Nutritional and Possible Pharmaceutical Aspects of Tree Exudates Eaten by Lemurs of Madagascar’s Dry Forests †
Highlights
- Tree exudates can represent valuable food resources for primates and other animals, especially during dry seasons.
- Tree exudates can contain high protein concentrations and provide high energy contents if animals can break down ß-linked glycosides.
- Exudates from some trees are consumed even though they have very low protein and energy contents.
- These low nutritional exudates have antibacterial activity and contain components with pharmaceutical properties.
- Tree exudates could be screened for pharmaceutical properties in view of self-medication of primates as well as for human applications.
Abstract
:1. Introduction
- Do facultative gum feeders consume gum of different nutritional quality than obligate gum-feeding species?
- Are the protein, sugar, and energy content of gum relevant for food selection?
- Are consumed gums with low protein, sugar, and/or energy content more likely to display antibacterial activity than gums of higher nutritional value?
2. Materials and Methods
2.1. Comparison of Obligate and Facultative Gum Feeders
2.2. Relevance of Protein, Sugar, and Energy Content for Gum Consumption of Microcebus griseorufus
2.3. Antibacterial Properties and Chemical Composition of Consumed Gum
2.3.1. Chemical Analyses
2.3.2. Antibacterial Activity
2.3.3. Statistics
2.4. Ethical Note
3. Results
3.1. Regional Study
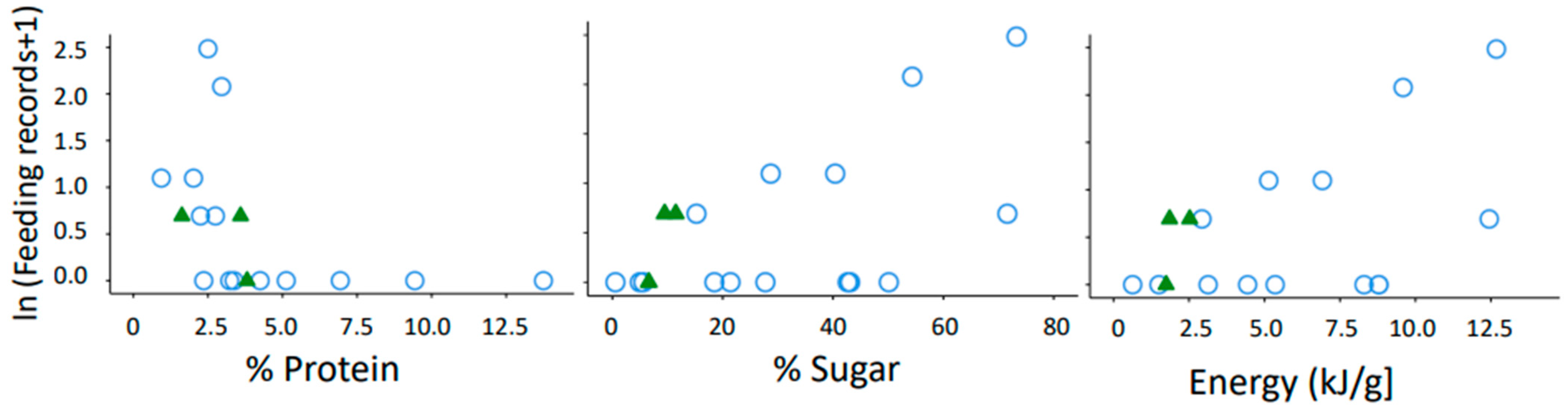
3.2. Relevance of Protein, Sugar and Energy Content for Gum Consumption of Microcebus griseorufus
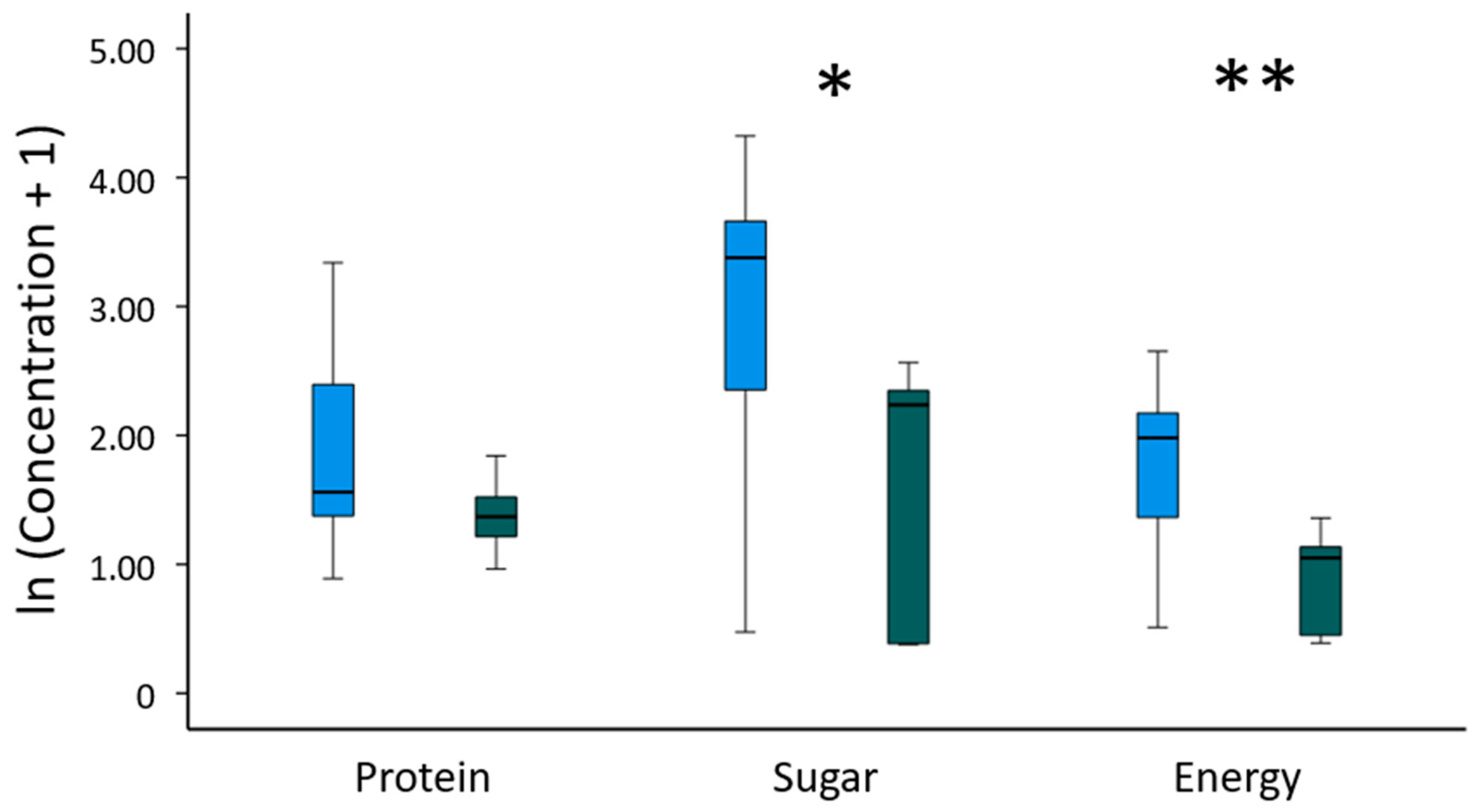
3.3. Antibacterial Properties and Chemical Composition of Consumed Gum
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Tree Species | Site of Collection | Year Collected | Consumer Species | Protein % | Sugar % | Energy kJ_g |
|---|---|---|---|---|---|---|
| Acacia bellula | Tsimanampetsotse | 2008 | Mg | 10.44 | 34.55 | 7.53 |
| Acacia bellula | Tsimanampetsotse | 2008 | Mg | 7.13 | 34.40 | 6.95 |
| Acacia bellula | Tsimanampetsotse | 2008 | Mg | 3.31 | 59.00 | 10.43 |
| Alantsilodendron alluaudianum | Berenty | 2006 | Mg | 21.38 | 10.01 | 5.25 |
| Alantsilodendron alluaudianum | Berenty | 2006 | Mg | 19.81 | 41.00 | 10.18 |
| Alantsilodendron alluaudianum | Berenty | 2006 | Mg | 19.06 | 27.49 | 7.79 |
| Alantsilodendron alluaudianum | Berenty | 2006 | Mg | 18.44 | 47.63 | 11.06 |
| Alantsilodendron alluaudianum | Berenty | 2006 | Mg | 12.88 | 48.68 | 10.30 |
| Albiza tulearensis | Tsimanampetsotse | 2012 | Mg | 4.13 | 0.65 | 0.80 |
| Albiza tulearensis | Tsimanampetsotse | 2012 | Mg | 2.94 | 0.47 | 0.57 |
| Albiza tulearensis | Tsimanampetsotse | 2012 | Mg | 2.63 | 0.57 | 0.53 |
| Albizia mainaea | Analabe | 2007 | Mm, Pp | 11.31 | 28.35 | 6.64 |
| Albizia microphylla | Berenty | 2011 | Mg | 2.19 | 8.04 | 1.71 |
| Astrotrichilia asterotricha | Ankarafantsika | 2009 | Mm, Mr | 1.75 | 21.93 | 3.96 |
| Azima tetracantha | Berenty | 2004 | Mg | 5.25 | 65.62 | 11.86 |
| Commiphora 1 | Berenty | 2004 | Mg | 4.00 | 32.23 | 6.06 |
| Commiphora 3 | Berenty | 2004 | Mg | 2.81 | 31.37 | 5.72 |
| Commiphora 5 | Berenty | 2004 | Mg | 2.75 | 30.42 | 5.55 |
| Commiphora aprevalii | Berenty | 2006 | Mg | 4.56 | 59.08 | 10.65 |
| Commiphora arafy | Analabe | 2007 | Mm, Pp | 15.25 | 30.81 | 7.71 |
| Commiphora arafy | Kirindy | 2000 | Pp | 32.82 | ||
| Commiphora arafy | Kirindy | 1990 | Pp | 16.70 | ||
| Commiphora arafy | Kirindy | 1989 | Pp | 17.10 | ||
| Commiphora humbertii | Tsimanampetsotse | 2008 | Mg | 6.05 | ||
| Commiphora humbertii | Berenty | 2006 | Mg | 1.94 | 28.98 | 5.17 |
| Commiphora lamii | Tsimanampetsotse | 2008 | Mg | 9.44 | 41.53 | 8.53 |
| Commiphora lamii | Berenty | 2006 | Mg | 5.00 | 31.4 | 6.09 |
| Commiphora marchandii | Tsimanampetsotse | 2008 | Mg | 9.44 | 53.73 | 10.57 |
| Commiphora marchandii | Tsimanampetsotse | 2008 | Mg | 25.67 | ||
| Commiphora marchandii | Tsimanampetsotse | 2008 | Mg | 49.94 | ||
| Commiphora monstruosa | Tsimanampetsotse | 2008 | Mg | 24.66 | ||
| Commiphora orbicularis | Berenty | 2006 | Mg | 11.38 | 39.33 | 8.49 |
| Commiphora orbicularis | Berenty | 2006 | Mg | 11.31 | 55.94 | 11.26 |
| Commiphora orbicularis | Berenty | 2006 | Mg | 10.06 | 32.51 | 7.12 |
| Commiphora simplicifolia | Tsimanampetsotse | 2008 | Mg | 4.69 | 9.17 | 2.32 |
| Commiphora simplicifolia | Tsimanampetsotse | 2008 | Mg | 4.31 | 15.22 | 3.27 |
| Commiphora simplicifolia | Tsimanampetsotse | 2008 | Mg | 4.00 | 9.88 | 2.32 |
| Commiphora simplicifolia | Tsimanampetsotse | 2012 | Mg | 3.81 | 4.90 | 1.46 |
| Commiphora simplicifolia | Tsimanampetsotse | 2012 | Mg | 3.81 | 6.02 | 1.65 |
| Commiphora simplicifolia | Tsimanampetsotse | 2008 | Mg | 2.94 | 12.35 | 2.56 |
| Commiphora simplicifolia | Tsimanampetsotse | 2008 | Mg | 1.38 | 17.39 | 3.14 |
| Commiphora simplicifolia | Tsimanampetsotse | 2008 | Mg | 4.76 | ||
| Commiphora simplicifolia | Tsimanampetsotse | 2008 | Mg | 5.81 | ||
| Commiphora simplicifolia | Tsimanampetsotse | 2012 | Mg | 8.92 | ||
| Commiphora simplicifolia | Tsimanampetsotse | 2008 | Mg | 10.7 | ||
| Commiphora sp. | Berenty | 2006 | Mg | 2.63 | 50.17 | 8.84 |
| Commiphora stellulata | Kirindy | 1990 | Pp | 26.10 | ||
| Commiphora trifolia | Berenty | 2003 | Mg | 16.88 | 2.13 | 3.18 |
| Commiphora_Anka1 | Ankarafantsika | 2009 | Mm | 17.75 | 38.17 | 9.36 |
