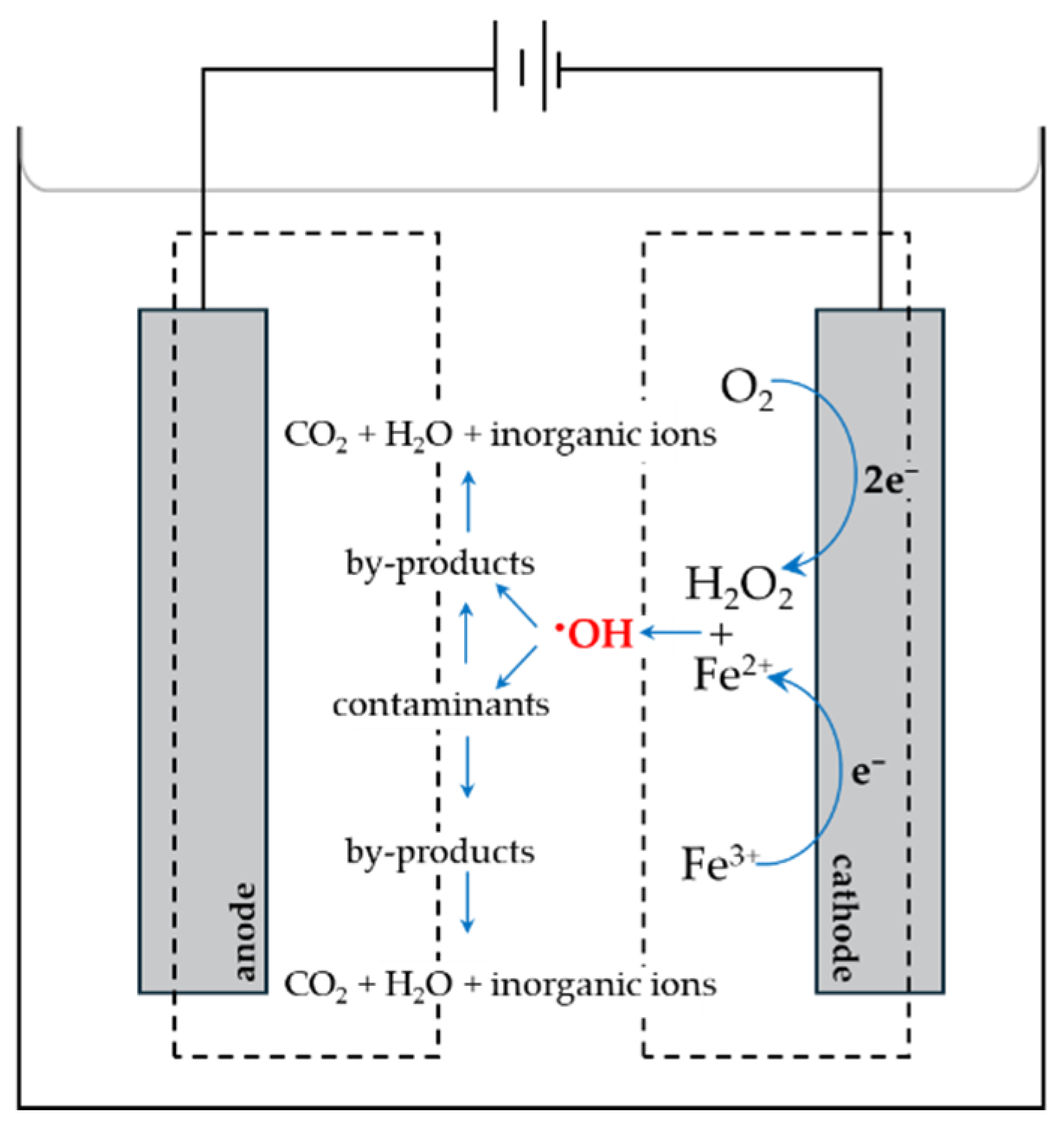
5.3. Electrochemical Types of AOPs
5.3.1. Anodic Oxidation Technique
5.3.2. Electro-Fenton Technique
5.4. Sonochemical Types of AOPs
Sonocatalysis
6. Disinfection
6.1. Chemical Disinfection
6.1.1. Sodium Hypochlorite
6.1.2. Peracetic Acid
6.1.3. Performic Acid
6.2. Physical Means of Disinfection
6.2.1. UV Irradiation
6.2.2. Sonolysis
6.3. Use of AOPs as a Disinfection Method
6.4. Electrochemical Disinfection
7. Assessment of Reviewed Treatment Methods
8. Conclusions
- The existing literature is dominated by water scarcity and water stress, which are caused by rapid urban expansion, development of the world economy, demographic changes, deforestation and climate change.
- According to the predictions, the water scarcity and drought events are likely to be more frequent in the future. Due to these facts, the missing water resources must be replaced by a suitable water source.
- Quaternary treated urban wastewater has been proposed as an alternative water source for irrigation in Europe. For quaternary treatment, various additional processes can be used, such as filtration, coagulation, adsorption, ozonation, advanced oxidation processes and disinfection. The choice of the specific process depends on various factors, including wastewater characteristics and treatment goals. According to the existing literature, we recommend for quaternary urban wastewater treatment, a combination of coagulation, membrane filtration (UF or NF) and UV disinfection. These processes are relatively well known and commercially available with high removal efficiencies of micropollutants and microorganisms.
- The quaternary treated wastewater reuse has the following innovativeness in the field of water management: an efficient use of water resources by citizens, industry and agriculture; promoting water saving and reuse; water-efficient technologies in all sectors; fitting in the context of the 2020 Circular Economy Action Plan; development of the huge potential for safe wastewater reuse in line with the new EU Regulation on water reuse; contribution to reduce greenhouse gas emissions; reduction the use of additional fertilizers resulting in savings for the environment, farmers and wastewater treatment; and the creation of green jobs in the water-related industry.
- Barriers to the reuse of quaternary treated urban wastewater are well characterized, and they mainly include concerns about microbial risk and presence of micropollutants; high investments for modernization of urban wastewater treatment plants; and a lack of financial incentives for quaternary treated wastewater reuse in agriculture.
- This review article serves as a basis for knowledge development, provides a comprehensive understanding of the current state of quaternary treatment of urban wastewater for its reuse, creates guidelines for practice, has the capacity to engender new ideas and serve as the grounds for future research directions. It helps researchers to identify key themes and concepts, evaluate the strengths and weaknesses of previous studies and determine areas where further research is needed.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| •OH | hydroxyl radical |
| •OOH | hydroperoxide radical |
| AOPs | advanced oxidation processes |
| ARB&ARGs | antibiotic resistant bacteria and genes |
| BAC | biological activated carbon |
| BDD | boron-doped diamond electrode |
| CEC | contaminants of emerging concern |
| COD | chemical oxygen demand |
| DBD | dielectric barrier discharge |
| DBP | disinfection byproducts |
| DBPFP | disinfection byproducts formation potential |
| DNA | deoxyribonucleic acid |
| DOC | dissolved organic carbon |
| DPD | diethylphenylenediamine |
| e– | electrons |
| ED | electrochemical disinfection |
| EU | European Union |
| GAC | granular activated charcoal |
| GDP | gross domestic product |
| GO | graphene oxide |
| h+ | holes |
| HAA | haloacetic acid |
| HAN | haloacetonitril |
| HK | haloketone |
| HOCl | hypochloride |
| IUPAC | international union of pure and applied chemistry |
| LED | light-emitting diode |
| MBR | membrane bioreactor |
| MF | microfiltration |
| MP | microplastic |
| NF | nanofiltration |
| NO• | nitric oxide |
| NOMs | natural organic matters |
| NTU | nephelometric turbidity unit |
| O2•− | superoxide anion radical |
| OMP | organic micropollutant |
| PAA | peracetic acid |
| PAC | poly aluminum chloride |
| PBT | persistent, bioaccumulative and toxic |
| PE | polyethylene |
| PFA | performic acid |
| PFC | poly ferric chloride |
| PFO | pseudo-first order |
| PFS | poly ferrous sulphate |
| PMT | persistent, mobile and toxic |
| PSF | polysulfone |
| PSiAS | poly-aluminum silicate sulphate |
| PSiTS | poly-titanium silicate sulphate |
| PSO | pseudo-second order |
| PVC | polyvinyl chloride |
| RNA | ribonucleic acid |
| RO | reverse osmosis |
| RO• | alkoxyl radical |
| ROO• | peroxyl radical |
| ROOH | organic hydroperoxides |
| ROS | reactive oxygen species |
| ROS• | sulphonyl radicals |
| RS• | thiyl radicals |
| RSF | rapid sand filtration |
| RSOO• | thiyl peroxyl |
| SDBS | sodium dodecyl benzenesulfonate |
| SHE | standard hydrogen electrode |
| SO4•− | sulphate radicals |
| SUVA | specific ultraviolet absorbance |
| TCNM | trichloronitromethane |
| THM | trihalomethane |
| TOC | total organic carbon |
| UF | ultrafiltration |
| US | ultrasound |
| UV | ultraviolet |
| UVA254 | absorbance of light in the UV part of the light spectrum at 254 nm |
| WWTP | wastewater treatment plant |
References
- Khan, S.A.R.; Ponce, P.; Yu, Z.; Golpîra, H.; Mathew, M. Environmental technology and wastewater treatment: Strategies to achieve environmental sustainability. Chemosphere 2022, 286, 131532. [Google Scholar] [CrossRef]
- Falco, C.; Galeotti, M.; Olper, A. Climate change and migration: Is agriculture the main channel? Glob. Environ. Change 2019, 59, 101995. [Google Scholar] [CrossRef]
- International Decade for Action: Water for Life—United Nations. Available online: http://www.un.org/waterforlifedecade/scarcity.shtml (accessed on 15 June 2024).
- The United Nations World Water Development Report 2020: Water and Climate Change—UNESCO. Available online: https://unesdoc.unesco.org/ark:/48223/pf0000372985 (accessed on 15 June 2024).
- Capocelli, M.; Piemonte, V. Technologies for Water Reuse: Current status and future challenges. Water 2021, 13, 832. [Google Scholar] [CrossRef]
- The United Nations World Water Development Report 2017—UNESCO. Available online: https://www.unwater.org/publications/un-world-water-development-report-2017 (accessed on 15 June 2024).
- Water in Agriculture—World Bank Group. Available online: https://www.worldbank.org/en/news/infographic/2023/07/26/water-in-agriculture (accessed on 15 June 2024).
