The Removal of Organic Contaminants from Condensed Wastewater Using Electrolysis Combined with Ozonation: A Pilot-Scale Study
Abstract
:1. Introduction
2. Experimental
2.1. Experimental Apparatus
2.2. Pilot Experimental Materials
2.3. Analytical Methods
3. Results and Discussion
3.1. The Analysis of the Wastewater
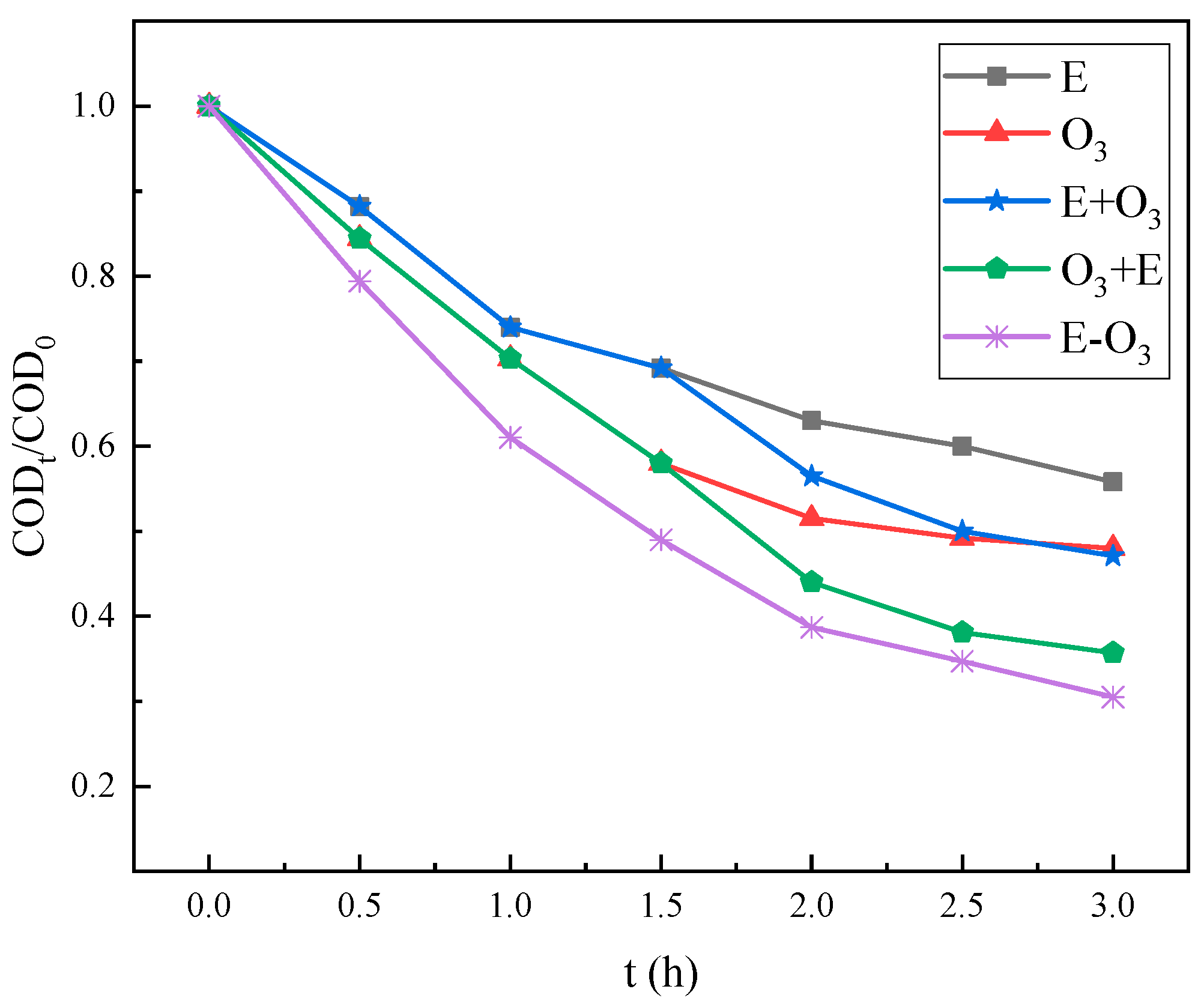