| Delonix floribunda | Tsimanampetsotse | 2008 | Mg | 4.56 | 21.99 | 4.44 |
| Delonix floribunda | Tsimanampetsotse | 2008 | Mg | 4.25 | 26.71 | 5.18 |
| Delonix floribunda | Tsimanampetsotse | 2008 | Mg | 2.75 | 71.61 | 12.44 |
| Delonix floribunda | Tsimanampetsotse | 2008 | Mg | 2.50 | 58.14 | 10.15 |
| Delonix floribunda | Tsimanampetsotse | 2008 | Mg | 1.44 | 8.03 | 1.58 |
| Delonix floribunda | Tsimanampetsotse | 2008 | Mg | 1.31 | 50.69 | 8.70 |
| Delonix floribunda | Tsimanampetsotse | 2012 | Mg | 1.25 | 56.03 | 9.59 |
| Delonix floribunda | Tsimanampetsotse | 2008 | Mg | 0.75 | 34.12 | 5.84 |
| Delonix floribunda | Tsimanampetsotse | 2012 | Mg | 0.63 | 24.77 | 4.25 |
| Delonix floribunda | Tsimanampetsotse | 2008 | Mg | 20.59 | ||
| Delonix floribunda | Tsimanampetsotse | 2008 | Mg | 35.93 | ||
| Delonix floribunda | Analabe | 2007 | Mm, Pp | 3.88 | 47.59 | 8.61 |
| Dichrostachys | Berenty | 2004 | Mg | 30.94 | 18.68 | 8.30 |
| Dichrostachys | Berenty | 2004 | Mg | 11.88 | ||
| Dichrostachys | Berenty | 2004 | Mg | 22.94 | 18.49 | 6.93 |
| Dichrostachys | Berenty | 2004 | Mg | 18.19 | 32.39 | 8.46 |
| Dichrostachys | Berenty | 2004 | Mg | 22.83 | ||
| Dichrostachys | Berenty | 2004 | Mg | 22.31 | 21.21 | 7.28 |
| Dichrostachys | Berenty | 2004 | Mg | 22.19 | ||
| Dichrostachys | Berenty | 2004 | Mg | 21.67 | ||
| Dichrostachys | Berenty | 2004 | Mg | 30.85 | ||
| Dichrostachys | Berenty | 2004 | Mg | 19.55 | ||
| Dichrostachys | Berenty | 2004 | Mg | 28.20 | ||
| Dichrostachys | Berenty | 2004 | Mg | 24.91 | ||
| Dichrostachys | Berenty | 2004 | Mg | 22.61 | ||
| Dichrostachys | Berenty | 2004 | Mg | 2.63 | ||
| Dichrostachys | Berenty | 2004 | Mg | 21.13 | ||
| Dichrostachys | Berenty | 2004 | Mg | 22.81 | 26.51 | 8.25 |
| Dichrostachys | Berenty | 2004 | Mg | 22.56 | 32.3 | 9.18 |
| Dichrostachys | Berenty | 2004 | Mg | 38.53 | ||
| Grewia_Tabarike | Berenty | 2004 | Mg | 7.00 | 16.64 | 3.96 |
| Grewia_Tabarike | Berenty | 2004 | Mg | 7.50 | 14.01 | 3.60 |
| Grewia_Taolankafotsy | Berenty | 2004 | Mg | 11.25 | 2.49 | 2.30 |
| Hymenodictyon decaryi | Kirindy | 2000 | Pp | 3.50 | 5.55 | 1.51 |
| Neobeguea mahafaliensis | Tsimanampetsotse | 2012 | Mg | 5.13 | 21.42 | 4.44 |
| Neobeguea mahafaliensis | Tsimanampetsotse | 2008 | Mg | 3.38 | 11.08 | 2.42 |
| Neobeguea mahafaliensis | Tsimanampetsotse | 2008 | Mg | 2.25 | 6.15 | 1.41 |
| Neobeguea mahafaliensis | Tsimanampetsotse | 2008 | Mg | 2.00 | 10.71 | 2.13 |
| Neobeguea mahafaliensis | Tsimanampetsotse | 2008 | Mg | 0.17 | ||
| Neobeguea mahafaliensis | Tsimanampetsotse | 2008 | Mg | 2.70 | ||
| Neobeguea mahafaliensis | Tsimanampetsotse | 2008 | Mg | 9.53 | ||
| Neobeguea mahafaliensis | Kirindy | 2000 | Pp | 37.25 | ||
| Neobeguea mahafaliensis | Analabe | 2007 | Pp | 3.38 | 2.83 | 1.04 |
| Operculicarya gummifera | Kirindy | 2001 | Pp | 0.63 | ||
| Operculicarya gummifera | Kirindy | 2001 | Pp | 0.81 | ||
| Operculicarya gummifera | Kirindy | Pp | 26.01 | |||
| Operculicarya hyphaenoides | Tsimanampetsotse | 2008 | Mg | 2.44 | 65.60 | 11.39 |
| Operculicarya hyphaenoides | Tsimanampetsotse | 2008 | Mg | 2.13 | 74.72 | 12.86 |
| Operculicarya hyphaenoides | Tsimanampetsotse | 2008 | Mg | 1.69 | 40.67 | 7.09 |
| Operculicarya hyphaenoides | Tsimanampetsotse | 2008 | Mg | 58.18 | ||
| Operculicarya hyphaenoides | Tsimanampetsotse | 2008 | Mg | 73.64 | ||
| Operculicarya hyphaenoides | Tsimanampetsotse | 2008 | Mg | 3.19 | 11.43 | 2.45 |
| Poupartia silvatica | Ankarafantsika | 2009 | Mm, Mr | 28.21 | 6.45 | |
| Poupartia silvatica | Kirindy | 2000 | Pp | 27.25 | 7.95 | 5.89 |
| Poupartia silvatica | Kirindy | 1999 | Pp | 2.31 | 56.64 | 9.87 |
| Poupartia silvatica | Kirindy | 1999 | Pp | 1.38 | 25.08 | 4.43 |
| Poupartia silvatica | Kirindy | 1990 | Pp | 16.70 | ||
| Poupartia silvatica | Kirindy | 1990 | Pp | 23.30 | ||
| Poupartia silvatica | Kirindy | 1993 | Pp | 39.59 | ||
| Quivisianthe papinae | Berenty | 2011 | (Mg) | 2.38 | 0.46 | 0.47 |
| Rhopalocarpus lucidus | Zombitse | 2011 | (Mg) | 2.75 | 13.51 | 2.72 |
| Rhopalocarpus similis | Ankarafantsika | 2009 | Mm | 2.56 | 64.76 | 11.27 |
| Rhopalocarpus sp | Analabe | 2007 | Mm | 3.94 | 38.15 | 7.04 |
| Terminalia disjuncta | Tsimanampetsotse | 2008 | Mg | 3.94 | 78.76 | 13.84 |
| Terminalia disjuncta | Tsimanampetsotse | 2012 | Mg | 3.69 | 48.66 | 8.76 |
| Terminalia disjuncta | Tsimanampetsotse | 2012 | Mg | 3.19 | 62.86 | 11.05 |
| Terminalia disjuncta | Tsimanampetsotse | 2008 | Mg | 3.13 | 61.60 | 10.83 |
| Terminalia disjuncta | Tsimanampetsotse | 2008 | Mg | 3.00 | 80.63 | 14.00 |
| Terminalia disjuncta | Tsimanampetsotse | 2008 | Mg | 2.38 | 85.94 | 14.78 |
| Terminalia disjuncta | Tsimanampetsotse | 2008 | Mg | 2.13 | 53.25 | 9.27 |
| Terminalia disjuncta | Tsimanampetsotse | 2012 | Mg | 2.00 | 51.57 | 8.97 |
| Terminalia disjuncta | Tsimanampetsotse | 2008 | Mg | 1.63 | 9.48 | 1.86 |
| Terminalia disjuncta | Tsimanampetsotse | 2008 | Mg | 55.76 | ||
| Terminalia mantaliopsis | Analabe | 2007 | Mm, Pp | 2.38 | 26.06 | 4.76 |
| Terminalia mantaly | Analabe | 2007 | Mm, Pp | 1.44 | 25.15 | 4.45 |
| Terminalia sp. | Kirindy | 2001 | Pp | 2.69 | 17.88 | 3.44 |
| Terminalia sp. | Kirindy | 2001 | Pp | 2.44 | 26.91 | 4.91 |
| Terminalia sp. | Kirindy | 2000 | Pp | 1.56 | 64.70 | 11.09 |
| Terminalia sp. | Kirindy | 2000 | Pp | 1.44 | 1.41 | 0.48 |