- Kesari, K.K.; Soni, R.; Jamal, Q.M.S.; Tripathi, J.; Lal, J.A.; Jha, N.K.; Siddiqui, M.H.; Kumar, P.; Tripathi, V.; Ruokolainen, J. Wastewater treatment and Reuse: A review of its applications and health implications. Water Air Soil Pollut. 2021, 232, 208. [Google Scholar] [CrossRef]
- Proposal for a Revised Urban Wastewater Treatment Directive—European Commission. Available online: https://environment.ec.europa.eu/publications/proposal-revised-urban-wastewater-treatment-directive_en (accessed on 15 June 2024).
- Wang, H.; Wang, J.; Yu, X. Wastewater irrigation and crop yield: A meta-analysis. J. Integr. Agric. 2022, 21, 1215–1224. [Google Scholar] [CrossRef]
- Rizzo, L.; Gernjak, W.; Krzeminski, P.; Malato, S.; McArdell, C.S.; Perez, J.A.S.; Schaar, H.; Fatta-Kassinos, D. Best available technologies and treatment trains to address current challenges in urban wastewater reuse for irrigation of crops in EU countries. Sci. Total Environ. 2020, 710, 136312. [Google Scholar] [CrossRef]
- Hristov, J.; Barreiro-Hurle, J.; Salputra, G.; Blanco, M.; Witzke, P. Reuse of treated water in European agriculture: Potential to address water scarcity under climate change. Agric. Water Manag. 2021, 251, 106872. [Google Scholar] [CrossRef]
- Duckett, D.; Troldborg, M.; Hendry, S.; Cousin, H. Making waves: Promoting municipal water reuse without a prevailing scarcity driver. Water Res. 2024, 249, 120965. [Google Scholar] [CrossRef]
- Bilal, M.; Ashraf, S.S.; Barceló, D.; Iqbal, H.M. Biocatalytic degradation/redefining removal fate of pharmaceutically active compounds and antibiotics in the aquatic environment. Sci. Total Environ. 2019, 691, 1190–1211. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.; Barceló, D. Persistence of pesticides-based contaminants inthe environment and their effective degradation using laccase-assisted biocatalytic systems. Sci. Total Environ. 2019, 695, 133896. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Yan, X.; Zhu, Q.; Liao, C. The utilization of reclaimed water: Possible risks arising from waterborne contaminants. Env. Pollut 2019, 254, 113020. [Google Scholar] [CrossRef] [PubMed]
- Vojtěchovská Šrámková, M.; Diaz-Sosa, V.; Wanner, J. Experimental verification of tertiary treatment process in achieving effluent quality required by wastewater reuse standards. J. Water Process Eng. 2018, 22, 41–45. [Google Scholar] [CrossRef]
- Regulation (EU) 2020/741 of the European Parliament and of the Council of 25 May 2020 on Minimum Requirements for Water Reuse. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020R0741 (accessed on 15 June 2024).
- The EU and the United Nations—Common Goals for a Sustainable Future. Available online: https://commission.europa.eu/strategy-and-policy/sustainable-development-goals/eu-and-united-nations-common-goals-sustainable-future_en (accessed on 15 June 2024).
- Faour-Klingbeil, D.; Todd, E.C.D. The impact of climate change on treated and untreated wastewater use for agriculture, especially in arid regions: A Review. Foodborne Pathog. Dis. 2018, 15, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Gray, N.F. Filtration methods. In Microbiology of Waterborne Diseases; Academic Press: Cambridge, MA, USA, 2014; pp. 631–650. [Google Scholar] [CrossRef]
- Cescon, A.; Jiang, J.Q. Filtration process and alternative filter media material in water treatment. Water 2020, 12, 3377. [Google Scholar] [CrossRef]
- Guchi, E. Review on slow sand filtration in removing microbial contamination and particles from drinking water. Am. J. Food Nutr. 2015, 3, 47–55. [Google Scholar] [CrossRef]
- Jaeel, A.J.; Abdulkathum, S. Sustainable pollutants removal from wastewater using sand filter: A review. In Proceedings of the 2018 International Conference on Advance of Sustainable Engineering and its Application (ICASEA) IEEE, Wasit, Iraq, 14–15 March 2018; pp. 179–183. [Google Scholar] [CrossRef]
- Verma, S.; Daverey, A.; Sharma, A. Slow sand filtration for water and wastewater treatment—A review. Environ. Technol. Rev. 2017, 6, 47–58. [Google Scholar] [CrossRef]
- Sabale, R.; Mujawar, S. Improved rapid sand filter for performance enhancement. Int. J. Sci. Res. 2014, 3, 1031–1033. [Google Scholar]
- Hakami, M.W.; Alkhudhiri, A.; Al-Batty, S.; Zacharof, M.P.; Maddy, J.; Hilal, N. Ceramic microfiltration membranes in wastewater treatment: Filtration behavior, fouling and prevention. Membranes 2020, 10, 248. [Google Scholar] [CrossRef]
- Cevallos-Mendoza, J.; Amorim, C.G.; Rodríguez-Díaz, J.M.; Montenegro, M.D.C.B. Removal of contaminants from water by membrane filtration: A review. Membranes 2022, 12, 570. [Google Scholar] [CrossRef]
- Melin, T.; Jefferson, B.; Bixio, D.; Thoeye, C.; De Wilde, W.; De Koning, J.; van der Graaf, J.; Wintgens, T. Membrane bioreactor technology for wastewater treatment and reuse. Desalination 2006, 187, 271–282. [Google Scholar] [CrossRef]
- Al-Asheh, S.; Bagheri, M.; Aidan, A. Membrane bioreactor for wastewater treatment: A review. Case Stud. Chem. Environ. Eng. 2021, 4, 100109. [Google Scholar] [CrossRef]
- Goswami, L.; Kumar, R.V.; Borah, S.N.; Manikandan, N.A.; Pakshirajan, K.; Pugazhenthi, G. Membrane bioreactor and integrated membrane bioreactor systems for micropollutant removal from wastewater: A review. J. Water Process Eng. 2018, 26, 314–328. [Google Scholar] [CrossRef]
- Rajbongshi, A.; Gogoi, S.B. Microfiltration, ultrafiltration and nanofiltration as a post-treatment of biological treatment process with references to oil field produced water of Moran oilfield of Assam. Pet. Res. 2024, 9, 143–154. [Google Scholar] [CrossRef]
- Wang, J.; Cahyadi, A.; Wu, B.; Pee, W.; Fane, A.G.; Chew, J.W. The roles of particles in enhancing membrane filtration: A review. J. Membr. Sci. 2020, 595, 117570. [Google Scholar] [CrossRef]
- Sembiring, E.; Fajar, M.; Handajani, M. Performance of rapid sand filter–single media to remove microplastics. Water Supply 2021, 21, 2273–2284. [Google Scholar] [CrossRef]
- Wolff, S.; Weber, F.; Kerpen, J.; Winklhofer, M.; Engelhart, M.; Barkmann, L. Elimination of microplastics by downstream sand filters in wastewater treatment. Water 2020, 13, 33. [Google Scholar] [CrossRef]
- Bayo, J.; López-Castellanos, J.; Olmos, S. Membrane bioreactor and rapid sand filtration for the removal of microplastics in an urban wastewater treatment plant. Mar. Pollut. Bull. 2020, 156, 111211. [Google Scholar] [CrossRef]
- Conesa, J.A.; Ortuño, N. Reuse of water contaminated by microplastics, the effectiveness of filtration processes: A review. Energies 2022, 15, 2432. [Google Scholar] [CrossRef]
- Goswami, K.P.; Pugazhenthi, G. Credibility of polymeric and ceramic membrane filtration in the removal of bacteria and virus from water: A review. J. Environ. Manag. 2020, 268, 110583. [Google Scholar] [CrossRef]
- Marsono, B.D.; Yuniarto, A.; Purnomo, A.; Soedjono, E.S. Comparison performances of microfiltration and rapid sand filter operated in water treatment plant. IOP Conf. Ser. Earth Environ. Sci. 2022, 1111, 012048. [Google Scholar] [CrossRef]
- Kacprzyńska-Gołacka, J.; Kowalik-Klimczak, A.; Woskowicz, E.; Wieciński, P.; Łożyńska, M.; Sowa, S.; Barszcz, W.; Kaźmierczak, B. Microfiltration Membranes Modified with Silver Oxide by Plasma Treatment. Membranes 2020, 10, 133. [Google Scholar] [CrossRef] [PubMed]
- Maryam, B.; Buscio, V.; Odabasi, S.U.; Buyukgungor, H. A study on behavior, interaction and rejection of Paracetamol, Diclofenac and Ibuprofen (PhACs) from wastewater by nanofiltration membranes. Environ. Technol. Innov. 2020, 18, 100641. [Google Scholar] [CrossRef]
- Couto, C.F.; Santos, A.V.; Amaral, M.C.S.; Lange, L.C.; de Andrade, L.H.; Foureaux, A.F.S.; Fernandes, B.S. Assessing potential of nanofiltration, reverse osmosis and membrane distillation drinking water treatment for pharmaceutically active compounds (PhACs) removal. J. Water Process Eng. 2020, 33, 101029. [Google Scholar] [CrossRef]
- Vieira, W.T.; de Farias, M.B.; Spaolonzi, M.P.; da Silva, M.G.C.; Vieira, M.G.A. Removal of endocrine disruptors in waters by adsorption, membrane filtration and biodegradation. A review. Environ. Chem. Lett. 2020, 18, 1113–1143. [Google Scholar] [CrossRef]
