Phytochemical Analysis and In Vitro Effects on Isolated Murine Lymphocytes and Macrophages of Polymeric Micelles Loaded with Cycloartane Saponin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Saponin Isolation
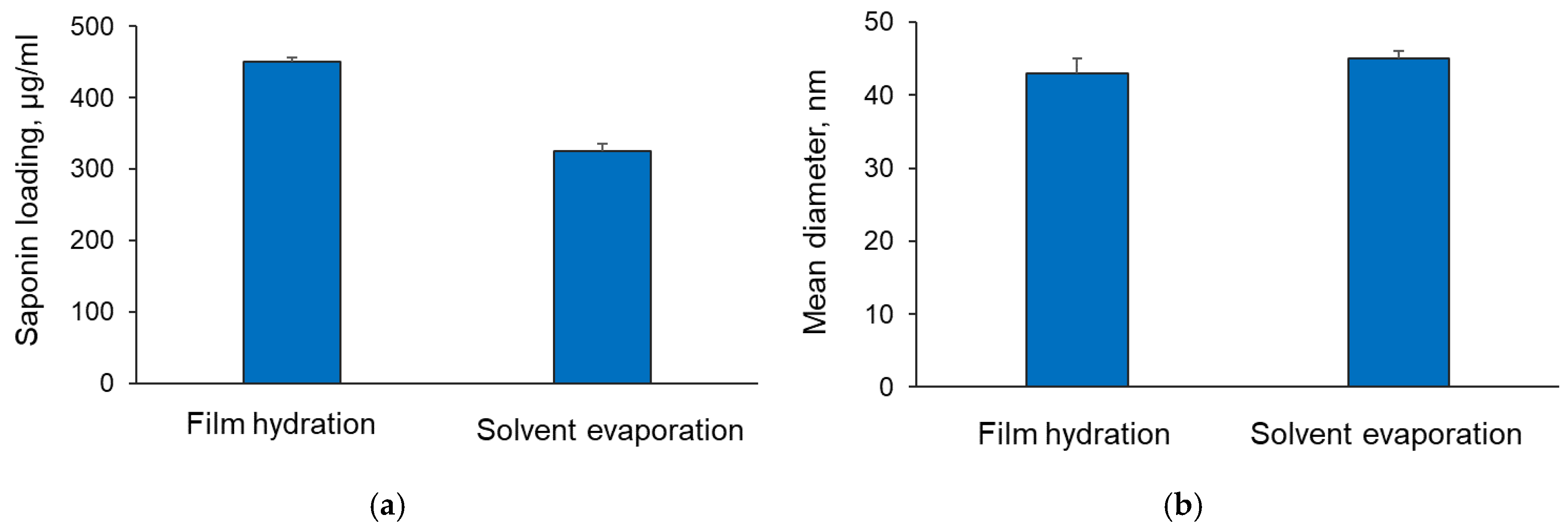
2.2. Formulation of Saponin-Loaded Copolymeric Micelles
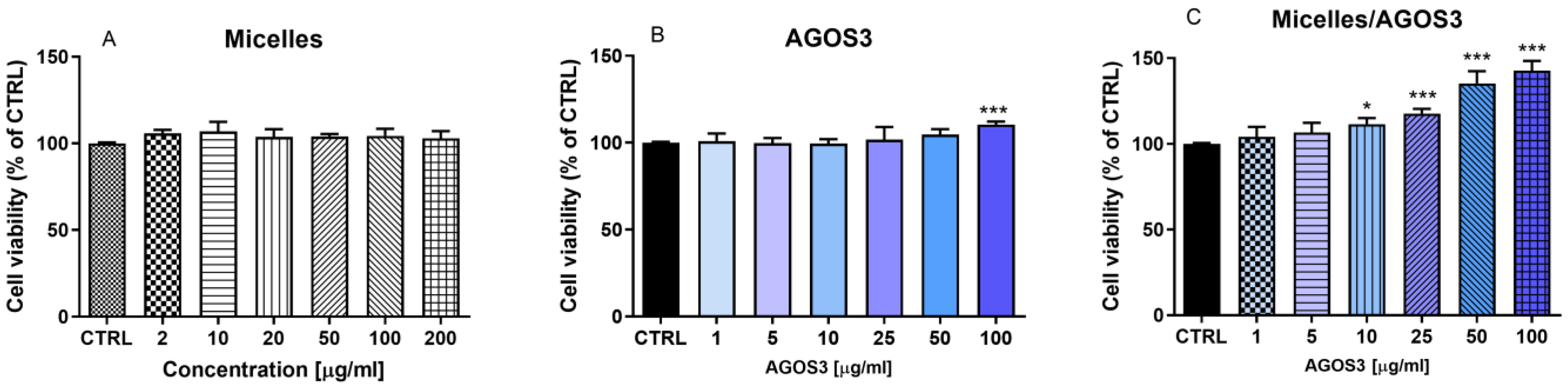
2.3. Pharmacological Evaluation
2.4. LC-MS Analysis
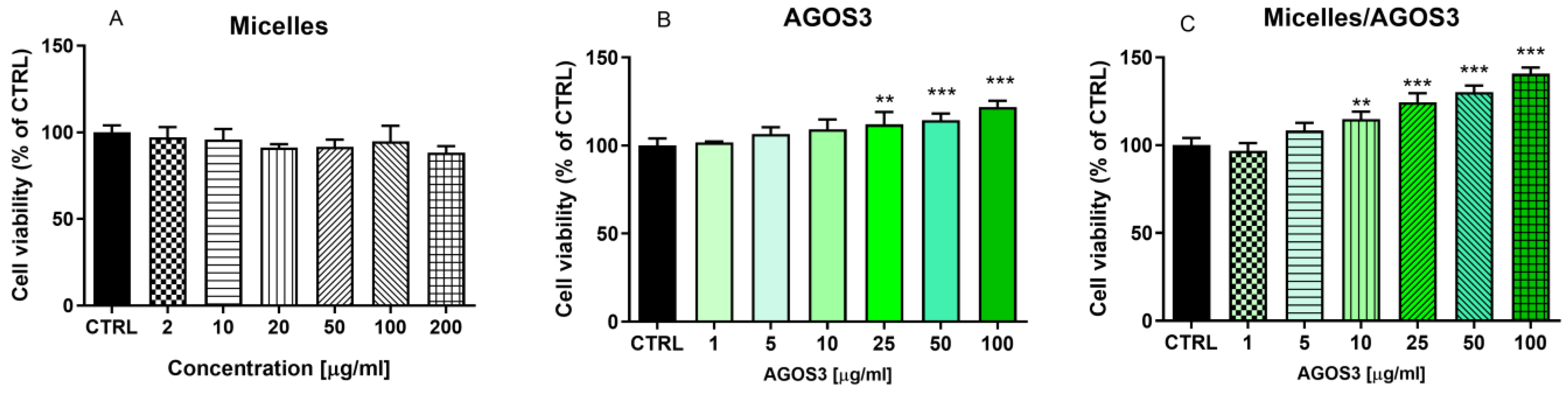
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Stambolov, I.; Shkondrov, A.; Krasteva, I. Astragalus Glycyphyllos L.: Phytochemical Constituents, Pharmacology, and Biotechnology. Pharmacia 2023, 70, 635–641. [Google Scholar] [CrossRef]
- Linnek, J.; Mitaine-Offer, A.-C.; Miyamoto, T.; Tanaka, C.; Paululat, T.; Avunduk, S.; Alankuş-Çalişkan, Ö.; Lacaille-Dubois, M.-A. Cycloartane Glycosides from Three Species of Astragalus (Fabaceae). Helv. Chim. Acta 2011, 94, 230–237. [Google Scholar] [CrossRef]
- Shkondrov, A.; Krasteva, I.; Bucar, F.; Kunert, O.; Kondeva-Burdina, M.; Ionkova, I. Flavonoids and Saponins from Two Bulgarian Astragalus Species and Their Neuroprotective Activity. Phytochem. Lett. 2018, 26, 44–49. [Google Scholar] [CrossRef]
- Shkondrov, A.; Krasteva, I.; Bucar, F.; Kunert, O.; Kondeva-Burdina, M.; Ionkova, I. A New Tetracyclic Saponin from Astragalus glycyphyllos L. and Its Neuroprotective and HMAO-B Inhibiting Activity. Nat. Prod. Res. 2020, 34, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Stambolov, I.; Shkondrov, A.; Kunert, O.; Bucar, F.; Kondeva-Burdina, M.; Krasteva, I. Cycloartane Saponins from Astragalus glycyphyllos and Their In Vitro Neuroprotective, Antioxidant, and HMAO-B-Inhibiting Effects. Metabolites 2023, 13, 857. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Liu, Y.; Zhang, Z.