| Terminalia sp. | Kirindy | 2000 | Pp | 2.28 | ||
| Terminalia sp. | Kirindy | 2000 | Pp | 6.32 | ||
| Terminalia sp. | Kirindy | 2000 | Pp | 6.67 | ||
| Terminalia sp. | Kirindy | 2001 | Pp | 8.40 | ||
| Terminalia sp. | Kirindy | 2000 | Pp | 11.21 | ||
| Terminalia sp. | Kirindy | 2000 | Pp | 12.19 | ||
| Terminalia sp. | Kirindy | 2000 | Pp | 15.48 | ||
| Terminalia sp. | Kirindy | 2000 | Pp | 19.32 | ||
| Terminalia sp. | Kirindy | 2001 | Pp | 21.89 | ||
| Terminalia sp. | Kirindy | 2000 | Pp | 26.66 | ||
| Terminalia sp. | Kirindy | 2001 | Pp | 51.5 | ||
| Terminalia sp.2 | Kirindy | 2000 | Pp | 0.66 | ||
| Terminalia ulexoides | Tsimanampetsotse | 2012 | Mg | 13.75 | 4.68 | 3.08 |
| Terminalia ulexoides | Tsimanampetsotse | 2008 | Mg | 3.00 | 11.90 | 2.49 |
| Terminalia ulexoides | Tsimanampetsotse | 2008 | Mg | 1.75 | 9.86 | 1.94 |
| Terminalia ulexoides | Tsimanampetsotse | 2008 | Mg | 1.50 | 18.60 | 3.36 |
| Terminalia ulexoides | Tsimanampetsotse | 2012 | Mg | 5.28 | ||
| Terminalia ulexoides | Tsimanampetsotse | 2008 | Mg | 18.50 | ||
| unknown sp1 | Kirindy | 1999 | Pp | 0.38 | 23.52 | 4.00 |
| unknown sp2 | Kirindy | 2000 | Pp | 1.50 | 0.84 | 0.39 |
| unknown sp3 | Zombitse | 2011 | Pp | 5.31 | 12.01 | 2.90 |
| Zanthoxylum sp. | Kirindy | 1999 | Pp | 4.13 | 54.39 | 9.79 |
| Zanthoxylum sp. | Kirindy | 2001 | Pp | 3.50 | 45.21 | 8.15 |
| Zanthoxylum sp. | Kirindy | 2001 | Pp | 60.65 |
Appendix B
| Dry Season | Wet Season | Dry Season | Wet Season | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Freq. | N % | Sugar % | AB | Freq. | N | Sugar | AB | Freq. | N % | Sugar % | AB | Freq. | N | Sugar | AB | |
| Acacia bellula | 0 | 1.11 | 42.65 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | ||||||
| Albizia tulearensis | 2 | 0 | 0 | 0.52 | 0.56 | 0 | 0 | 0 | 1 | 0 | ||||||
| Commiphora marchandi | 0 | 1.51 | 43.11 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | ||||||
| Commiphora simplicifolia | 1 | 0.58 | 11.49 | 1 | 0 | 0.61 | 6.61 | 1 | 0 | 1 | 0 | 1 | ||||
| Delonix floribunda | 0 | 0.68 | 27.74 | 0 | 1 | 0.44 | 71.61 | 0 | 2 | 0.32 | 28.71 | 0 | 2 | 0.15 | 40.40 | 0 |
| Neobeguea mahafaliensis | 0 | 0.54 | 5.63 | 0 | 0 | 0.82 | 21.42 | 0 | 3 | 0 | 0 | 0 | ||||
| Operculicarya hyphaenoides | 0 | 0 | 0 | 0.38 | 50.12 | 0 | 0 | |||||||||
| Terminalia disjuncta | 1 | 0.26 | 9.48 | 1 | 7 | 0.47 | 54.36 | 0 | 11 | 0.40 | 73.27 | 0 | 3 | 0 | ||
| Terminalia ulexoïdes | 1 | 0.36 | 15.25 | 0 | 0 | 2.2 | 4.98 | 0 | 0 | 18.50 | 0 | 0 | 0 | |||
Appendix C
| Tree Species | Sample No. | ESCH 006 | PSMN 028 | MICO 004 | SFCO 002 |
|---|---|---|---|---|---|
| Acacia bellula | P08-127 | 0 | - | - | - |
| Acacia bellula | P08-128 | 0 | - | - | - |
| Acacia burkei | F11-4 | - | - | 0 | - |
| Albizia tulearensis | B12-10 | - | - | 0 | - |
| Albizia tulearensis | B12-11 | - | - | 0 | - |
| Albizia tulearensis | B12-12 | - | - | 0 | - |
| Combretum molle | F11-5 | 0 | - | - | - |
| Commiphora simplicifolia | P08-120 | 0 | 0 | 0 | 0 |
| Commiphora sp. | F07-7 | 0 | - | 0 | - |
| Commiphora sp. | F07-8 | 0 | - | 0 | - |
| Delonix floribunda | B12-15 | - | - | 0 | - |
| Delonix floribunda | B12-16 | - | - | 0 | - |
| Harpephyllum caffrum | F11-7 | 0 | - | - | - |
| Neobeguea mahafaliensis | P08-104 | - | - | 0 | - |
| Neobeguea mahafaliensis | B12-13 | - | - | 0 | - |
| Neobeguea mahafaliensis | B12-14 | - | - | 0 | - |
| Quivisianthe papinae | F11-8 | - | 0 | 0 | - |
| Terminalia disjuncta | P08-78 | - | - | 0 | - |
| Terminalia mantaliopsis | F07-3 | - | - | 0 | - |
| Terminalia ulexoides | B12-1 | - | - | 0 | - |
| Terminalia ulexoides | B12-2 | - | - | 0 | - |
| Terminalia ulexoides | B12-3 | - | - | 0 | - |
| Unknown species | F11-11 | - | - | 0 | - |
Appendix D
| Tree Species | Sample No. | ESCH 006 | PSMN 028 | MICO 001 | MICO 003 | MICO 004 | MICO 005 | MICO 006 | SFCO 002 | STCO 001 |
|---|---|---|---|---|---|---|---|---|---|---|
| Acacia bellula | P08-127 | - | - | - | - | 0 | - | - | - | - |
| Acacia bellula | P08-128 | - | - | - | - | 0 | - | - | - | - |
| Acacia burkei | F11-4 | - | - | - | - | 0 | - | - | - | - |
| Acacia robusta | F11-6 | - | - | - | - | 0 | - | - | - | - |
| Albizia mainaea | F07-5 | 0 | - | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Albizia tulearensis | B12-10 | 0 | - | - | - | 0 | - | - | - | - |
| Albizia tulearensis | B12-11 | 0 | - | - | - | 0 | - | - | - | - |
| Albizia tulearensis | B12-12 | 0 | - | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Combretum molle | F11-5 | - | - | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Commiphora guillaumini | S00-19 | - | - | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Commiphora marchandii | P08-111 | - | - | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Commiphora marchandii | P08-114 | - | - | - | - | 0 | - | - | - | - |
| Commiphora simplicifolia | P08-119 | 0 | - | - | - | 2 | - | - | - | - |
| Commiphora simplicifolia | P08-120 | 0 | 0 | 0 | 1 | 2 | 1 | 0 | 0 | 0 |
| Commiphora simplicifolia | P08-125 | 0 | - | - | - | 2 | - | - | - | - |
| Commiphora simplicifolia | B12-7 | - | - | - | 2 | 2 | 1 | - | 1 | - |
| Commiphora simplicifolia | B12-8 | 0 | 0 | - | 1 | 2 | 1 | - | 1 | - |
| Commiphora sp. | F07-7 | - | - | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Commiphora sp. | F07-8 | - | - | - | - | 0 | - | - | - | - |
| Delonix floribunda | F07-4 | - | - | - | - | 0 | - | - | - | - |