- Zhang, J.; Nguyen, M.N.; Li, Y.; Yang, C.; Schäfer, A.I. Steroid hormone micropollutant removal from water with activated carbon fiber-ultrafiltration composite membranes. J. Hazard. Mater. 2020, 391, 122020. [Google Scholar] [CrossRef]
- Nakada, N.; Shinohara, H.; Murata, A.; Kiri, K.; Managaki, S.; Sato, N.; Takada, H. Removal of selected pharmaceuticals and personal care products (PPCPs) and endocrine-disrupting chemicals (EDCs) during sand filtration and ozonation at a municipal sewage treatment plant. Water Res. 2007, 41, 4373–4382. [Google Scholar] [CrossRef]
- Jatoi, A.S.; Hashmi, Z.; Adriyani, R.; Yuniarto, A.; Mazari, S.A.; Akhter, F.; Mubarak, N.M. Recent trends and future challenges of pesticide removal techniques—A comprehensive review. J. Environ. Chem. Eng. 2021, 9, 105571. [Google Scholar] [CrossRef]
- Mukherjee, A.; Mehta, R.; Saha, S.; Bhattacharya, A.; Biswas, P.K.; Kole, R.K. Removal of multiple pesticide residues from water by low-pressure thin-film composite membrane. Appl. Water Sci. 2020, 10, 244. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, B.; Fang, C.; Liu, Z.; Fang, J.; Zhu, L. Macroporous membranes doped with micro-mesoporous β-cyclodextrin polymers for ultrafast removal of organic micropollutants from water. Carbohydr. Polym. 2019, 222, 114970. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Qi, Y.; Zhu, L.; Shen, X.; Sotto, A.; Gao, C.; Shen, J. Polythyleneimine-modified original positive charged nanofiltration membrane: Removal of heavy metal ions and dyes. Sep. Purif. Technol. 2019, 222, 117–124. [Google Scholar] [CrossRef]
- Moradi, G.; Zinadini, S.; Rajabi, L.; Derakhshan, A.A. Removal of heavy metal ions using a new high performance nanofiltration membrane modified with curcumin boehmite nanoparticles. Chem. Eng. J. 2020, 390, 124546. [Google Scholar] [CrossRef]
- Rezaee, R.; Nasseri, S.; Mahvi, A.H.; Nabizadeh, R.; Mousavi, S.A.; Rashidi, A.; Jafari, A.; Nazmara, S. Fabrication and characterization of a polysulfone-graphene oxide nanocomposite membrane for arsenate rejection from water. J. Environ. Health Sci. Eng. 2015, 13, 61. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Min, X.; Tang, C.J.; Sillanpää, M.; Zhao, F. Recent advances in membrane filtration for heavy metal removal from wastewater: A mini review. J. Water Process Eng. 2022, 49, 103023. [Google Scholar] [CrossRef]
- Saleh, T.A.; Mustaqeem, M.; Khaled, M. Water treatment technologies in removing heavy metal ions from wastewater: A review. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100617. [Google Scholar] [CrossRef]
- El-Taweel, R.M.; Mohamed, N.; Alrefaey, K.A.; Husien, S.; Abdel-Aziz, A.B.; Salim, A.I.; Mostafa, N.G.; Said, L.A.; Fahim, I.R.; Radwan, A.G. A review of coagulation explaining its definition, mechanism, coagulant types, and optimization models; RSM, and ANN. Curr. Res. Green Sustain. Chem. 2023, 6, 100358. [Google Scholar] [CrossRef]
- Zahmatkesh, S.; Karimian, M.; Chen, Z.; Ni, B.J. Combination of coagulation and adsorption technologies for advanced wastewater treatment for potable water reuse: By ANN, NSGA-II, and RSM. J. Environ. Manag. 2024, 349, 119429. [Google Scholar] [CrossRef]
- Gautam, S.; Saini, G. Use of natural coagulants for industrial wastewater treatment. Glob. J. Environ. Sci. Manag. 2020, 6, 553–578. [Google Scholar] [CrossRef]
- Precious Sibiya, N.; Rathilal, S.; Kweinor Tetteh, E. Coagulation treatment of wastewater: Kinetics and natural coagulant evaluation. Molecules 2021, 26, 698. [Google Scholar] [CrossRef]
- Bahrodin, M.B.; Zaidi, N.S.; Hussein, N.; Sillanpää, M.; Prasetyo, D.D.; Syafiuddin, A. Recent advances on coagulation-based treatment of wastewater: Transition from chemical to natural coagulant. Curr. Pollut. Rep. 2021, 7, 379–391. [Google Scholar] [CrossRef]
- Sonal, S.; Mishra, B.K. Role of coagulation/flocculation technology for the treatment of dye wastewater: Trend and future aspects. In Water Pollution and Management Practices; Springer: Singapore, 2021; pp. 303–331. [Google Scholar] [CrossRef]
- Abujazar, M.S.S.; Karaağaç, S.U.; Amr, S.S.A.; Alazaiza, M.Y.; Bashir, M.J. Recent advancement in the application of hybrid coagulants in coagulation-flocculation of wastewater: A review. J. Clean. Prod. 2022, 345, 131133. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, J.; Yan, Y.; Yang, L.; Xing, G.; Li, H.; Wu, P.; Wang, M.; Zheng, H. Application of coagulation/flocculation in oily wastewater treatment: A review. Sci. Total Environ. 2021, 765, 142795. [Google Scholar] [CrossRef]
- Tang, W.; Li, H.; Fei, L.; Wei, B.; Zhou, T.; Zhang, H. The removal of microplastics from water by coagulation: A comprehensive review. Sci. Total Environ. 2022, 851, 158224. [Google Scholar] [CrossRef]
- Hjorth, M.; Jørgensen, B.U. Polymer flocculation mechanism in animal slurry established by charge neutralization. Water Res. 2012, 46, 1045–1051. [Google Scholar] [CrossRef]
- Sukmana, H.; Bellahsen, N.; Pantoja, F.; Hodur, C. Adsorption and coagulation in wastewater treatment–Review. Prog. Agric. Eng. Sci. 2021, 17, 49–68. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Abdullah, S.R.S.; Imron, M.F.; Said, N.S.M.; Ismail, N.I.; Hasan, H.A.; Othman, A.R.; Purwanti, I.F. Challenges and opportunities of biocoagulant/bioflocculant application for drinking water and wastewater treatment and its potential for sludge recovery. Int. J. Environ. Res. Public Health 2020, 17, 9312. [Google Scholar] [CrossRef]
- Al-Sahari, M.; Al-Gheethi, A.A.S.; Radin Mohamed, R.M.S. Natural coagulates for wastewater treatment; A review for application and mechanism. In Prospects of Fresh Market Wastes Management in Developing Countries; Springer: Cham, Germany, 2020; pp. 17–31. [Google Scholar] [CrossRef]
- Othmani, B.; Rasteiro, M.G.; Khadhraoui, M. Toward green technology: A review on some efficient model plant-based coagulants/flocculants for freshwater and wastewater remediation. Clean Technol. Environ. Policy 2020, 22, 1025–1040. [Google Scholar] [CrossRef]
- Ang, W.L.; Mohammad, A.W. State of the art and sustainability of natural coagulants in water and wastewater treatment. J. Clean. Prod. 2020, 262, 121267. [Google Scholar] [CrossRef]
- Desta, W.M.; Bote, M.E. Wastewater treatment using a natural coagulant (Moringa oleifera seeds): Optimization through response surface methodology. Heliyon 2021, 7, e08451. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Zhang, M.; Wang, T.; Tang, H.; Jia, Y. The effect of pH/PAC on the coagulation of anionic surfactant wastewater generated in the cosmetic production. J. Environ. Chem. Eng. 2023, 11, 109312. [Google Scholar] [CrossRef]
- Iwuozor, K.O. Prospects and challenges of using coagulation-flocculation method in the treatment of effluents. Adv. J. Chem.-Sect. A 2019, 2, 105–127. [Google Scholar] [CrossRef]
- Ahmad, T.; Ahmad, K.; Ahad, A.; Alam, M. Characterization of water treatment sludge and its reuse as coagulant. J. Environ. Manag. 2016, 182, 606–611. [Google Scholar] [CrossRef]
- Na, S.H.; Kim, M.J.; Kim, J.; Batool, R.; Cho, K.; Chung, J.; Lee, S.; Kim, E. J Fate and potential risks of microplastic fibers and fragments in water and wastewater treatment processes. J. Hazard. Mater. 2024, 463, 132938. [Google Scholar] [CrossRef]
- Mao, Y.; Hu, Z.; Li, H.; Zheng, H.; Yang, S.; Yu, W.; Tang, B.; Yang, H.; He, R.; Guo, W.; et al. Recent advances in microplastic removal from drinking water by coagulation: Removal mechanisms and influencing factors. Environ. Pollut. 2024, 349, 123863. [Google Scholar] [CrossRef]
- Ziembowicz, S.; Kida, M.; Koszelnik, P. Elimination of a Mixture of Microplastics Using Conventional and Detergent-Assisted Coagulation. Materials 2023, 16, 4070. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhao, J.; Ge, W.; Li, W.; Yuan, H. Coagulation-flocculation performance and floc properties for microplastics removal by magnesium hydroxide and PAM. J. Environ. Chem. Eng. 2022, 10, 107263. [Google Scholar] [CrossRef]