-P.; Wu, J.-T.; Wang, J.; Pan, J.; Guan, W.; Kuang, H.; Yang, B. Seven New Triterpenoid Saponins from Astragalus membranaceus Var. Mongholicus and the Inhibition of High-Glucose Induced SV40 MES 13 Cells. New J. Chem. 2023, 47, 8776–8784. [Google Scholar] [CrossRef]
- Lacaille-Dubois, M.-A. Saponins as Immunoadjuvants and Immunostimulants. In Immunomodulatory Agents from Plants; Wagner, H., Ed.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 243–272. ISBN 3764358483. [Google Scholar]
- Wang, P. Natural and Synthetic Saponins as Vaccine Adjuvants. Vaccines 2021, 9, 222. [Google Scholar] [CrossRef]
- Ionkova, I.; Shkondrov, A.; Zarev, Y.; Kozuharova, E.; Krasteva, I. Anticancer Secondary Metabolites: From Ethnopharmacology and Identification in Native Complexes to Biotechnological Studies in Species of Genus Astragalus L. and Gloriosa L. Curr. Issues Mol. Biol. 2022, 44, 3884–3904. [Google Scholar] [CrossRef]
- Navarro del Hierro, J.; Piazzini, V.; Reglero, G.; Martin, D.; Bergonzi, M.C. In Vitro Permeability of Saponins and Sapogenins from Seed Extracts by the Parallel Artificial Membrane Permeability Assay: Effect of in Vitro Gastrointestinal Digestion. J. Agric. Food Chem. 2020, 68, 1297–1305. [Google Scholar] [CrossRef]
- Ikram, M.; Jo, M.H.; Choe, K.; Khan, A.; Ahmad, S.; Saeed, K.; Kim, M.W.; Kim, M.O. Cycloastragenol, a Triterpenoid Saponin, Regulates Oxidative Stress, Neurotrophic Dysfunctions, Neuroinflammation and Apoptotic Cell Death in Neurodegenerative Conditions. Cells 2021, 10, 2719. [Google Scholar] [CrossRef]
- Sasaki, H.; Sunagawa, Y.; Takahashi, K.; Imaizumi, A.; Fukuda, H.; Hashimoto, T.; Wada, H.; Katanasaka, Y.; Kakeya, H.; Fujita, M.; et al. Innovative Preparation of Curcumin for Improved Oral Bioavailability. Biol. Pharm. Bull. 2011, 34, 660–665. [Google Scholar] [CrossRef]