| Delonix floribunda | P08-76 | - | - | - | - | 0 | - | - | - | - |
| Delonix floribunda | P08-51 | - | - | - | - | 0 | - | - | - | - |
| Delonix floribunda | B12-15 | 0 | - | - | - | 0 | - | - | - | - |
| Delonix floribunda | B12-16 | 0 | - | - | - | 0 | - | - | - | - |
| Harpephyllum caffrum | F11-7 | - | - | - | - | 0 | - | - | - | - |
| Hymenodictyon decaryi | S00-3 | - | - | - | - | 0 | - | - | - | - |
| Neobeguea mahafaliensis | P08-100 | - | - | - | - | 0 | - | - | - | - |
| Neobeguea mahafaliensis | P08-104 | - | - | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Neobeguea mahafaliensis | B12-13 | 0 | - | - | - | 0 | - | - | - | - |
| Neobeguea mahafaliensis | B12-14 | 0 | - | - | - | 0 | - | - | - | - |
| Operculicarya gummifera | S00-10 | - | - | - | - | 0 | - | - | - | - |
| Operculicarya hyphaenoides | P08-108 | - | - | - | - | 0 | - | - | - | - |
| Poupartia silvatica | S00-2 | - | - | - | - | 0 | - | - | - | - |
| Quivisianthe papinae | F11-8 | 0 | 0 | 0 | 0 | nr | nr | 0 | 0 | 0 |
| Rhopalocarpus sp. | F07-6 | - | - | - | - | 0 | - | - | - | - |
| Terminalia disjuncta | P08-30 | - | - | - | - | nr | - | - | - | - |
| Terminalia disjuncta | P08-53 | - | - | - | - | 0 | - | - | - | - |
| Terminalia disjuncta | P08-78 | - | - | - | - | 0 | - | - | - | - |
| Terminalia mantaliopsis | F07-3 | - | - | - | - | 0 | - | - | - | - |
| Terminalia mentaly | F07-2 | - | - | - | - | 0 | - | - | - | - |
| Terminalia ulexoides | B12-1 | 0 | - | - | - | 0 | - | - | - | - |
| Terminalia ulexoides | B12-2 | 0 | - | - | - | 0 | - | - | - | - |
| Terminalia ulexoides | B12-3 | 0 | - | - | - | 0 | - | - | - | - |
| Zanthoxylum sp. | S00-13 | - | - | - | - | 0 | - | - | - | - |
| Unknown species | F11-11 | 0 | 0 | - | - | 2 | nr | - | 0 | 0 |
Appendix E
| Tree Species | Sample No. | ESCH 006 | PSMN 028 | MICO 003 | MICO 004 | MICO 005 | SFCO 002 |
|---|---|---|---|---|---|---|---|
| Albizia tulearensis | B12-12 | - | - | - | nr | - | 0 |
| Commiphora guillaumini | S00-19 | - | - | 0 | 0 | - | - |
| Commiphora simplicifolia | P08-120 | 0 | 0 | 1 | 2 | nr | 1; 2 |
| Commiphora simplicifolia | B12-8 | - | - | nr | 1 | Nr | nr |
| Quivisianthe papinae | F11-8 | - | - | nr | nr | Nr | nr |
| Terminalia disjuncta | P08-30 | - | - | - | 0 | - | 0 |
Appendix F
| Ab | Am | At | Df | Ca | Cs | Hd | Nm | Oh | Ps | Qp | Rs | Td | Tm | T ma | Tu | Z sp | Pharmacology [44] | Pharmacology NIH National Library of Medicine https://pubchem.ncbi.nlm.nih.gov/ (accessed on 1 November 2023) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antimicrobial effect in the present study | 0 | 0 | 0/1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0/1 | 0 | 0 | 0 | 0 | ||
| Part used according to [52,53] | Ar | Br | Lx | Gum | Ar | Sb,Tr | Ar | Sb | Sb | ||||||||||
| Acetophenone | 1 | 1 | None | Photosensitizing | |||||||||||||||
| Allocimene | 1 | Not listed | Possibly toxic | ||||||||||||||||
| Andrographolide | 1 | Anti-inflammatory | Antiprotozoal Anti-inflammatory Antiviral Platelet Aggregation Inhibitor | ||||||||||||||||
| Anethole | 1 | Antitussive; flavoring agent in food | Flavoring agent | ||||||||||||||||
| Anisic acid | 1 | Condiment and flavor in foods; carminative; expectorant | Flavoring agent | ||||||||||||||||
| Aromadendrene | 1 | Not listed | None | ||||||||||||||||
| trans-α- Begamoten | 1 | Not listed | None | ||||||||||||||||
| Benzaldehyd | 1 | ||||||||||||||||||
| Bourbonene | 1 | 1 | Not listed | Flavouring agent | |||||||||||||||
| Bulnesene | 1 | Not listed | None | ||||||||||||||||
| Cadinene | 1 | 1 | None | None | |||||||||||||||
| Calorene | 1 | Not listed | Not listed | ||||||||||||||||
| Camphen | 1 | None | None | ||||||||||||||||
| Caprylic acid (Octanoic Acid) | 1 | Antifungal Toxic | Possibly against seizures | ||||||||||||||||
| Caryo-phyllene | 1 | 1 | 1 | None | Flavouring agent | ||||||||||||||
| Cendrene | 1 | Not listed | Not listed | ||||||||||||||||
| Chamigrene | 1 | 1 | Not listed | None | |||||||||||||||
| Chavicol | 1 | 1 | None | None | |||||||||||||||
| Copaene | 1 | 1 | None | None | |||||||||||||||
| Cubebene | 1 | Urinary antiseptic; expectorant | None | ||||||||||||||||
| Curcumene | 1 | None | None | ||||||||||||||||
| Curzene | 1 | Not listed | Not listed | ||||||||||||||||
| Cyclolanost-24en-3ol | 1 | Not listed | Not listed | ||||||||||||||||
| Cymen | 1 | None | None | ||||||||||||||||
| Elemene | 1 | 1 | 1 | Not listed | None | ||||||||||||||
| Elemol | 1 | Not listed | None | ||||||||||||||||
| Enanthic acid | 1 | Not listed; Enanthotoxin highly toxic | None | ||||||||||||||||
| Ergostene | 1 | None | Not listed | ||||||||||||||||
| Eudesmol | 1 | Not listed | None | ||||||||||||||||
| Eugenol | 1 | 1 | Toxic | Anti-infective agent | |||||||||||||||
| Falcarinol | 1 | Not listed | Unclear | ||||||||||||||||
| Farnesene | 1 | 1 | None | None | |||||||||||||||
| Germacrene | 1 | Not listed | None | ||||||||||||||||
| Guaiacol | 1 | Expectorant | Not listed | ||||||||||||||||
| Himachalene | 1 | Not listed | None | ||||||||||||||||
| Hydrochinon | 1 | Not listed | Antioxidant Mutagen | ||||||||||||||||
| Isoeugenol | 1 | None | None | ||||||||||||||||
| Isoledene | 1 | Not listed | None | ||||||||||||||||
| Isolongifolene | 1 | Not listed | None | ||||||||||||||||
| Lauric acid | 1 | None | None | ||||||||||||||||