- Hu, P.; Ren, J.; Ren, W.; Sun, Y.; Yang, H. The feasibility and mechanism of poly-aluminum/titanium silicate composite coagulants for the efficient removal of nano-and micro-sized plastics. Chem. Eng. J. 2024, 482, 149095. [Google Scholar] [CrossRef]
- Gong, Y.; Bai, Y.; Zhao, D.; Wang, Q. Aggregation of carboxyl-modified polystyrene nanoplastics in water with aluminum chloride: Structural characterization and theoretical calculation. Water Res. 2022, 208, 117884. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ding, S.; Xiao, R.; An, G.; Fang, C.; Chu, W. Enhanced coagulation for mitigation of disinfection by-product precursors: A review. Adv. Colloid Interface Sci. 2021, 296, 102518. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.L.; Ika, A.R. Minimization of halogenated DBP precursors by enhanced PACl coagulation: The impact of organic molecule fraction changes on DBP precursors destabilization with Al hydrates. Sci. Total Environ. 2020, 703, 134936. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ding, S.; An, G.; Qu, R.; Liu, X.; Fang, C.; Chu, W. Removal of disinfection by-product precursors by Al-based coagulants: A comparative study on coagulation performance. J. Hazard. Mater. 2021, 420, 126558. [Google Scholar] [CrossRef]
- Dahasahastra, A.V.; Balasundaram, K.; Latkar, M.V. Turbidity removal from synthetic turbid water using coagulant recovered from water treatment sludge: A potential method to recycle and conserve aluminium. Hydrometallurgy 2022, 213, 105939. [Google Scholar] [CrossRef]
- Okoro, B.U.; Sharifi, S.; Jesson, M.A.; Bridgeman, J. Natural organic matter (NOM) and turbidity removal by plant-based coagulants: A review. J. Environ. Chem. Eng. 2021, 9, 106588. [Google Scholar] [CrossRef]
- Zedan, T.; Mossad, M.; Fouad, M.; Mahanna, H. Potential application of natural coagulant extraction from walnut seeds for water turbidity removal. Water Pract. Technol. 2022, 17, 684–698. [Google Scholar] [CrossRef]
- Ahmad, A.; Abdullah, S.R.S.; Hasan, H.A.; Othman, A.R.; Ismail, N.I. Potential of local plant leaves as natural coagulant for turbidity removal. Environ. Sci. Pollut. Res. 2022, 29, 2579–2587. [Google Scholar] [CrossRef] [PubMed]
- Tahraoui, H.; Belhadj, A.E.; Triki, Z.; Boudellal, N.R.; Seder, S.; Amrane, A.; Zhang, J.; Moula, N.; Tifoura, A.; Ferhat, R.; et al. Mixed coagulant-flocculant optimization for pharmaceutical effluent pretreatment using response surface methodology and Gaussian process regression. Process Saf. Environ. Prot. 2023, 169, 909–927. [Google Scholar] [CrossRef]
- Alazaiza, M.Y.; Albahnasawi, A.; Ali, G.A.; Bashir, M.J.; Nassani, D.E.; Al Maskari, T.; Amr, S.S.A.; Abujazar, M.S.S. Application of natural coagulants for pharmaceutical removal from water and wastewater: A review. Water 2022, 14, 140. [Google Scholar] [CrossRef]
- Nonfodji, O.M.; Fatombi, J.K.; Ahoyo, T.A.; Osseni, S.A.; Aminou, T. Performance of Moringa oleifera seeds protein and Moringa oleifera seeds protein-polyaluminum chloride composite coagulant in removing organic matter and antibiotic resistant bacteria from hospital wastewater. J. Water Process Eng. 2020, 33, 101103. [Google Scholar] [CrossRef]
- Iloamaeke, I.M.; Julius, C.O. Treatment of pharmaceutical effluent using seed of phoenix dactylifera as a natural coagulant. J. Basic Phys. Res. 2019, 9, 91–100. [Google Scholar]
- Liao, Z.L.; Zhao, Z.C.; Zhu, J.C.; Chen, H.; Meng, D.Z. Complexing characteristics between Cu (Ⅱ) ions and dissolved organic matter in combined sewer overflows: Implications for the removal of heavy metals by enhanced coagulation. Chemosphere 2021, 265, 129023. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.D.; Girinathannair, P.; Ohlinger, K.N.; Ritchie, S.; Teuber, L.; Kirby, J. Enhanced removal of heavy metals in primary treatment using coagulation and flocculation. Water Environ. Res. 2008, 80, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Skotta, A.; Jmiai, A.; Elhayaoui, W.; El-Asri, A.; Tamimi, M.; Assabbane, A.; El Issami, S. Suspended matter and heavy metals (Cu and Zn) removal from water by coagulation/flocculation process using a new Bio-flocculant: Lepidium sativum. J. Taiwan Inst. Chem. Eng. 2023, 145, 104792. [Google Scholar] [CrossRef]
- Shekho, M.S.; Hassan, N.E. A Review on Techniques for the Cleaning of Wastewater. GSC Adv. Res. Rev. 2024, 18, 118–128. [Google Scholar] [CrossRef]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef]
- Hasan, F.; Kandhan, K.; Liu, Y.; Ren, M.; Jaleel, A.; Abdul, M. Wastewater Irrigation: A Promising Way for Future Sustainable Agriculture and Food Security in the United Arab Emirates. Water 2023, 15, 2284. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Omoarukhe, F.O.; Ojukwu, V.E.; Iwuozor, K.O.; Igwegbe, C.A. Cost of Adsorbent Preparation and Usage in Wastewater Treatment: A Review. Clean. Chem. Eng. 2022, 3, 100042. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Oveisi, M.; Taghizadeh, A.; Taghizadeh, M. Novel Magnetic Amine Functionalized Carbon Nanotube/Metal-Organic Framework Nanocomposites: From Green Ultrasound-Assisted Synthesis to Detailed Selective Pollutant Removal Modelling from Binary Systems. J. Hazard. Mater. 2019, 368, 746–759. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, N.M.; Oveisi, M.; Taghizadeh, A.; Taghizadeh, M. Synthesis of Pearl Necklace-like ZIF-8@Chitosan/PVA Nanofiber with Synergistic Effect for Recycling Aqueous Dye Removal. Carbohydr. Polym. 2020, 227, 115364. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Taghizadeh, M.; Taghizadeh, A. Mesoporous Activated Carbons of Low-Cost Agricultural Bio-Wastes with High Adsorption Capacity: Preparation and Artificial Neural Network Modeling of Dye Removal from Single and Multicomponent (Binary and Ternary) Systems. J. Mol. Liq. 2018, 269, 217–228. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Williams, R.T. Physisorption Hysteresis Loops and the Characterization of Nanoporous Materials. Adsorpt. Sci. Technol. 2004, 22, 773–782. [Google Scholar] [CrossRef]
- Verma, C.; Aslam, J.; Khan, M.E. Adsorption through Advanced Nanoscale Materials; Elsevier: Amsterdam, The Netherlands, 2023; pp. 8–9. [Google Scholar]
- Xiong, Q.; Li, K.; Yang, D.; Yu, H.; Pan, Z.; Song, Y. Characterizing Coal Pore Space by Gas Adsorption, Mercury Intrusion, FIB–SEM and µ-CT. Environ. Earth Sci. 2020, 79, 209. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, Y.-P.; Guo, J.-S. SPR for Water Pollutant Detection and Water Process Analysis. In Comprehensive Analytical Chemistry; Surface Plasmon Resonance in Bioanalysis; Elsevier: Amsterdam, The Netherlands, 2021; pp. 145–183. [Google Scholar] [CrossRef]
- Tseng, R.-L.; Tran, H.N.; Juang, R.-S. Revisiting Temperature Effect on the Kinetics of Liquid–Phase Adsorption by the Elovich Equation: A Simple Tool for Checking Data Reliability. J. Taiwan Inst. Chem. Eng. 2022, 136, 104403. [Google Scholar] [CrossRef]
- Mudoi, M.P.; Sharma, P.; Khichi, A.S. A Review of Gas Adsorption on Shale and the Influencing Factors of CH4 and CO2 Adsorption. J. Pet. Sci. Eng. 2022, 217, 110897. [Google Scholar] [CrossRef]
- Tien, C. Introduction to Adsorption; Elsevier: Amsterdam, The Netherlands, 2018; pp. 8–11. [Google Scholar]
- Markoš, J.; Steltenpohl, P. Separation Processes II; Slovak Chemical Library: Bratislava, Slovakia, 2017; p. 25. [Google Scholar]
- Shukla, V.; Kumar, N. Environmental Concerns and Sustainable Development. Volume 1, Air, Water and Energy Resources; Springer: Singapore, 2020; p. 372. [Google Scholar]
- Iftekhar, S.; Srivastava, V.; Sillanpää, M. Synthesis of Hybrid Bionanocomposites and Their Application for the Removal of Rare-Earth Elements from Synthetic Wastewater. In Advanced Water Treatment; Elsevier Ebooks; Elsevier: Amsterdam, The Netherlands, 2020; pp. 505–564. [Google Scholar] [CrossRef]
- Chakhtouna, H.; Benzeid, H.; Zari, N.; Qaiss, A.e.K.; Bouhfid, R. Recent Advances in Eco-Friendly Composites Derived from Lignocellulosic Biomass for Wastewater Treatment. Biomass Convers. Biorefinery 2022, 14, 12085–12111. [Google Scholar] [CrossRef]