- de Oliveira, R.S.S.; Marín Huachaca, N.S.; Lemos, M.; Santos, N.F.; Feitosa, E.; Salay, L.C. Molecular Interactions between Pluronic F127 and Saponin in Aqueous Solution. Colloid Polym. Sci. 2020, 298, 113–122. [Google Scholar] [CrossRef]
- Radeva, L.; Yordanov, Y.; Spassova, I.; Kovacheva, D.; Tzankova, V.; Yoncheva, K. Double-Loaded Doxorubicin/Resveratrol Polymeric Micelles Providing Low Toxicity on Cardiac Cells and Enhanced Cytotoxicity on Lymphoma Cells. Pharmaceutics 2023, 15, 1287. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Sari, G.; Ozdal, T.; Capanoglu, E. Guidelines for Cell Viability Assays. Food Front. 2020, 1, 332–349. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Tava, A.; Biazzi, E.; Mella, M.; Quadrelli, P.; Avato, P. Artefact Formation during Acid Hydrolysis of Saponins from Medicago spp. Phytochemistry 2017, 138, 116–127. [Google Scholar] [CrossRef]
- Oleszek, W.A. Chromatographic Determination of Plant Saponins. J. Chromatogr. A 2002, 967, 147–162. [Google Scholar] [CrossRef]
- Abhijith, B.; Natakkakath Kaliyathan Raveena, M.V.R.; Lankalapalli, R.S. Artifacts from the Methanolic Extract of Solanum Nigrum Linn. Nat. Prod. Res. 2023, 38, 2896–2900. [Google Scholar] [CrossRef]
- ICH Harmonised Tripartite Guideline. Validation of Analytical Procedures: Text and Methodology Q2 (R1); International Council on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use: Geneva, Switzerland, 2005; pp. 11–12. [Google Scholar]
- Savarino, P.; Demeyer, M.; Decroo, C.; Colson, E.; Gerbaux, P. Mass Spectrometry Analysis of Saponins. Mass Spectrom. Rev. 2023, 42, 954–983. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Kord, A. UHPLC Method Development. In Ultra-High Performance Liquid Chromatography and Its Applications; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2013; pp. 1–30. ISBN 9781118533956. [Google Scholar]