| Limonene | 1 | 1 | 1 | None | None | ||||||||||||||
| Longifolenaldehyde | 1 | None | None | ||||||||||||||||
| Maltol | 1 | Flavoring agent | None | ||||||||||||||||
| p-Mentadien | 1 | 1 | 1 | Not listed | Not listed | ||||||||||||||
| Muurolen | 1 | Not listed | None | ||||||||||||||||
| Myrcene | 1 | None | None | ||||||||||||||||
| Myristic acid | 1 | Antifoaming | None | ||||||||||||||||
| Neoisolongifolene | 1 | Not listed | None | ||||||||||||||||
| Nerolidol | 1 | 1 | None | None | |||||||||||||||
| Palmitic acid | 1 | None | Enzyme inhibitor | ||||||||||||||||
| Pelargonic acid | 1 | Strong irritant | Antifungal | ||||||||||||||||
| Pelargonaldehyde | 1 | Not listed | None | ||||||||||||||||
| Phenanthrene | 1 | Photosensitization of skin | None | ||||||||||||||||
| Phenanthrenol | 1 | Not listed | None | ||||||||||||||||
| α-Pinene | 1 | Toxic | None | ||||||||||||||||
| ß-Pinene | 1 | None | None | ||||||||||||||||
| Pinocarveol | 1 | Not listed | None | ||||||||||||||||
| Podocarp 7-en-3one | 1 | Not listed | Not listed | ||||||||||||||||
| Resorcinol | 1 | Toxic; antiseptic | Unclear | ||||||||||||||||
| Salicylic acid | 1 | Antireumatic, analgesic | Anti-infective Antifungal Keratolytic | ||||||||||||||||
| Santolinatriene | 1 | Not listed | None | ||||||||||||||||
| Selinene | 1 | Not listed | None | ||||||||||||||||
| Spathulenol | 1 | 1 | Not listed | Part of essential oils | |||||||||||||||
| Syringol | 1 | 1 | Not listed | None | |||||||||||||||
| Tetradecanal | 1 | Not listed | None | ||||||||||||||||
| Valeric acid | 1 | None | None | ||||||||||||||||
| Valerolactone | 1 | Not listed | None | ||||||||||||||||
| Veratraldehyde | 1 | None | None | ||||||||||||||||
| Veratric acid | 1 | None | Prevention of neoplasm associated with HPV | ||||||||||||||||
| Veratril | 1 | 1 | Not listed | None | |||||||||||||||
| Verbenone | 1 | None | Not listed | ||||||||||||||||
| Vertraldehyde | 1 | Not listed | Not listed | ||||||||||||||||
| Viridiflorol | 1 | 1 | 1 | Not listed | Growth inhibitor (animal cells, micro-organisms) |
References
- Murphy, H.N. (Ed.) Chemical Constituents and Applications of Gums, Resins, and Latexes of Plant Origin. In Gums, Resins and Latexes of Plant Origin; Springer: Cham, Switzerland, 2021; pp. 1–21. [Google Scholar]
- Burrows, A.M.; Nash, L.T. The Evolution of Exudativory in Primates; Springer: New York, NY, USA, 2010. [Google Scholar]
- Cabana, F.; Dierenfeld, E.S.; Wirdateti; Donati, G.; Nekaris, K.A.I. Exploiting a readily available but hard to digest resource: A review of exudativorous mammals identified thus far and how they cope in captivity. Integr. Zool. 2018, 13, 94–111. [Google Scholar] [CrossRef] [PubMed]
- Bearder, S.K.; Martin, R.D. Acacia gum and its use by lesser bushbabies, Galago senegalensis (Primates: Lorisidae). Int. J. Primatol. 1980, 1, 102–128. [Google Scholar] [CrossRef]
- Heymann, E.W.; Smith, A.C. When to feed on gums: Temporal patterns of gummivory in wild tamarins, Saguinus mystax and Saguinus fuscicollis (Callitrichidae). Zoo Biol. 1999, 18, 459–471. [Google Scholar] [CrossRef]
- Nash, T.L.; Burrows, A.M. (Eds.) Introduction: Advances and remaining sticky issues in the understanding of exudativory in primates. In The Evolution of Exudativory in Primates; Springer: New York, NY, USA, 2010; pp. 1–23. [Google Scholar]
- Génin, F.G.S.; Masters, J.C.; Ganzhorn, J.U. Gummivory in cheirogaleids: Primitive retention or adaptation to hypervariable environments? In The Evolution of Exudativory in Primates; Burrows, A.M., Nash, L.T., Eds.; Springer: New York, NY, USA, 2010; pp. 123–140. [Google Scholar]
- Charles-Dominique, P. Ecology and Behaviour of Nocturnal Prosimians; Duckworth: London, UK, 1977. [Google Scholar]
- Hladik, C.M. Diet and ecology of prosimians. In The Study of Prosimian Behavior; Doyle, G.A., Martin, R.D., Eds.; Academic Press: London, UK, 1979; pp. 307–357. [Google Scholar]
- Isbell, L.A.; Rothman, J.M.; Young, P.J.; Rudolph, K. Nutritional benefits of Crematogaster mimosae ants and Acacia drepanolobium gum for patas monkeys and vervets in laikipia, Kenya. Amer. J. Phys. Anthropol. 2013, 150, 286–300. [Google Scholar] [CrossRef]
- Harborne, J.B. Introduction to Ecological Biochemistry; Academic Press: London, UK, 2014. [Google Scholar]
- Lieberei, R.; Reisdorff, C. Nutzpflanzenkunde; Georg Thieme Verlag KG: Stuttgart, Germany, 2007. [Google Scholar]
- Dewi, T.; Imron, M.A.; Lukmandaru, G.; Hedger, K.; Campera, M.; Nekaris, K.A.I. The sticky tasty: The nutritional content of the exudativorous diet of the Javan slow loris in a lowland forest. Primates 2022, 63, 93–102. [Google Scholar] [CrossRef]
- Das, N.; Nekaris, K.A.I.; Bhattacharjee, P.C. Medicinal plant exudativory by the Bengal slow loris Nycticebus bengalensis. Endanger. Species Res. 2014, 23, 149–157. [Google Scholar] [CrossRef]
- Chapman, C.A.; Rothman, J.M.; Lambert, J.E. Food as a selective force in primates. In The Evolution of Primate Societies; Kappeler, P.M., Ed.; The University of Chicago Press: Chicago, IL, USA, 2012; pp. 149–168. [Google Scholar]
- Ganzhorn, J.U.; Arrigo-Nelson, S.J.; Carrai, V.; Chalise, M.K.; Donati, G.; Droescher, I.; Eppley, T.M.; Irwin, M.T.; Koch, F.; Koenig, A.; et al. The importance of protein in leaf selection of folivorous primates. Am. J. Primatol. 2017, 79, e22550. [Google Scholar] [CrossRef]