- Samadi, A.; Kong, L.; Guo, W.; Sillanpää, M.; Boztepe, I.; Song, C.; Zeng, Q.; Zhao, S. Standardized Methodology for Performance Evaluation in Using Polyaniline-Based Adsorbents to Remove Aqueous Contaminants. J. Environ. Chem. Eng. 2024, 12, 112650. [Google Scholar] [CrossRef]
- Zhang, J. Physical Insights into Kinetic Models of Adsorption. Sep. Purif. Technol. 2019, 229, 115832. [Google Scholar] [CrossRef]
- Alves, S.C.; Araújo, R.F.; Moura, T.A.; Sousa, H.; Beatriz, S.; Fregolente, L.G.; Ferreira, O.P.; Avelino, F. Coconut Shell-Based Biochars Produced by an Innovative Thermochemical Process for Obtaining Improved Lignocellulose-Based Adsorbents. Int. J. Biol. Macromol. 2024, 275, 133685. [Google Scholar] [CrossRef]
- Sharafian, A.; Bahrami, M. Assessment of Adsorber Bed Designs in Waste-Heat Driven Adsorption Cooling Systems for Vehicle Air Conditioning and Refrigeration. Renew. Sustain. Energy Rev. 2014, 30, 440–451. [Google Scholar] [CrossRef]
- Zhang, Y.; Palomba, V.; Frazzica, A. Understanding the Effect of Materials, Design Criteria and Operational Parameters on the Adsorption Desalination Performance—A Review. Energy Convers. Manag. 2022, 269, 116072. [Google Scholar] [CrossRef]
- Gutierrez, M.; Mutavdžić Pavlović, D.; Stipaničev, D.; Repec, S.; Avolio, F.; Zanella, M.; Verlicchi, P. A Thorough Analysis of the Occurrence, Removal and Environmental Risks of Organic Micropollutants in a Full-Scale Hybrid Membrane Bioreactor Fed by Hospital Wastewater. Sci. Total Environ. 2024, 914, 169848. [Google Scholar] [CrossRef] [PubMed]
- van der Hoek, J.P.; Deng, T.; Spit, T.; Luimstra, V.; de Kreuk, M.; van Halem, D. Bromate Removal in an Ozone—Granular Activated Carbon Filtration Process for Organic Micropollutants Removal from Wastewater. J. Water Process Eng. 2024, 58, 104877. [Google Scholar] [CrossRef]
- Qin, W.; Dong, Y.; Jiang, H.; Loh, W.H.; Imbrogno, J.; Swenson, T.M.; Garcia-Rodriguez, O.; Lefebvre, O. A New Approach of Simultaneous Adsorption and Regeneration of Activated Carbon to Address the Bottlenecks of Pharmaceutical Wastewater Treatment. Water Res. 2024, 252, 121180. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Weng, Y.; Luo, T.; Wang, Q.; Yang, G.; Jin, Y. Antimicrobial and the Resistances in the Environment: Ecological and Health Risks, Influencing Factors, and Mitigation Strategies. Toxics 2023, 11, 185. [Google Scholar] [CrossRef]
- Deere, J.R.; Streets, S.; Jankowski, M.D.; Ferrey, M.L.; Chenaux-Ibrahim, Y.; Convertino, M.; Isaac, E.J.; Phelps, N.B.D.; Primus, A.; Servadio, J.L.; et al. A Chemical Prioritization Process: Applications to Contaminants of Emerging Concern in Freshwater Ecosystems (Phase I). Sci. Total Environ. 2021, 772, 146030. [Google Scholar] [CrossRef] [PubMed]
- Tiedeken, E.J.; Tahar, A.; McHugh, B.; Rowan, N.J. Monitoring, Sources, Receptors, and Control Measures for Three European Union Watch List Substances of Emerging Concern in Receiving Waters—A 20 Year Systematic Review. Sci. Total Environ. 2017, 574, 1140–1163. [Google Scholar] [CrossRef]
- Sousa, J.C.G.; Ribeiro, A.R.; Barbosa, M.O.; Pereira, M.F.R.; Silva, A.M.T. A Review on Environmental Monitoring of Water Organic Pollutants Identified by EU Guidelines. J. Hazard. Mater. 2018, 344, 146–162. [Google Scholar] [CrossRef]
- Guarin, T.C.; Li, L.; Haak, L.; Teel, L.; Pagilla, K.R. Contaminants of Emerging Concern Reduction and Microbial Community Characterization across a Three-Barrier Advanced Water Treatment System. Sci. Total Environ. 2024, 912, 169637. [Google Scholar] [CrossRef]
- Rizzo, L.; Fiorentino, A.; Grassi, M.; Attanasio, D.; Guida, M. Advanced Treatment of Urban Wastewater by Sand Filtration and Graphene Adsorption for Wastewater Reuse: Effect on a Mixture of Pharmaceuticals and Toxicity. J. Environ. Chem. Eng. 2015, 3, 122–128. [Google Scholar] [CrossRef]
- Emanuele, D.V.D.; Oliveira, M.G.; Spaolonzi, M.P.; Heloisa, P.S.C.; Thiago, L.S.; Melissa, G.A.V. Adsorption of Pharmaceutical Products from Aqueous Solutions on Functionalized Carbon Nanotubes by Conventional and Green Methods: A Critical Review. J. Clean. Prod. 2022, 372, 133743. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Vaccari, M.; van Hullebusch, E.D.; Amrane, A.; Rtimi, S. Mechanisms and Adsorption Capacities of Biochar for the Removal of Organic and Inorganic Pollutants from Industrial Wastewater. Int. J. Environ. Sci. Technol. 2021, 18, 3273–3294. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Tao, X.; Wang, H.; Li, W.; Ding, X.; Chu, H. Biochar as a Low-Cost Adsorbent for Aqueous Heavy Metal Removal: A Review. J. Anal. Appl. Pyrolysis 2021, 155, 105081. [Google Scholar] [CrossRef]
- Ghasaq, A.A.; Salih, M.; Ghsoon, A.F.; Fahim, R. Adsorption Technique for the Removal of Heavy Metals from Wastewater Using Low-Cost Natural Adsorbent. IOP Conf. Ser. Earth Environ. Sci. 2023, 1129, 012012. [Google Scholar] [CrossRef]
- ElSayed, E.E. Natural Diatomite as an Effective Adsorbent for Heavy Metals in Water and Wastewater Treatment (a Batch Study). Water Sci. 2018, 32, 32–43. [Google Scholar] [CrossRef]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.-H.; Show, P.L. A Review on Conventional and Novel Materials towards Heavy Metal Adsorption in Wastewater Treatment Application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Khushk, S.; Zhang, L.; Pirzada, A.M.; Irfan, M.; Li, A. Cr(VI) Heavy Metal Adsorption from Aqueous Solution by KOH Treated Hydrochar Derived from Agricultural Wastes. AIP Conf. Proc. 2019, 2119, 020003. [Google Scholar] [CrossRef]
- Khanzada, A.K.; Al-Hazmi, H.E.; Kurniawan, T.A.; Majtacz, J.; Piechota, G.; Kumar, G.; Ezzati, P.; Saeb, M.R.; Rabiee, N.; Karimi-Maleh, H.; et al. Hydrochar as a Bio-Based Adsorbent for Heavy Metals Removal: A Review of Production Processes, Adsorption Mechanisms, Kinetic Models, Regeneration and Reusability. Sci. Total Environ. 2024, 945, 173972. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, L.; Hu, S.; Liu, Y. Optimized Synthesis of Novel Hydrogel for the Adsorption of Copper and Cobalt Ions in Wastewater. RSC Adv. 2019, 9, 16058–16068. [Google Scholar] [CrossRef]
- Al-Senani, G.M.; Al-Fawzan, F.F. Adsorption Study of Heavy Metal Ions from Aqueous Solution by Nanoparticle of Wild Herbs. Egypt. J. Aquat. Res. 2018, 44, 187–194. [Google Scholar] [CrossRef]
- Li, J.; Dong, X.; Liu, X.; Xu, X.; Duan, W.; Park, J.; Gao, L.; Lu, Y. Comparative Study on the Adsorption Characteristics of Heavy Metal Ions by Activated Carbon and Selected Natural Adsorbents. Sustainability 2022, 14, 15579. [Google Scholar] [CrossRef]
- Teo, Y.S.; Jafari, I.; Liang, F.; Jung, Y.; Van der Hoek, J.P.; Ong, S.L.; Hu, J. Investigation of the Efficacy of the UV/Chlorine Process for the Removal of Trimethoprim: Effects of Operational Parameters and Artificial Neural Networks Modelling. Sci. Total Environ. 2022, 812, 152551. [Google Scholar] [CrossRef]
- Zhong, Y.; Chen, Y.; Ong, S.L.; Hu, J.; Balakrishnan, V.; Ang, W.S. Disinfection By-Products Control in Wastewater Effluents Treated with Ozone and Biological Activated Carbon Followed by UV/Chlor(Am)Ine Processes. Sci. Total Environ. 2024, 922, 171317. [Google Scholar] [CrossRef]
- Shao, B.; Shen, L.; Liu, Z.; Tang, L.; Tan, X.; Wang, D.; Zeng, W.; Wu, T.; Pan, Y.; Zhang, X.; et al. Disinfection Byproducts Formation from Emerging Organic Micropollutants during Chlorine-Based Disinfection Processes. Chem. Eng. J. 2023, 455, 140476. [Google Scholar] [CrossRef]
- Al Bsoul, A.; Hailat, M.; Abdelhay, A.; Tawalbeh, M.; Al-Othman, A.; Al-kharabsheh, I.N.; Al-Taani, A.A. Efficient Removal of Phenol Compounds from Water Environment Using Ziziphus Leaves Adsorbent. Sci. Total Environ. 2021, 761, 143229. [Google Scholar] [CrossRef] [PubMed]
- Vasseghian, Y.; Dragoi, E.-N.; Almomani, F.; Le, V.T. Graphene Derivatives in Bioplastic: A Comprehensive Review of Properties and Future Perspectives. Chemosphere 2022, 286, 131892. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sedighi, M.; Lea-Langton, A. Filtration of Microplastic Spheres by Biochar: Removal Efficiency and Immobilisation Mechanisms. Water Res. 2020, 184, 116165. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Khandelwal, N.; Ganie, Z.A.; Tiwari, E.; Darbha, G.K. Eco-Friendly Magnetic Biochar: An Effective Trap for Nanoplastics of Varying Surface Functionality and Size in the Aqueous Environment. Chem. Eng. J. 2021, 418, 129405. [Google Scholar] [CrossRef]