- Figueiras, A.; Domingues, C.; Jarak, I.; Santos, A.I.; Parra, A.; Pais, A.; Alvarez-Lorenzo, C.; Concheiro, A.; Kabanov, A.; Cabral, H.; et al. New Advances in Biomedical Application of Polymeric Micelles. Pharmaceutics 2022, 14, 1700. [Google Scholar] [CrossRef]
- Swartz, M.E.; Krull, I.S. Analytical Method Development and Validation; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Gao, S.; Basu, S.; Yang, Z.; Deb, A.; Hu, M. Bioavailability Challenges Associated with Development of Saponins as Therapeutic and Chemopreventive Agents. Curr. Drug Targets 2012, 13, 1885–1899. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Liu, J.-Y.; Liu, Z.-Q.; Xiao, D.; Chen, J. The Role of ADCY1 in Regulating the Sensitivity of Platinum-Based Chemotherapy in NSCLC. Pharmaceuticals 2024, 17, 1118. [Google Scholar] [CrossRef]
- Bahcecioglu, G.; Basara, G.; Ellis, B.W.; Ren, X.; Zorlutuna, P. Breast Cancer Models: Engineering the Tumor Microenvironment. Acta Biomater. 2020, 106, 1–21. [Google Scholar] [CrossRef]
- Lee, J.; Lim, J.-H.; Jung, G.-Y.; Kang, J.; Jo, I.; Kang, K.; Kim, J.-H.; Kim, B.-S.; Yang, H. Triterpenoid Saponins from Camellia sinensis Roots with Cytotoxic and Immunomodulatory Effects. Phytochemistry 2023, 212, 113688. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, D.; Ma, C.; Wang, R.; Wang, W. Free Radical Scavenging Effect and Immunomodulatory Activity of Total Saponins Extract of Ginseng Fibrous Roots. Molecules 2024, 29, 2770. [Google Scholar] [CrossRef]
- Li, C.-X.; Liu, Y.; Zhang, Y.-Z.; Li, J.-C.; Lai, J. Astragalus Polysaccharide: A Review of Its Immunomodulatory Effect. Arch. Pharm. Res. 2022, 45, 367–389. [Google Scholar] [CrossRef]