- Donati, G.; Santini, L.; Eppley, T.M.; Arrigo-Nelson, S.J.; Balestri, M.; Boinski, S.; Bollen, A.; Bridgeman, L.L.; Campera, M.; Carrai, V.; et al. Low levels of fruit nitrogen as drivers for the evolution of Madagascar’s primate communities. Sci. Rep. 2017, 7, 14406. [Google Scholar] [CrossRef]
- Chivers, D.J.; Wood, B.A.; Bilsborough, A. Food Acquisition and Processing in Primates; Plenum Press: New York, NY, USA, 1984. [Google Scholar]
- Nevo, O.; Valenta, K.; Helman, A.; Ganzhorn, J.U.; Ayasse, M. Fruit scent as an honest signal for fruit quality. BMC Ecol. Evol. 2022, 22, 139. [Google Scholar] [CrossRef] [PubMed]
- Robbins, C.T. Wildlife Feeding and Nutrition; Academic Press: New York, NY, USA, 1994. [Google Scholar]
- National Research Council. Nutrient Requirements of Non-Human Primates, 2nd ed.; The National Academies Press: Washington, DC, USA, 2003. [Google Scholar]
- Terborgh, J. Diversity and the Tropical Rain Forest; Scientific American Library: New York, NY, USA, 1992. [Google Scholar]
- Huffman, M.A. Animal self-medication: Ethnoveterinary medicine without human cultural bias? Planta Medica 2016, 82, S1–S381. [Google Scholar] [CrossRef]
- Wrangham, R.W. Relationship of chimpanzee leaf-swallowing to a tapeworm infection. Am. J. Primatol. 1995, 37, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Krief, S.; Huffman, M.A.; Sevenet, T.; Hladik, C.M.; Grellier, P.; Loiseau, P.M.; Wrangham, R.W. Bioactive properties of plant species ingested by chimpanzees (Pan troglodytes schweinfurthii) in the Kibale National Park, Uganda. Am. J. Primatol. 2006, 68, 51–71. [Google Scholar] [CrossRef]
- Waeber, P.O.; Wilmé, L.; Ramamonjisoa, B.; Garc¡a, G.; Rakotomalala, D.; Rabemananjara, Z.H.; Kull, C.A.; Ganzhorn, J.U.; Sorg, J.P. Dry forests in Madagascar: Neglected and under pressure. Int. For. Rev. 2015, 16, 127–148. [Google Scholar] [CrossRef]
- Thoren, S.; Quietzsch, F.; Schwochow, D.; Sehen, L.; Meusel, C.; Meares, K.; Radespiel, U. Seasonal changes in feeding ecology and activity patterns of two sympatric mouse lemur species, the Gray mouse lemur (Microcebus murinus) and the Golden-brown mouse lemur (M. ravelobensis), in northwestern Madagascar. Int. J. Primatol. 2011, 32, 566–586. [Google Scholar] [CrossRef]
- Hladik, C.M.; Charles-Dominique, P.; Petter, J.J. Feeding strategies of five nocturnal prosimians in the dry forest of the west coast of Madagascar. In Nocturnal Malagasy Primates; Charles-Dominique, P., Cooper, H.M., Hladik, A., Hladik, C.M., Pages, E., Pariente, G.F., Petter-Rousseaux, A., Petter, J.J., Schilling, A., Eds.; Academic Press: New York, NY, USA, 1980; pp. 41–73. [Google Scholar]
- Ganzhorn, J.U.; Kappeler, P.M. Lemurs of the Kirindy Forest. Primate Rep. 1996, 46, 257–274. [Google Scholar]
- Schülke, O.; Kappeler, P.M. So near and yet so far: Territorial pairs but low cohesion between pair partners in a nocturnal lemur, Phaner furcifer. Anim. Behav. 2003, 65, 331–343. [Google Scholar] [CrossRef]
- Goodman, S.M.; Raherilalao, M.J.; Wohlhauser, S. Les Aires Protégées Terrestres de Madagascar: Leur Histoire, Description et Biote/The Terrestrial Protected Areas of Madagascar: Their History, Description, and Biota; Association Vahatra: Antananarivo, Madagascar, 2018. [Google Scholar]
- Bohr, Y.E.M.B.; Giertz, P.; Ratovonamana, Y.R.; Ganzhorn, J.U. Gray-brown mouse lemurs (Microcebus griseorufus) as an example of distributional constraints through increasing desertification. Int. J. Primatol. 2011, 32, 901–913. [Google Scholar] [CrossRef]
- Ratovonamana, Y.R.; Rajeriarison, C.; Edmond, R.; Ganzhorn, J.U. Phenology of different vegetation types in Tsimanampetsotsa National Park, south-western Madagascar. Malagasy Nat. 2011, 5, 14–38. [Google Scholar]
- Génin, F. Life in unpredictable environments: First investigation of the natural history of Microcebus griseorufus. Int. J. Primatol. 2008, 29, 303–321. [Google Scholar] [CrossRef]
- Andrews, C.A. A Comparative Evolutionary Approach to Gum-Feeding in Galago moholi and Microcebus griseorufus. Master’s Thesis, University of Fort Hare, Alice, South Africa, 24 February 2014. [Google Scholar]
- Steffens, K.J.E. Lemur food plants as options for forest restoration in Madagascar. Restor. Ecol. 2020, 28, 1517–1527. [Google Scholar] [CrossRef]
- Moat, J.; Smith, P. Atlas of the Vegetation of Madagascar. Atlas de la Végétation de Madagascar; Kew Publishing, Royal Botanic Gardens: Kew, UK, 2007. [Google Scholar]
- Rakotondranary, J.S.; Ratovonamana, Y.R.; Ganzhorn, J.U. Distributions et caractéristiques des microhabitats de Microcebus griseorufus (Cheirogaleidae) dans le Parc National de Tsimanampetsotsa (Sud-ouest de Madagascar). Malagasy Nat. 2010, 4, 55–64. [Google Scholar]
- Ratovonamana, R.Y. Analyse Floristique et Structurale des Différentes Formations Végétales, Habitats de Microcebus griseorufus dans le Parc National de Tsimanampetsotse. Doctoral Thesis, Faculté des Sciences, Département de Biologie et Ecologie Végétales, Université d’Antananarivo, Antananarivo, Madagascar, 2016. [Google Scholar]
- Abel, C.; Giertz, P.; Ratovonamana, Y.R.; Püttker, T.; Rakotondranary, S.J.; Scheel, B.M.; Lenz, T.L.; Ganzhorn, J.U. Habitat quality affects the social organization in mouse lemurs (Microcebus griseorufus). Behav. Ecol. Sociobiol. 2023, 77, 65. [Google Scholar] [CrossRef]
- Milton, K.; Dintzis, F.R. Nitrogen-to-protein conversion factors for tropical plant samples. Biotropica 1981, 13, 177–181. [Google Scholar] [CrossRef]