- Ji, G.; Xing, Y.; You, T. Biochar as Adsorbents for Environmental Microplastics and Nanoplastics Removal. J. Environ. Chem. Eng. 2024, 12, 113377. [Google Scholar] [CrossRef]
- Siipola, V.; Pflugmacher, S.; Romar, H.; Wendling, L.; Koukkari, P. Low-Cost Biochar Adsorbents for Water Purification Including Microplastics Removal. Appl. Sci. 2020, 10, 788. [Google Scholar] [CrossRef]
- Giannakis, S.; Rtimi, S.; Pulgarin, C. Light-Assisted Advanced Oxidation Processes for the Elimination of Chemical and Microbiological Pollution of Wastewaters in Developed and Developing Countries. Molecules 2017, 22, 1070. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, J.; Lodh, B.K.; Sharma, R.; Mahata, N.; Shah, M.P.; Mandal, S.; Ghanta, S.; Bhunia, B. Advanced Oxidation Process for the Treatment of Industrial Wastewater: A Review on Strategies, Mechanisms, Bottlenecks and Prospects. Chemosphere 2023, 345, 140473. [Google Scholar] [CrossRef] [PubMed]
- Mahbub, P.; Duke, M. Scalability of Advanced Oxidation Processes (AOPs) in Industrial Applications: A Review. J. Environ. Manag. 2023, 345, 118861. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, A. Advanced oxidation process: A Remediation Technique for Organic and Non-Biodegradable Pollutant. Results Surf. Interfaces 2023, 11, 100122. [Google Scholar] [CrossRef]
- Rayaroth, M.P.; Aravindakumar, C.T.; Shah, N.S.; Boczkaj, G. Advanced Oxidation Processes (AOPs) Based Wastewater Treatment—Unexpected Nitration Side Reactions—A Serious Environmental Issue: A Review. Chem. Eng. J. 2021, 430, 133002. [Google Scholar] [CrossRef]
- Ghime, D.; Ghosh, P. Advanced Oxidation Processes: A Powerful Treatment Option for the Removal of Recalcitrant Organic Compounds. In Advanced Oxidation Processes—Applications, Trends, and Prospects; IntechOpen: London, UK, 2020. [Google Scholar]
- Oturan, N.; Oturan, M.A. Electro-Fenton Process: Background, New Developments, and Applications. In Electrochemical Water and Wastewater Treatment; Martínez-Huitle, C.A., Rodrigo, M.A., Scialdone, O., Eds.; Butterworth-Heinemann: Oxford, UK, 2018; pp. 193–221. [Google Scholar] [CrossRef]
- Pliego, G.; Zazo, J.A.; Garcia-Muñoz, P.; Munoz, M.; Casas, J.A.; Rodriguez, J.J. Trends in the Intensification of the Fenton Process for Wastewater Treatment: An Overview. Crit. Rev. Env. Sci. Technol. 2015, 45, 2611–2692. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J. Metal Organic Framework with Coordinatively Unsaturated Sites as Efficient Fenton-like Catalyst for Enhanced Degradation of Sulfamethazine. Environ. Sci. Technol. 2018, 52, 5367–5377. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Reactive Species in Advanced Oxidation Processes: Formation, Identification and Reaction Mechanism. Chem. Eng. J. 2020, 401, 126158. [Google Scholar] [CrossRef]
- Zhang, M.; Dong, H.; Zhao, L.; Wang, D.; Meng, D. A Review on Fenton Process for Organic Wastewater Treatment Based on Optimization Perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, Z.; Sun, H.; Zhao, Q.; Huang, Z.; Zhang, C.; Wang, Y.; Yang, C.; Zhou, Z. Comparison on Molecular Transformation of Dissolved Organic Matter during Fenton and Activated Carbon Adsorption Processes for Chemical Cleaning Wastewater Treatment. Sep. Purif. Technol. 2024, 344, 127226. [Google Scholar] [CrossRef]
- Wang, X.; Dai, Y.; Li, Y.; Yin, L. Application of Advanced Oxidation Processes for the Removal of Micro/Nanoplastics from Water: A Review. Chemosphere 2024, 346, 140636. [Google Scholar] [CrossRef]
- Ortiz, D.; Munoz, M.; Nieto-Sandoval, J.; Romera-Castillo, C.; de Pedro, Z.M.; Casas, J.A. Insights into the Degradation of Microplastics by Fenton Oxidation: From Surface Modification to Mineralization. Chemosphere 2022, 309, 136809. [Google Scholar] [CrossRef]
- Ziembowicz, S.; Kida, M. Limitations and Future Directions of Application of the Fenton-like Process in Micropollutants Degradation in Water and Wastewater Treatment: A Critical Review. Chemosphere 2022, 296, 134041. [Google Scholar] [CrossRef]
- Sobczak, M.; Bujnowicz, S.; Bilińska, L. Fenton and Electro-Fenton Treatment for Industrial Textile Wastewater Recycling. Comparison of By-Products Removal, Biodegradability, Toxicity, and Re-Dyeing. Water Resour. Ind. 2024, 31, 100256. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Q.; Li, H.; Yang, P. Enhanced Heterogeneous Electro-Fenton Degradation of Salicylic Acid by Different Fe3O4 Loaded Carriers. Desalination Water Treat. 2024, 320, 100723. [Google Scholar] [CrossRef]
- Zhu, Y.; Xie, Q.; Deng, F.; Ni, Z.; Lin, Q.; Cheng, L.; Chen, X.; Qiu, R.; Zhu, R. The Differences in Heterogeneous Fenton Catalytic Performance and Mechanism of Various Iron Minerals and Their Influencing Factors: A Review. Sep. Purif. Technol. 2023, 325, 124702. [Google Scholar] [CrossRef]
- Liu, Z.; Demeestere, K.; Hulle, S.V. Comparison and Performance Assessment of Ozone-Based AOPs in View of Trace Organic Contaminants Abatement in Water and Wastewater: A Review. J. Environ. Chem. Eng. 2021, 9, 105599. [Google Scholar] [CrossRef]
- Derco, J.; Gotvajn, A.Ž.; Čižmárová, O.; Dudáš, J.; Sumegová, L.; Šimovičová, K. Removal of Micropollutants by Ozone-Based Processes. Processes 2021, 9, 1013. [Google Scholar] [CrossRef]
- Tripathi, S.C.; Hussain, T. Water and Wastewater Treatment through Ozone-Based Technologies. In Development in Wastewater Treatment Research and Processes; Shah, M., Rodriguez-Couto, S., Biswas, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 139–172. [Google Scholar] [CrossRef]
- Rekhate, C.V.; Srivastava, J.K. Recent Advances in Ozone-Based Advanced Oxidation Processes for Treatment of Wastewater- a Review. Chem. Eng. J. Adv. 2020, 3, 100031. [Google Scholar] [CrossRef]
- Derco, J.; Dudáš, J.; Valičková, M.; Šimovičová, K.; Kecskés, J. Removal of Micropollutants by Ozone Based Processes. Chem. Eng. Process. Process Intensif. 2015, 94, 78–84. [Google Scholar] [CrossRef]
- Deng, L.-Z.; Mujumdar, A.S.; Pan, Z.; Vidyarthi, S.K.; Xu, J.; Zielinska, M.; Xiao, H.-W. Emerging Chemical and Physical Disinfection Technologies of Fruits and Vegetables: A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2019, 60, 2481–2508. [Google Scholar] [CrossRef] [PubMed]
- Gou, X.; Yuan, D.; Wang, L.; Xie, L.; Wei, L.; Zhang, G. Enhancing Ozone Production in Dielectric Barrier Discharge Utilizing Water as Electrode. Vacuum 2023, 212, 112047. [Google Scholar] [CrossRef]
- Joseph, C.G.; Farm, Y.Y.; Taufiq-Yap, Y.H.; Pang, C.K.; Nga, J.L.H.; Li Puma, G. Ozonation Treatment Processes for the Remediation of Detergent Wastewater: A Comprehensive Review. J. Environ. Chem. Eng. 2021, 9, 106099. [Google Scholar] [CrossRef]
- Presumido, P.H.; Ribeirinho-Soares, S.; Montes, R.; Quintana, J.B.; Rodil, R.; Ribeiro, M.; Neuparth, T.; Santos, M.M.; Feliciano, M.; Nunes, O.C.; et al. Ozone Membrane Contactor for Tertiary Treatment of Urban Wastewater: Chemical, Microbial and Toxicological Assessment. Sci. Total. Environ. 2023, 892, 164492. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Choi, S.; Kim, H.; Lee, W.; Lee, M.; Son, H.J.; Chang, H.L.; Cho, M.; Lee, Y. Efficiency of Ozonation and O3/H2O2 as Enhanced Wastewater Treatment Processes for Micropollutant Abatement and Disinfection with Minimized Byproduct Formation. J. Hazard. Mater. 2023, 454, 131436. [Google Scholar] [CrossRef] [PubMed]
- Miklos, D.B.; Wang, W.-L.; Linden, K.G.; Drewes, J.E.; Hübner, U. Comparison of UV-AOPs (UV/H2O2, UV/PDS and UV/Chlorine) for TOrC Removal from Municipal Wastewater Effluent and Optical Surrogate Model Evaluation. Chem. Eng. J. 2019, 362, 537–547. [Google Scholar] [CrossRef]