- Ding, G.; Gong, Q.; Ma, J.; Liu, X.; Wang, Y.; Cheng, X. Immunosuppressive Activity Is Attenuated by Astragalus polysaccharides through Remodeling the Gut Microenvironment in Melanoma Mice. Cancer Sci. 2021, 112, 4050–4063. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Liu, Y.-Q.; Liu, D.; Zhang, L.; Qin, J.; Zhang, Z.; Su, Y.; Yan, C.; Luo, Y.-L.; Li, J.; et al. The Effects of Astragalus Polysaccharide on Bone Marrow-Derived Mesenchymal Stem Cell Proliferation and Morphology Induced by A549 Lung Cancer Cells. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 4110–4121. [Google Scholar] [CrossRef]
- Dong, M.; Li, J.; Yang, D.; Li, M.; Wei, J. Biosynthesis and Pharmacological Activities of Flavonoids, Triterpene Saponins and Polysaccharides Derived from Astragalus Membranaceus. Molecules 2023, 28, 5018. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, L.; Gao, C.; Chen, W.; Vong, C.T.; Yao, P.; Yang, Y.; Li, X.; Tang, X.; Wang, S.; et al. Astragali Radix (Huangqi): A Promising Edible Immunomodulatory Herbal Medicine. J. Ethnopharmacol. 2020, 258, 112895. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Meng, T.; Hao, N.; Tao, H.; Zou, S.; Li, M.; Ming, P.; Ding, H.; Dong, J.; Feng, S.; et al. Immune Regulation Mechanism of Astragaloside IV on RAW264.7 Cells through Activating the NF-ΚB/MAPK Signaling Pathway. Int. Immunopharmacol. 2017, 49, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Krasteva, I.N.; Toshkova, R.A.; Nikolov, S.D. Protective Effect of Astragalus corniculatus Saponins against Myeloid Graffi Tumour in Hamsters. Phyther. Res. 2004, 18, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Anang, V.; Kumari, K.; Kottarath, S.K.; Verma, C. Chapter Eleven—Role of