- Kates, M. Techniques in lipidology. In Laboratory Techniques in Biochemistry and Molecular Biology; Work, T.S., Work, E., Eds.; North Holland Publishing Company: Amsterdam, The Netherlands, 1972; pp. 267–610. [Google Scholar]
- Irwin, M.T.; Raharison, J.-L.; Raubenheimer, D.; Chapman, C.A.; Rothman, J.M. Nutritional correlates of the “lean season”: Effects of seasonality and frugivory on the nutritional ecology of Diademed Sifakas. Am. J. Phys. Anthropol. 2014, 153, 78–91. [Google Scholar] [CrossRef]
- O’Neil, M.J. (Ed.) The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals; RSC Publ., Royal Soc. of Chemistry: Cambridge, UK, 2013. [Google Scholar]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Montero, B.K.; Wasimuddin; Schwensow, N.; Gillingham, M.A.F.; Ratovonamana, Y.R.; Rakotondranary, S.J.; Corman, V.; Drosten, C.; Ganzhorn, J.U.; Sommer, S. Evidence of MHC class I and II influencing viral and helminth infection via the microbiome in a non-human primate. PLoS Pathog. 2021, 17, e1009675. [Google Scholar] [CrossRef]
- Schmid, D.W.; Fackelmann, G.; Wasimuddin; Rakotondranary, S.J.; Ratovonamana, Y.R.; Montero, B.K.; Ganzhorn, J.U.; Sommer, S. A framework for testing the impact of co-infections on host gut microbiomes. Anim. Microbiome 2022, 4, 48. [Google Scholar] [CrossRef] [PubMed]
- Wasimuddin; Corman, V.M.; Ganzhorn, J.U.; Rakotondranary, S.J.; Ratovonamana, Y.R.; Drosten, C.; Sommer, S. Adenovirus infection is associated with altered gut microbial communities in a non-human primate. Sci. Rep. 2019, 9, 13410. [Google Scholar] [CrossRef]
- Wasimuddin; Malik, H.; Ratovonamana, Y.R.; Rakotondranary, S.J.; Ganzhorn, J.U.; Sommer, S. Anthropogenic disturbance impacts gut microbiome homeostasis in a Malagasy primate. Front. Microbiol. 2022, 13, 911275. [Google Scholar] [CrossRef]
- Clayton, J.B.; Gomez, A.; Amato, K.; Knights, D.; Travis, D.A.; Blekhman, R.; Knight, R.; Leigh, S.; Stumpf, R.; Wolf, T.; et al. The gut microbiome of nonhuman primates: Lessons in ecology and evolution. Am. J. Primatol. 2018, 80, e22867. [Google Scholar] [CrossRef]
- Veith, T.; Bleicker, T.; Eschbach-Bludau, M.; Brünink, S.; Mühlemann, B.; Schneider, J.; Beheim-Schwarzbach, J.; Rakotondranary, S.J.; Ratovonamana, Y.R.; Tsagnangara, C.; et al. Non-structural genes of novel lemur adenoviruses reveal recent parallel evolution of virus and host. Virus Evol. 2023, 9, vead024. [Google Scholar] [CrossRef] [PubMed]
- Andriamparany, J.N.; Brinkmann, K.; Jeannoda, V.; Buerkert, A. Effects of socio-economic household characteristics on traditional knowledge and usage of wild yams and medicinal plants in the Mahafaly region of south-western Madagascar. J. Ethnobiol. Ethnomed. 2014, 10, 82. Available online: http://www.ethnobiomed.com/content/10/1/82 (accessed on 1 November 2022). [CrossRef] [PubMed]
- Favre, J.-C. Fiches et Listes des Essences Faisant L’objez D’une Collection par la Population du Village de Marofandilia Dans la Région de Morondava/Madagascar; ETH Zürich: Zürich, Switzerland, 1990; Volume 90/5. [Google Scholar]
- Power, M.L. Nutritional and digestive challenges to being a gum-feeding primate. In The Evolution of Exudativory in Primates; Burrows, A.M., Nash, L.T., Eds.; Springer: New York, NY, USA, 2010; pp. 25–44. [Google Scholar]
- Smith, A.C. Exudativory in primates: Interspecific patterns. In The Evolution of Exudativory in Primates; Burrows, A.M., Nash, L.T., Eds.; Springer: New York, NY, USA, 2010; pp. 45–87. [Google Scholar]
- Smith, A.C. Influences on gum feeding in primates. In The Evolution of Exudativory in Primates; Burrows, A.M., Nash, L.T., Eds.; Springer: New York, NY, USA, 2010; pp. 109–121. [Google Scholar]
- Cabana, F.; Dierenfeld, E.; Wirdateti, W.; Donati, G.; Nekaris, K.A.I. The seasonal feeding ecology of the javan slow loris (Nycticebus javanicus). Am. J. Phys. Anthropol. 2017, 162, 768–781. [Google Scholar] [CrossRef] [PubMed]
- Chivers, D.J.; Hladik, C.M. Morphology of the gastrointestinal tract in primates: Comparison with other mammals in relation to diet. J. Morphol. 1980, 166, 337–386. [Google Scholar] [CrossRef] [PubMed]
- Huffman, M.A. Current evidence for self-medication in primates: A multidisciplinary perspective. Yearb. Phys. Anthropol. 1997, 40, 171–200. [Google Scholar] [CrossRef]
- Krief, S.; Huffman, M.A.; Sevenet, T.; Guillot, J.; Bories, C.; Hladik, C.M.; Wrangham, R.W. Noninvasive monitoring of the health of Pan troglodytes schweinfurthii in the Kibale National Park, Uganda. Int. J. Primatol. 2005, 26, 467–490. [Google Scholar] [CrossRef]
- Tasdemir, D.; MacIntosh, A.J.J.; Stergiou, P.; Kaiser, M.; Mansour, N.R.; Bickle, Q.; Huffman, M.A. Antiprotozoal and antihelminthic properties of plants ingested by wild Japanese macaques (Macaca fuscata yakui) in Yakushima Island. J. Ethnopharmacol. 2020, 247, 112270. [Google Scholar] [CrossRef]
- Starr, C.; Nekaris, K.A.I. Obligate exudativory characterizes the diet of the Pygmy Slow Loris, Nycticebus pygmaeus. Am. J. Primatol. 2013, 75, 1054–1061. [Google Scholar] [CrossRef]
- Abdallah, E.M.; Khalid, A.S.; Ibrahim, N. Antibacterial activity of oleo-gum resins of Commiphora molmol and Boswellia papyrifera against methicillin resistant Staphylococcus aureus (MRSA). Sci. Res. Essays 2009, 4, 351–356. Available online: http://www.academicjournals.org/SRE (accessed on 20 June 2023).