- Miklos, D.B.; Hartl, R.; Philipp, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. UV/H2O2 Process Stability and Pilot-Scale Validation for Trace Organic Chemical Removal from Wastewater Treatment Plant Effluents. Water Res. 2018, 136, 169–179. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Pedrazzani, R.; Sorlini, S.; Abbà, A.; Bertanza, G. H2O2 Based Oxidation Processes for the Treatment of Real High Strength Aqueous Wastes. Sustainability 2017, 9, 244. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, J.; Li, C.; Tian, F.; Gao, N. Comparative Evaluation of Metoprolol Degradation by UV/Chlorine and UV/H2O2 Processes. Chemosphere 2020, 243, 125325. [Google Scholar] [CrossRef]
- Farzanehsa, M.S.Z.; Vaughan, L.; Zamyadi, A.; Khan, S.J. Comparison of UV-Cl and UV-H2O2 Advanced Oxidation Processes in the Degradation of Contaminants from Water and Wastewater: A Review. Water Environ. J. 2023, 37, 633–643. [Google Scholar] [CrossRef]
- Akerdi, A.T.; Bahrami, S.H. Application of Heterogeneous Nano-Semiconductors for Photocatalytic Advanced Oxidation of Organic Compounds: A Review. J. Environ. Chem. Eng. 2019, 7, 103283. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M.; Raha, S. ZnO Nanostructured Materials and Their Potential Applications: Progress, Challenges and Perspectives. Nanoscale Adv. 2022, 8, 1868–1925. [Google Scholar] [CrossRef]
- Martínez-Escudero, C.M.; Garrido, I.; Contreras, F.; Hellín, P.; Flores, P.; León-Morán, L.O.; Arroyo-Manzanares, N.; Campillo, N.; Pastor, M.; Viñas, P.; et al. Photodecomposition of Antibiotics and Their Transformation Products in Wastewaters Using ZnO and TiO2 with LED Lamps. J. Photochem. Photobiol. A Chem. 2024, 454, 115732. [Google Scholar] [CrossRef]
- Allé, P.H.; Garcia-Muñoz, P.; Adouby, K.; Keller, N.; Robert, D. Efficient Photocatalytic Mineralization of Polymethylmethacrylate and Polystyrene Nanoplastics by TiO2/β-SiC Alveolar Foams. Environ. Chem. Lett. 2020, 19, 1803–1808. [Google Scholar] [CrossRef]
- Domínguez-Jaimes, L.P.; Cedillo-González, E.I.; Luévano-Hipólito, E.; Acuña-Bedoya, J.D.; Hernández-López, J.M. Degradation of Primary Nanoplastics by Photocatalysis Using Different Anodized TiO2 Structures. J. Hazard. Mater. 2021, 413, 125452. [Google Scholar] [CrossRef]
- Beyazıt, N.; Karaca, H. Performance comparison of UV, UV/H2O2, UV/Fe2+, H2O2/Fe2+, UV/H2O2/Fe2+ processes in the removal of COD and color from textile wastewater. J. Sci. Rep.-A 2020, 45, 236–252. [Google Scholar]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical Advanced Oxidation Processes: A Review on Their Application to Synthetic and Real Wastewaters. Appl. Catal. B Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Martínez-Huitle, C.A.; Rodrigo, M.A. Renewable Energies Driven Electrochemical Wastewater/Soil Decontamination Technologies: A Critical Review of Fundamental Concepts and Applications. Appl. Catal. B Environ. 2020, 270, 118857. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, C.; Waite, T.D. Hydroxyl Radicals in Anodic Oxidation Systems: Generation, Identification and Quantification. Water Res. 2022, 217, 118425. [Google Scholar] [CrossRef]
- Hu, Z.; Cai, J.; Song, G.; Tian, Y.; Zhou, M. Anodic Oxidation of Organic Pollutants: Anode Fabrication, Process Hybrid and Environmental Applications. Curr. Opin. Electrochem. 2021, 26, 100659. [Google Scholar] [CrossRef]
- Kim, S.; Jeong, Y.; Park, M.-O.; Jang, Y.; Bae, J.-S.; Hong, K.-S.; Kim, S.; Song, P.; Yoon, J.H. Development of Boron Doped Diamond Electrodes Material for Heavy Metal Ion Sensor with High Sensitivity and Durability. J. Mater. Res. Technol. 2023, 23, 1375–1385. [Google Scholar] [CrossRef]
- Vinayagam, V.; Palani, K.N.; Ganesh, S.; Rajesh, S.; Akula, V.V.; Avoodaiappan, R.; Kushwaha, O.S.; Pugazhendhi, A. Recent developments on advanced oxidation processes for degradation of pollutants from wastewater with focus on antibiotics and organic dyes. Environ. Res. 2024, 240, 117500. [Google Scholar] [CrossRef]
- Brillas, E.; Garcia-Segura, S. Benchmarking Recent Advances and Innovative Technology Approaches of Fenton, Photo-Fenton, Electro-Fenton, and Related Processes: A Review on the Relevance of Phenol as Model Molecule. Sep. Purif. Technol. 2020, 237, 116337. [Google Scholar] [CrossRef]
- Brillas, E. Fenton, Photo-Fenton, Electro-Fenton, and Their Combined Treatments for the Removal of Insecticides from Waters and Soils. A Review. Sep. Purif. Technol. 2022, 284, 120290. [Google Scholar] [CrossRef]
- Monteil, H.; Péchaud, Y.; Oturan, N.; Oturan, M.A. A Review on Efficiency and Cost Effectiveness of Electro- and Bio-Electro-Fenton Processes: Application to the Treatment of Pharmaceutical Pollutants in Water. Chem. Eng. J. 2019, 376, 119577. [Google Scholar] [CrossRef]
- Ismail, S.A.; Ang, W.L.; Mohammad, A.W. Electro-Fenton Technology for Wastewater Treatment: A Bibliometric Analysis of Current Research Trends, Future Perspectives and Energy Consumption Analysis. J. Water Process Eng. 2021, 40, 101952. [Google Scholar] [CrossRef]
- Madhavan, J.; Theerthagiri, J.; Balaji, D.; Sunitha, S.; Choi, M.; Ashokkumar, M. Hybrid Advanced Oxidation Processes Involving Ultrasound: An Overview. Molecules 2019, 24, 3341. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Porras, J.; Torres-Palma, R.A. A Critical Review on the Sonochemical Degradation of Organic Pollutants in Urine, Seawater, and Mineral Water. Ultrason. Sonochem. 2022, 82, 105861. [Google Scholar] [CrossRef]
- Hayati, F.; Khodabakhshi, M.R.; Isari, A.A.; Moradi, S.; Kakavandi, B. LED-Assisted Sonocatalysis of Sulfathiazole and Pharmaceutical Wastewater Using N, Fe Co-Doped TiO2@SWCNT: Optimization, Performance and Reaction Mechanism Studies. J. Water Process Eng. 2020, 38, 101693. [Google Scholar] [CrossRef]
- Calcio Gaudino, E.; Canova, E.; Liu, P.; Wu, Z.; Cravotto, G. Degradation of Antibiotics in Wastewater: New Advances in Cavitational Treatments. Molecules 2021, 26, 617. [Google Scholar] [CrossRef]
- Saleh, R.; Taufik, A. Degradation of Methylene Blue and Congo-Red Dyes Using Fenton, Photo-Fenton, Sono-Fenton, and Sonophoto-Fenton Methods in the Presence of Iron(II,III) Oxide/Zinc Oxide/Graphene (Fe3O4/ZnO/Graphene) Composites. Sep. Purif. Technol. 2019, 210, 563–573. [Google Scholar] [CrossRef]
- Nicodemos, D.; Santana, C.S.; Silva, C.C.; Magalhães, F.; Aguiar, A. A Review on the Treatment of Textile Industry Effluents through Fenton Processes. Process Saf. Environ. Prot. 2021, 155, 366–386. [Google Scholar] [CrossRef]
- Ofori, S.; Puškáčová, A.; Růžičková, I.; Wanner, J. Treated Wastewater Reuse for Irrigation: Pros and Cons. Sci. Total Environ. 2021, 760, 144026. [Google Scholar] [CrossRef] [PubMed]
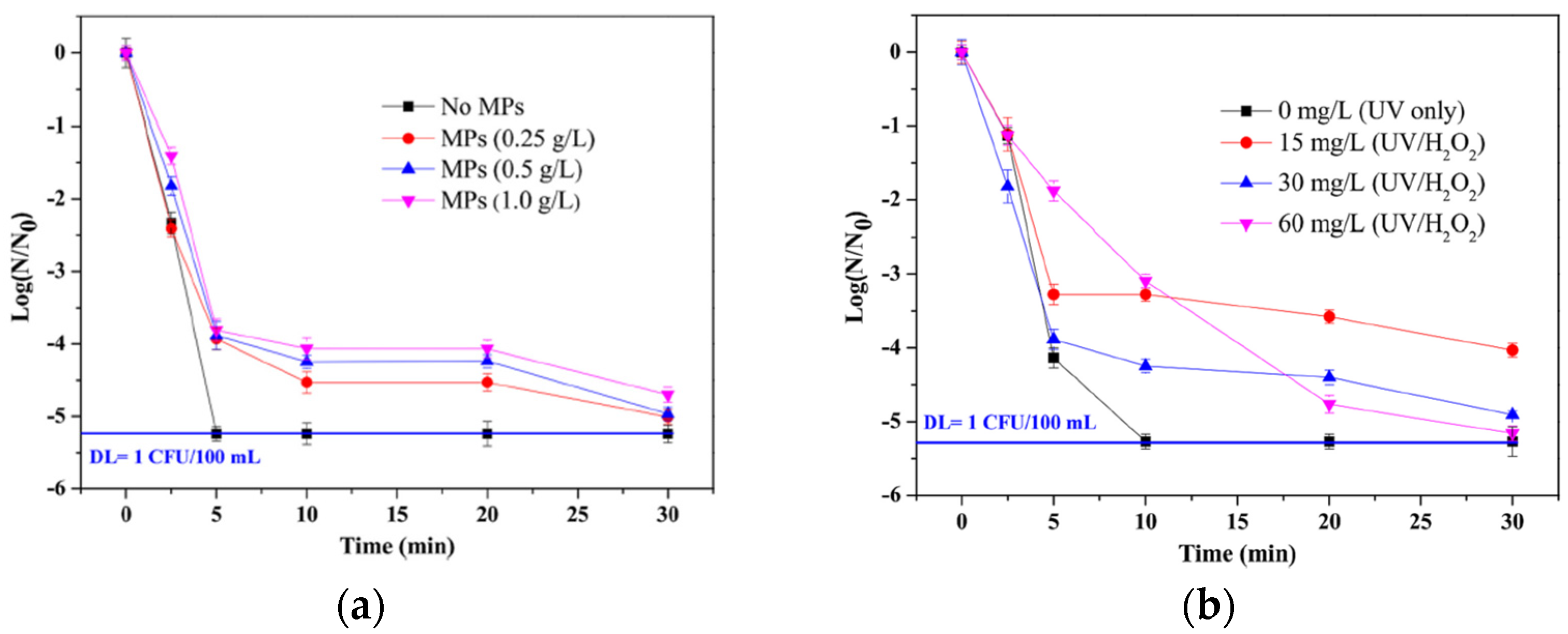
- Adeel, M.; Maniakova, G.; Rizzo, L. Tertiary/Quaternary Treatment of Urban Wastewater by UV/H2O2 or Ozonation: Microplastics May Affect Removal of E. Coli and Contaminants of Emerging Concern. Sci. Total Environ. 2024, 907, 167940. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Li, Z.; Jiang, L.; Liu, H.; Zhang, Y.; Sun, Y. Kinetics and Mechanisms of Bacteria Disinfection by Performic Acid in Wastewater: In Comparison with Peracetic Acid and Sodium Hypochlorite. Sci. Total. Environ. 2023, 878, 162606. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wei, X.; Gao, A.; Zhou, L.; Shi, X.; Zhou, X.; Bi, X.; Yang, T.; Huang, S. Performance and Mechanism of Sequential UV-NaClO Disinfection: Inactivation and Reactivation of Antibiotic-Resistant Bacteria, Disinfection Byproduct Formation and Microbial Community Variation. J. Water Process Eng. 2024, 58, 104824. [Google Scholar] [CrossRef]
- Li, Q.; Cui, X.; Gao, X.; Chen, X.; Zhao, H. Intelligent Dosing of Sodium Hypochlorite in Municipal Wastewater Treatment Plants: Experimental and Modeling Studies. J. Water Process Eng. 2024, 64, 105662. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Hypochlorous Acid Chemistry in Mammalian Cells—Influence on Infection and Role in Various Pathologies. Int. J. Mol. Sci. 2022, 23, 10735. [Google Scholar] [CrossRef]
- Kesar, S.; Bhatti, M.S. Chlorination of Secondary Treated Wastewater with Sodium Hypochlorite (NaOCl): An Effective Single Alternate to Other Disinfectants. Heliyon 2022, 8, e11162. [Google Scholar] [CrossRef]