Lymphocytes, Macrophages and Immune Receptors in Suppression of Tumor Immunity. In Receptor Endocytosis and Signalling in Health and Disease—Part A; Mani, I., Singh, V., Eds.; Progress in Molecular Biology and Translational Science; Academic Press: Cambridge, MA, USA, 2023; Volume 194, pp. 269–310. [Google Scholar]
- Sam-Yellowe, T.Y.; Sam-Yellowe, T.Y.; Sam-Yellowe, T. Immunology: Overview and Laboratory Manual; Springer: Basel, Switzerland, 2021. [Google Scholar]
- Wang, Q.; Atluri, K.; Tiwari, A.K.; Babu, R.J. Exploring the Application of Micellar Drug Delivery Systems in Cancer Nanomedicine. Pharmaceuticals 2023, 16, 433. [Google Scholar] [CrossRef] [PubMed]


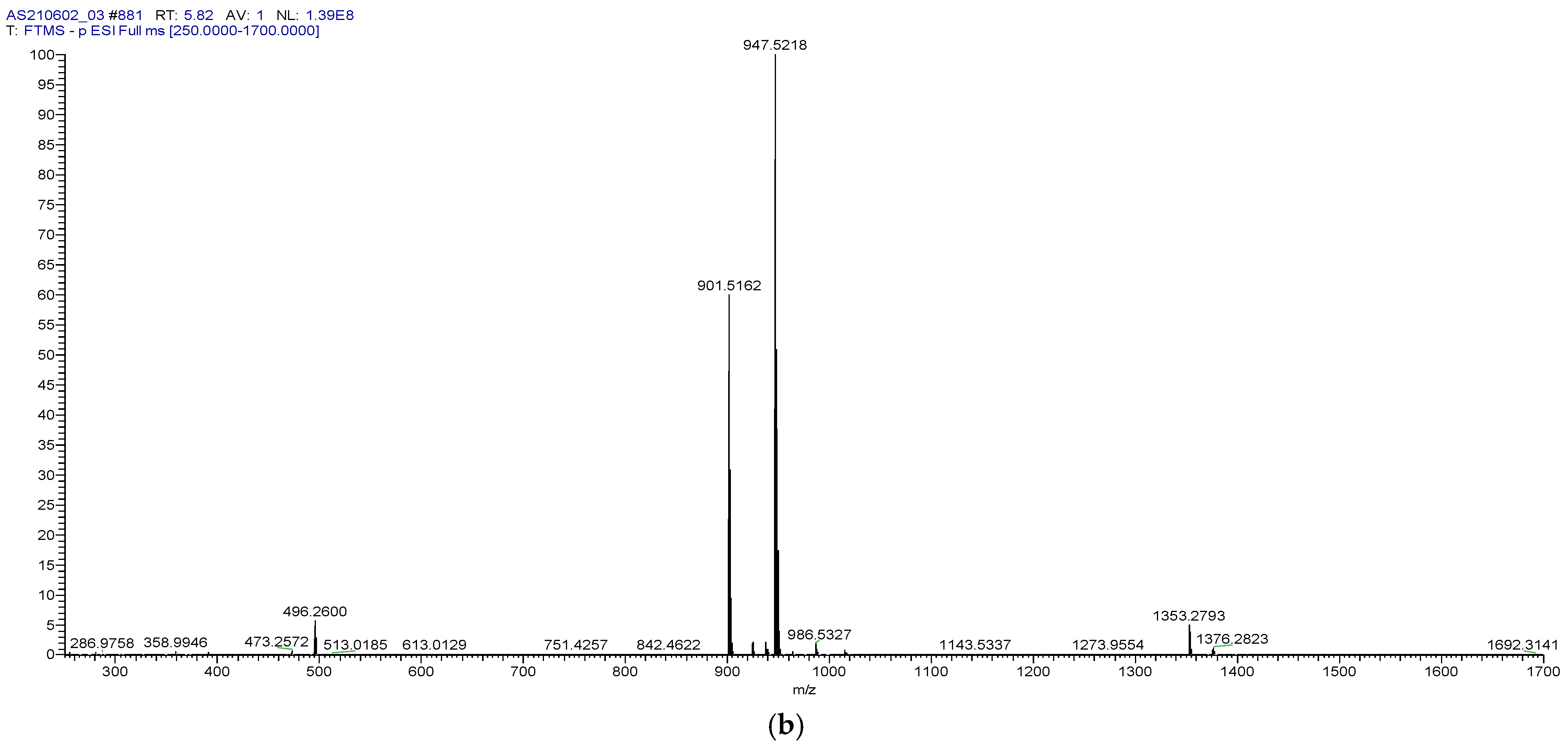
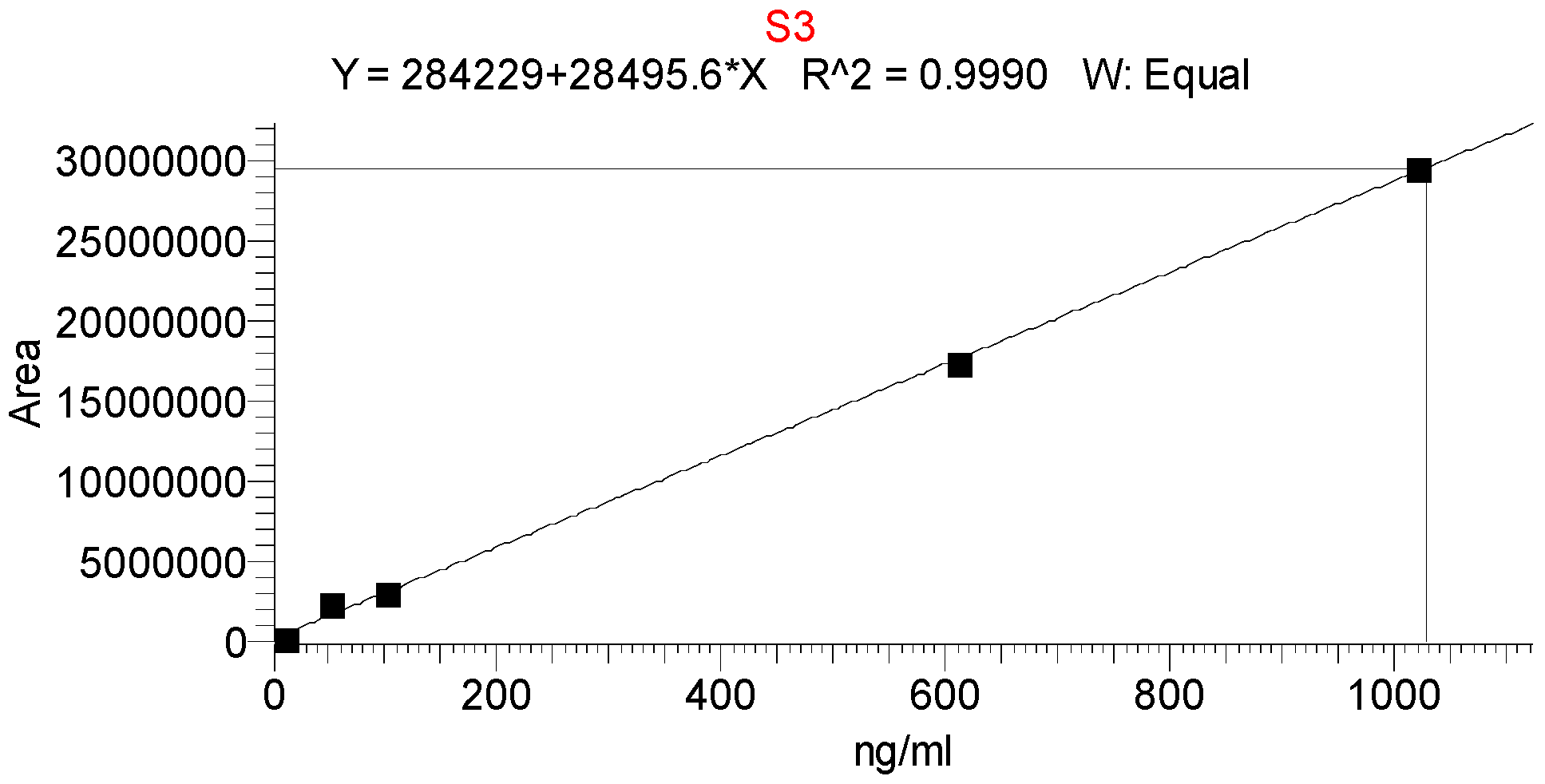
| Injection | Peak Area | tR, min | Theoretical Plates | Resolution |
|---|---|---|---|---|
| 1 | 29577210 | 5.82 | 32289 | 1.7 |
| 2 | 29580021 | 5.83 | 32125 | 1.7 |
| 3 | 29576564 | 5.84 | 32221 | 1.7 |
| 4 | 29577324 | 5.81 | 32254 | 1.6 |
| 5 | 29576499 | 5.82 | 32251 | 1.7 |
| 6 | 29580052 | 5.82 | 32218 | 1.7 |
| Mean | 29577945 | 5.82 | 32226 | 1.7 |
| SD | 1510 | 0.01 | 51 | 0.00 |
| %RSD | 0.01 | 0.16 | 0.16 | 0.22 |
| Indicator | Value |
|---|---|
| Linearity range, ng/mL | 10.2–1020 |
| Slope | 28,495.6 |
| y-intercept | 284,229 |
| Correlation coefficient r2 | 0.9990 |
| Theoretical Concentration, ng/mL | Found Concentration *, ng/mL | Recovery *, % ± SD | % RSD | Average Recovery, % |
|---|---|---|---|---|
| 1020.0 | 1032.2 | 101.2 ± 4.5 | 4.6 | 100.5 ± 1.1 |
| 510.0 | 508.0 | 99.6 ± 3.7 | 3.8 | |
| 102.0 | 102.2 | 100.2 ± 1.0 | 1.0 | |
| 10.2 | 10.3 | 100.6 ± 0.9 | 0.9 |
| Theoretical Concentration, ng/mL | Intraday Concentration *, ng/mL | Intraday Variance **, %RSD | Inter-Day Found Concentration *, ng/mL | Inter-Day Variance ***, %RSD | |