- Glander, K.E. The impact of plant secondary compounds on primate feeding behavior. Yearb. Phys. Anthropol. 1982, 25, 1–18. [Google Scholar] [CrossRef]
- Huffman, M.A.; Gotoh, S.; Turner, L.A.; Hamai, M.; Yoshida, K. Seasonal trends in intestinal nematode infection and medicinal plant use among chimpanzees in the Mahale Mountains, Tanzania. Primates 1997, 38, 111–125. [Google Scholar] [CrossRef]
- Villalba, J.J.; Provenca, F.D.; Shaw, R. Sheep self-medicate when challenged with illness-inducing foods. Anim. Behav. 2006, 71, 1131–1139. [Google Scholar] [CrossRef]
- Huffman, M.A.; Caton, J.M. Self-induced increase of gut motility and the control of parasitic infections in wild chimpanzees. Int. J. Primatol. 2001, 22, 329–346. [Google Scholar] [CrossRef]
- Wrangham, R.W.; Nishida, T. Aspilia spp. leaves: A puzzle in the feeding behavior of wild chimpanzees. Primates 1983, 24, 276–282. [Google Scholar] [CrossRef]
- Negre, A.; Tarnaud, L.; Roblot, J.F.; Gantier, J.C.; Guillot, J. Plants consumed by Eulemur fulvus in Comoros Islands (Mayotte) and potential effects on intestinal parasites. Int. J. Primatol. 2006, 27, 1495–1517. [Google Scholar] [CrossRef]
- Carrai, V.; Borgognini-Tarli, S.M.; Huffman, M.A.; Bardi, M. Increase in tannin consumption by sifaka (Propithecus verreauxi verreauxi) females during the birth season: A case for self-medication in prosimians? Primates 2003, 44, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Birkinshaw, C. Use of millipedes by Black lemurs to anoint their bodies. Folia Primatol. 1999, 70, 170–171. [Google Scholar] [CrossRef]
- Peckre, L.R.; Defolie, C.; Kappeler, P.M.; Fichtel, C. Potential self-medication using millipede secretions in red-fronted lemurs: Combining anointment and ingestion for a joint action against gastrointestinal parasites? Primates 2018, 59, 483–494. [Google Scholar] [CrossRef]
- Bouslama, L.; Kouidhi, B.; Alqurashi, Y.M.; Chaieb, K.; Papetti, A. Virucidal effect of guggulsterone isolated from Commiphora gileadensis. Planta Medica 2019, 85, 1225–1232. [Google Scholar] [CrossRef]
- Ullah, H.; Khan, A.; Rehman, N.U.; Halim, S.A.; Khan, H.; Khan, I.; Csuk, R.; Al-Rawahi, A.; Al-Hatmi, S.; Al-Harrasi, A. Lophenol and lathosterol from resin of Commiphora kua possess hepatoprotective effects in vivo. J. Ethnopharmacol. 2020, 252, 112558. [Google Scholar] [CrossRef]
- Hudson, J.B.; Lee, M.; Rasoanaivo, P. Antiviral activities in plants endemic to Madagascar. Pharm. Biol. 2000, 38, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.R.; Kaunda, J.S.; Zhu, H.T.; Wang, D.; Yang, C.R.; Zhang, Y.J. The genus Terminalia (Combretaceae): An ethnopharmacological, phytochemical and pharmacological review. Nat. Prod. Bioprospect. 2019, 9, 357–392. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Yoshikawa, M.; Kobayashi, S.; Sugihara, Y.; Suzuki, M.; Oominami, H.; Murakami, T.; Matsuda, H.; Doiphode, V.V. New triterpenes, myrrhanol A and myrrhanone A, from guggul-gum resins, and their potent anti-inflammatory effect on adjuvant-induced air-pouch granuloma of mice. Bioorg. Med. Chem. Lett. 2001, 11, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.B.; Niaz, M.A.; Ghosh, S. Hypolipidemic and antioxidant effects of Commiphora mukul as an adjunct to dietary therapy in patients with hypercholesterolemia. Cardiovasc. Drugs Ther. 1994, 8, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Dolara, P.; Corte, B.; Ghelardini, C.; Pugliese, A.M.; Cerbai, E.; Menichetti, S.; Lo Nostro, A. Local anaesthetic, antibacterial and antifungal properties of sesquiterpenes from myrrh. Planta Medica 2000, 66, 356–358. [Google Scholar] [CrossRef]
- Johns, T.; Nagarajan, M.; Parkipuny, M.L.; Jones, P.J.H. Maasai gummivory: Implications for paleolithic diets and contemporary health. Curr. Anthropol. 2000, 41, 453–459. Available online: https://www.journals.uchicago.edu/doi/epdf/10.1086/300152 (accessed on 20 June 2023). [CrossRef]
- Mulholland, D.A.; Taylor, D.A.H. Limonoid extractives from the genera Capuronianthus, Neobeguea and Quivisianthe. Phytochemistry 1988, 27, 1741–1743. [Google Scholar] [CrossRef]

| Lemur Species | Gum Specialization | Protein (%) | Sugar (%) | Energy (kJ/g) |
|---|---|---|---|---|
| Microcebus griseorufus | Obligate | 6.43 ± 5.66 | 27.06 ± 19.53 | 5.62 ± 3.41 |
| N = 25 | N = 26 | N = 25 | ||
| M. murinus | Facultative | 6.90 ± 6.12 | 33.96 ± 12.29 | 6.84 ± 2.24 |
| N = 10 | N = 10 | N = 10 | ||
| M. ravelobensis | Obligate | 1.75/10.31 | 21.93/28.21 | 3.96/6.45 |
| N = 2 | N = 2 | N = 2 | ||
| Phaner pallescens | Obligate | 4.96 ± 4.73 | 21.58 ± 13.92 | 4.90 ± 2.65 |
| N = 12 | N = 16 | N = 12 |
| Species | Protein | Sugar | Energy | Used in Traditional Medicine |
|---|---|---|---|---|
| No antibacterial activity | ||||
| Acacia bellula | 8.78 | 34.48 | 7.24 | A, F |
| Albizia mainaea | 11.31 | 28.35 | 6.64 | |
| Albizia tulearensis | 3.38 | 0.61 | 0.67 | A |
| Commiphora arafy | 15.25 | 30.81 | 7.71 | F |
| Commiphora guillaumini | 32.82 | |||
| Commiphora marchandii | 37.81 | A | ||
| Commiphora sp3 | 4.38 | 74.52 | 13.20 | |
| Delonix floribunda | 2.61 | 44.40 | 7.87 | A, R |
| Hymenodictyon decaryi | 3.50 | 5.55 | 1.51 | |
| Neobeguea mahafaliensis | 3.58 | 12.88 | 2.76 | A, R, F |
| Operculicarya gummifera | 0.63 | F | ||
| Operculicarya hyphaenoides | 3.19 | 11.43 | 2.45 | R |
| Poupartia sylvatica | 27.25 | 7.95 | 5.89 | |
| Rhopalocarpus sp | 3.94 | 38.15 | 7.04 | |
| Terminalia disjuncta | 2.75 | 73.77 | 12.81 | A |
| Terminalia mantaliopsis | 1.44 | 25.15 | 4.45 | |
| Terminalia mantaly | 2.38 | 26.06 | 4.76 | |
| Terminalia ulexoides | 13.75 | 4.98 | 3.08 | A |
| Zanthoxylum sp | 4.13 | 54.39 | 9.79 | |
| Antibacterial activity | ||||
| Albizia tulearensis | 2.94 | 0.47 | 0.57 | A |
| Commiphora simplicifolia | 3.58 | 8.37 | 2.11 | A, F |
| Quisvianthe papinae | 2.38 | 0.46 | 0.47 | |
| Terminalia disjuncta | 1.63 | 9.48 | 1.86 | A |
| Unknown tree species consumed by Phaner pallescens | 5.31 | 12.01 | 2.90 | na |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganzhorn, J.U.; Ratovonamana, Y.R.; Rother, M.; Giertz, P.; Andrews, C.A.; Baumann, S.; Bohr, Y.E.-M.B.; Kappeler, P.M.; Montero, B.K.; Pommerening-Röser, A.; et al. Nutritional and Possible Pharmaceutical Aspects of Tree Exudates Eaten by Lemurs of Madagascar’s Dry Forests. Separations 2023, 10, 575. https://doi.org/10.3390/separations10110575
Ganzhorn JU, Ratovonamana YR, Rother M, Giertz P, Andrews CA, Baumann S, Bohr YE-MB, Kappeler PM, Montero BK, Pommerening-Röser A, et al. Nutritional and Possible Pharmaceutical Aspects of Tree Exudates Eaten by Lemurs of Madagascar’s Dry Forests. Separations. 2023; 10(11):575. https://doi.org/10.3390/separations10110575
Chicago/Turabian StyleGanzhorn, Jörg U., Yedidya R. Ratovonamana, Melina Rother, Peggy Giertz, Curswan A. Andrews, Sabine Baumann, Yvonne E.-M. B. Bohr, Peter M. Kappeler, B. Karina Montero, Andreas Pommerening-Röser, and et al. 2023. "Nutritional and Possible Pharmaceutical Aspects of Tree Exudates Eaten by Lemurs of Madagascar’s Dry Forests" Separations 10, no. 11: 575. https://doi.org/10.3390/separations10110575
APA StyleGanzhorn, J. U., Ratovonamana, Y. R., Rother, M., Giertz, P., Andrews, C. A., Baumann, S., Bohr, Y. E. -M. B., Kappeler, P. M., Montero, B. K., Pommerening-Röser, A., Radespiel, U., Rakotondranary, S. J., Schülke, O., Steffens, K. J. E., Thorén, S., Timmermann, G., & Tomaschewski, I. (2023). Nutritional and Possible Pharmaceutical Aspects of Tree Exudates Eaten by Lemurs of Madagascar’s Dry Forests. Separations, 10(11), 575. https://doi.org/10.3390/separations10110575