- Shi, C.; Li, C.; Wang, Y.; Guo, J.; Barry, S.; Zhang, Y.; Marmier, N. Review of Advanced Oxidation Processes Based on Peracetic Acid for Organic Pollutants. Water 2022, 14, 2309. [Google Scholar] [CrossRef]
- Chen, G.-Y.; Lin, Y.-H.; Fu, C.-H.; Lin, C.-H.; Muthiah, B.; Espulgar, W.V.; Santos, G.N.; Yu, D.E.; Kasai, T. Quantification of Peracetic Acid (PAA) in the H2O2 + Acetic Acid Reaction by the Wavelength Shift Analysis in Near-UV/Visible Absorption Region. Anal. Sci. 2024, 40, 489–499. [Google Scholar] [CrossRef]
- Lin, Y.; He, Y.; Sun, Q.; Ping, Q.; Huang, M.; Wang, L.; Li, Y. Underlying the Mechanisms of Pathogen Inactivation and Regrowth in Wastewater Using Peracetic Acid-Based Disinfection Processes: A Critical Review. J. Hazard. Mater. 2024, 463, 132868. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, W.; Wang, T.; Reid, E.; Krall, C.; Kim, J.; Zhang, T.; Xie, X.; Huang, C.-H. Bacteria and Virus Inactivation: Relative Efficacy and Mechanisms of Peroxyacids and Chlor(Am)Ine. Environ. Sci. Technol. 2023, 57, 18710–18721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiang, J.-L.; Wang, J.; Du, H.-S.; Wang, T.; Huo, Z.-Y.; Wang, W.; Liu, M.; Du, Y. Ultraviolet-Based Synergistic Processes for Wastewater Disinfection: A Review. J. Hazard. Mater. 2023, 453, 131393. [Google Scholar] [CrossRef] [PubMed]
- Foschi, J.; Turolla, A.; Antonelli, M. Artificial Neural Network Modeling of Full-Scale UV Disinfection for Process Control Aimed at Wastewater Reuse. J. Environ. Manag. 2021, 300, 113790. [Google Scholar] [CrossRef] [PubMed]
- Manoli, K.; Naziri, A.; Ttofi, I.; Michael, C.; Allan, I.J.; Fatta-Kassinos, D. Investigation of the Effect of Microplastics on the UV Inactivation of Antibiotic-Resistant Bacteria in Water. Water Res. 2022, 222, 118906. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Z.; Xuan, X.; Ji, L.; Li, X.; Tao, Y.; Boczkaj, G.; Zhao, S.; Yoon, J.Y.; Chen, S. Disinfection Characteristics of an Advanced Rotational Hydrodynamic Cavitation Reactor in Pilot Scale. Ultrason. Sonochem. 2021, 73, 105543. [Google Scholar] [CrossRef]
- Suprakas, S.R.; Rashi, G.; Kumar, N. Water Purification Using Various Technologies and Their Advantages and Disadvantages. In Carbon Nanomaterial-Based Adsorbents for Water Purification; Elsevier: Amsterdam, The Netherlands, 2020; pp. 37–66. [Google Scholar] [CrossRef]
- Chen, Y.; Duan, X.; Zhou, X.; Wang, R.; Wang, S.; Ren, N.; Ho, S.-H. Advanced Oxidation Processes for Water Disinfection: Features, Mechanisms and Prospects. Chem. Eng. J. 2021, 409, 128207. [Google Scholar] [CrossRef]
- Li, Z.; Yang, D.; Li, S.; Yang, L.; Yan, W.; Xu, H. Advances on Electrochemical Disinfection Research: Mechanisms, Influencing Factors and Applications. Sci. Total Environ. 2024, 912, 169043. [Google Scholar] [CrossRef]
- Mosquera-Romero, S.; Prévoteau, A.; Louage, F.; Dominguez-Granda, L.; Korneel, R.; Diederik, P.L. Rousseau Fouling and Energy Consumption Impede Electrochemical Disinfection of Constructed Wetland Effluents. J. Environ. Chem. Eng. 2024, 12, 113348. [Google Scholar] [CrossRef]
- Li, H.; Dechesne, A.; He, Z.; Jensen, M.M.; Song, H.L.; Smets, B.F. Electrochemical Disinfection May Increase the Spread of Antibiotic Resistance Genes by Promoting Conjugal Plasmid Transfer. Sci. Total Environ. 2023, 858, 159846. [Google Scholar] [CrossRef] [PubMed]



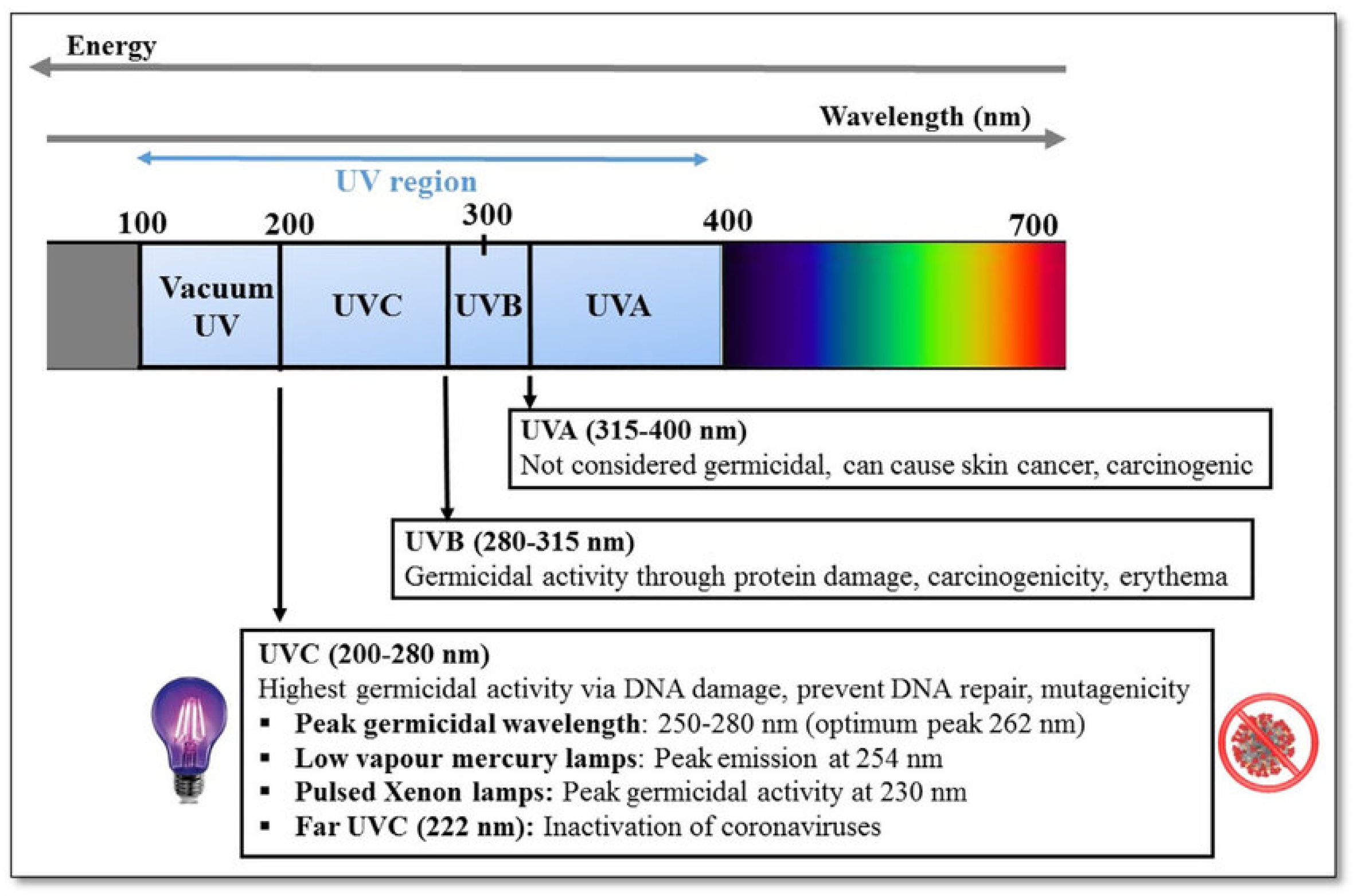
| Parameter | Symbol | Unit |
|---|---|---|
| Iodine number | IN | mg/g |
| Ash | % | |
| Porosity | εp | – |
| Skeletal density | ρHe | kg/m3 |
| Geometrical density | ρHg | kg/m3 |
| Bulk density | ρs | kg/m3 |
| Specific surface | SBET | m2/g |
| Particle size | d | mm |
| Pore size | dp | Å |
| Moisture | – | % |
| ROS as Free Oxygen Radicals | Non-Radical ROS |
|---|---|
| Hydroxyl radical •OH | Hydrogen peroxide H2O2 |
| Superoxide anion radical O2•− | Singlet oxygen 1O2 |
| Alkoxyl radical RO• | Ozone/trioxygen O3 |
| Peroxyl radical ROO• | Organic hydroperoxides ROOH |
| Hydroperoxide radical •OOH | Hypochloride HOCl |
| Nitric oxide NO• | Peroxynitrite ONO− |
| Thiyl radicals RS• | Nitrocarbonate anion O2NOCO2− |
| Sulphonyl radicals ROS• | Dinitrogen dioxide N2O2 |
| Thiyl peroxyl RSOO• | Nitronium NO2+ |
| Sulphate radicals SO4•− | Highly reactive lipid- or carbohydrate-derived carbonyl compounds |
| Method of Ozone Generation | Principle | Ozone Source |
|---|---|---|
| Electrical | Electrical discharge | Air or O2 |
| Electrochemical | Electrolysis | Water (highly purified) |
| Photochemical | Irradiation (λ < 185 nm) | Air, O2, water |
| Radiation chemistry | X-rays, radioactive γ-rays | Water (highly purified) |
| Thermal | Light arc ionization | Water |
| Method | Advantages | Disadvantages |
|---|---|---|
| Sand filtration | Chemicals free, simple in operation, no harmful byproducts, relatively low financial costs, relatively well known and commercially available. | Very low removal efficiency for micropollutants and other contaminants, backwash is needed, and disposal of used sand. |
| Membrane filtration | Well-defined and high removal efficiency of micropollutants, capable of removal of other contaminants and microorganisms, no toxic solid waste, chemicals free, no harmful byproducts and commercially available. | High energy demand, membrane fouling, disposal of concentrate, high water rejection, corrosive nature of the produced water, high-tech operation and maintenance, and relatively high financial costs. |
| Coagulation | Simple in operation, no harmful byproducts, relatively low financial costs, relatively well known and common chemicals are available. | Low removal efficiency for micropollutants, large amount of chemical sludge, introduction of coagulant salts in the aqueous phase, and sedimentation and filtration is needed. |
| Adsorption | High removal efficiency of micropollutants and other contaminants, simple in operation, chemical and sludge free, no harmful byproducts, relatively well known and commercially available. | Lower efficiency removal in the presence of NOMs, regeneration is needed, disposal of used carbon, production of toxic solid waste, desorption of sorbed contaminants, and relatively high financial costs. |
| Ozonation and other AOPs | Novel and promising technique, high removal efficiency of micropollutants, other contaminants and microorganisms, sludge free, ozonation is well known and commercially available. | High energy consumption, formation of harmful byproducts, interference of radical scavengers, strong developing is needed, focus on effective design and operation parameters is needed, other AOPs are not commercially available, and relatively high financial costs. |
| Chemical disinfection | Simple in operation, high removal efficiency of microorganisms, no toxic solid waste is produced, relatively low financial costs, relatively well known and commercially available. | Formation of harmful byproducts, not chemicals free, corrosive effects, requires understanding of principles of chemical disinfection, And does not prevent stored water from recontamination. |
| Physical disinfection (UV) | Simple in operation, high removal efficiency of microorganisms, no toxic solid waste, no harmful byproducts, chemicals free, relatively well known and commercially available. | Requires clear water, does not prevent stored water from recontamination, and relatively high financial costs. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurík, J.; Jankovičová, B.; Zakhar, R.; Šoltýsová, N.; Derco, J. Quaternary Treatment of Urban Wastewater for Its Reuse. Processes 2024, 12, 1905. https://doi.org/10.3390/pr12091905
Jurík J, Jankovičová B, Zakhar R, Šoltýsová N, Derco J. Quaternary Treatment of Urban Wastewater for Its Reuse. Processes. 2024; 12(9):1905. https://doi.org/10.3390/pr12091905
Chicago/Turabian StyleJurík, Jakub, Barbora Jankovičová, Ronald Zakhar, Nikola Šoltýsová, and Ján Derco. 2024. "Quaternary Treatment of Urban Wastewater for Its Reuse" Processes 12, no. 9: 1905. https://doi.org/10.3390/pr12091905