|---|---|---|---|---|---|
| 1st Measurement | 2nd Measurement | ||||
| 1020 | 1019 | 1021 | 1.2 | 1017 | 3.2 |
| 510 | 512 | 509 | 1.5 | 508 | 1.8 |
| 102 | 101 | 104 | 2.1 | 101 | 1.7 |
| Theoretical Concentration, ng/mL | 30 °C Column Temperature Found Concentration *, ng/mL ± SD | 35 °C Column Temperature Found Concentration *, ng/mL ± SD |
|---|---|---|
| 1020 | 1021 ± 2.8 | 1022 ± 1.9 |
| 510 | 511 ± 2.5 | 509 ± 2.1 |
| 102 | 101 ± 3.2 | 103 ± 2.0 |
| Theoretical Concentration, ng/mL | 4 °C Storage for 1 Month Found Concentration *, ng/mL ± SD | 25 °C Storage for 1 Month Found Concentration *, ng/mL ± SD |
|---|---|---|
| 1020 | 1020 ± 0.9 | 1017 ± 2.1 |
| 510 | 509 ± 1.1 | 508 ± 2.4 |
| 102 | 102 ± 0.8 | 99.9 ± 2.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shkondrov, A.; Stefanova, D.; Stambolov, I.; Yoncheva, K.; Tzankova, V.; Krasteva, I. Phytochemical Analysis and In Vitro Effects on Isolated Murine Lymphocytes and Macrophages of Polymeric Micelles Loaded with Cycloartane Saponin. Separations 2024, 11, 280. https://doi.org/10.3390/separations11100280
Shkondrov A, Stefanova D, Stambolov I, Yoncheva K, Tzankova V, Krasteva I. Phytochemical Analysis and In Vitro Effects on Isolated Murine Lymphocytes and Macrophages of Polymeric Micelles Loaded with Cycloartane Saponin. Separations. 2024; 11(10):280. https://doi.org/10.3390/separations11100280
Chicago/Turabian StyleShkondrov, Aleksandar, Denitsa Stefanova, Ivan Stambolov, Krassimira Yoncheva, Virginia Tzankova, and Ilina Krasteva. 2024. "Phytochemical Analysis and In Vitro Effects on Isolated Murine Lymphocytes and Macrophages of Polymeric Micelles Loaded with Cycloartane Saponin" Separations 11, no. 10: 280. https://doi.org/10.3390/separations11100280