3.2. The Synergistic Effect of E-O3
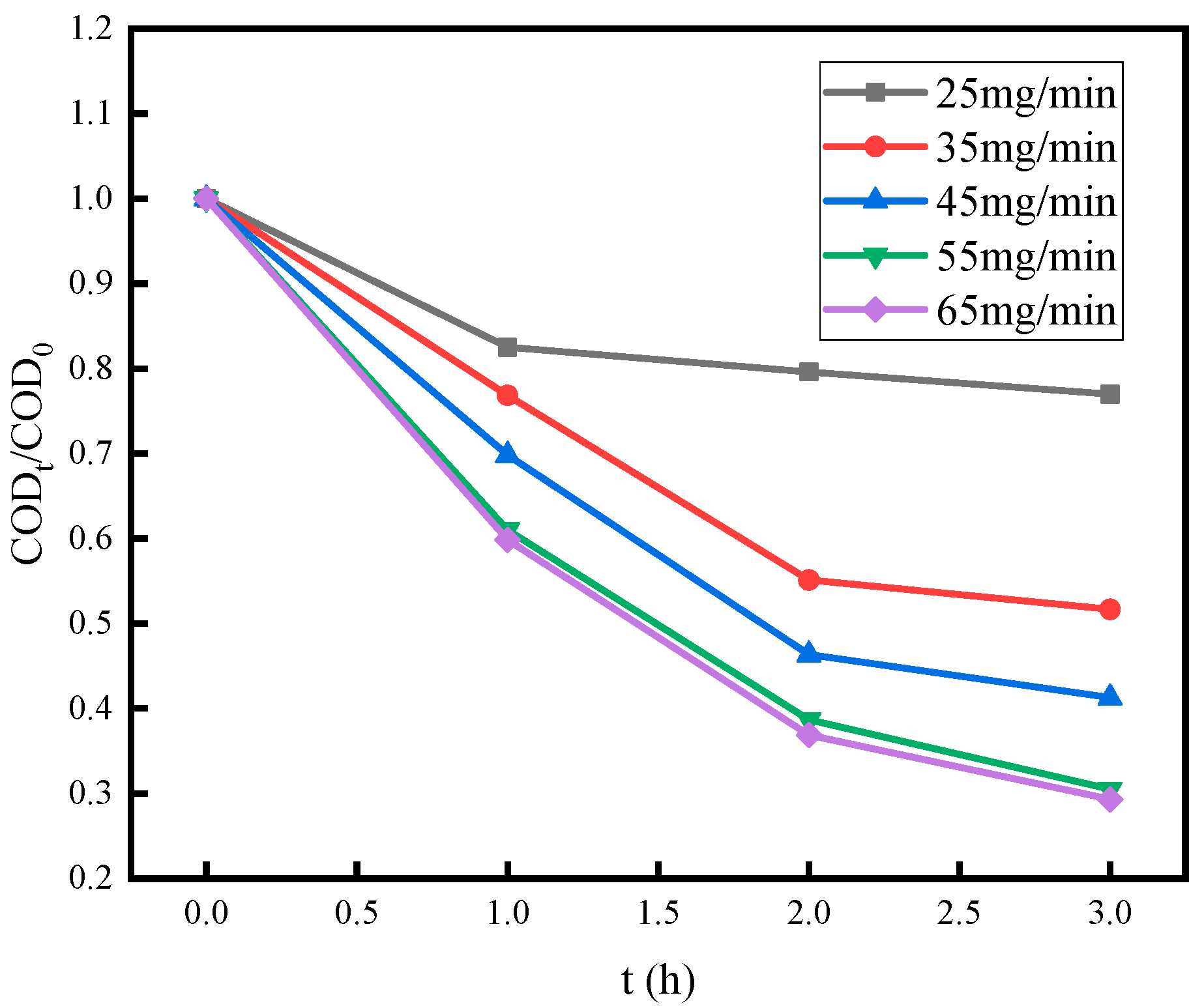
3.3. Effect of O3 Dosage
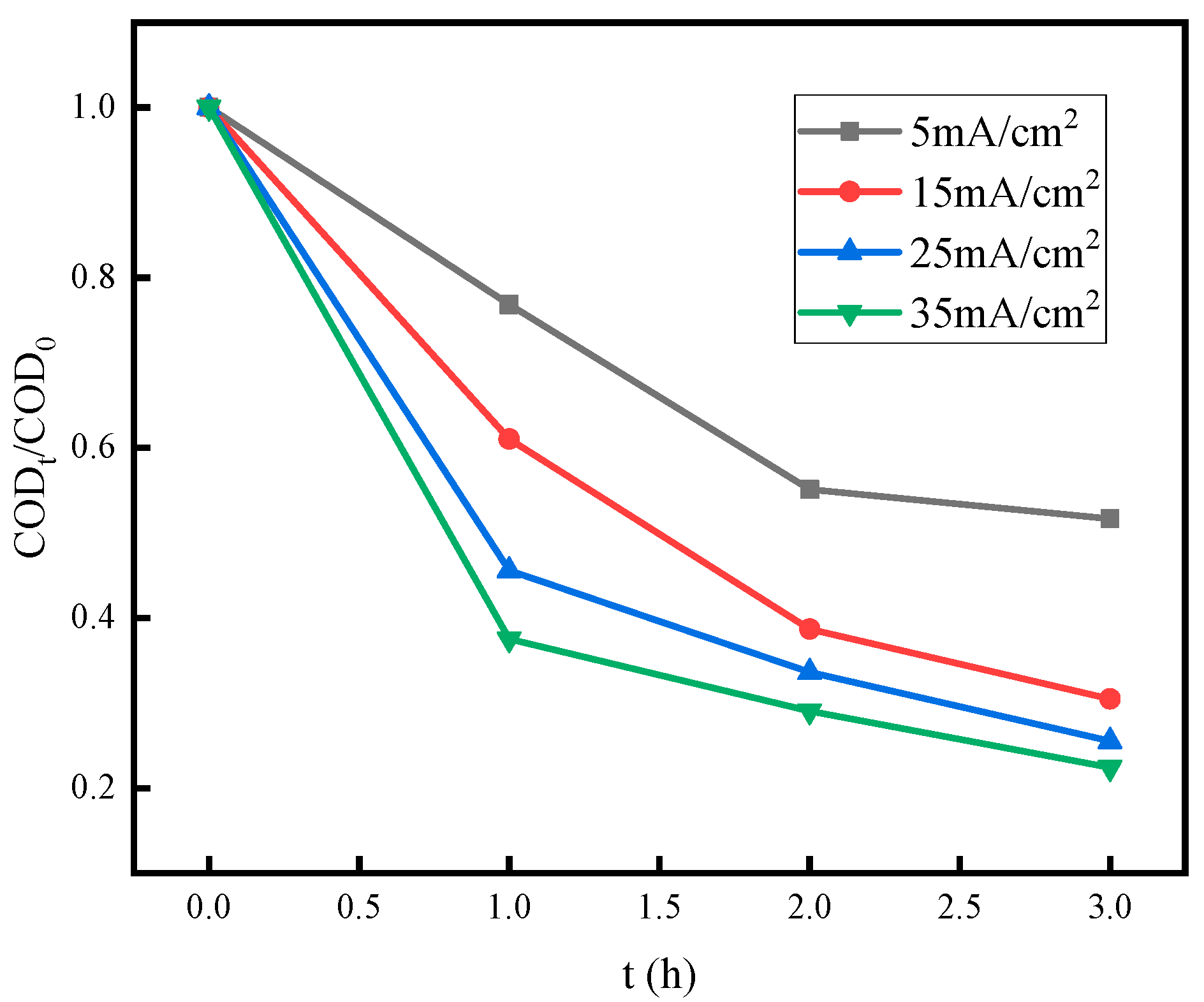
3.4. Effect of Current Density
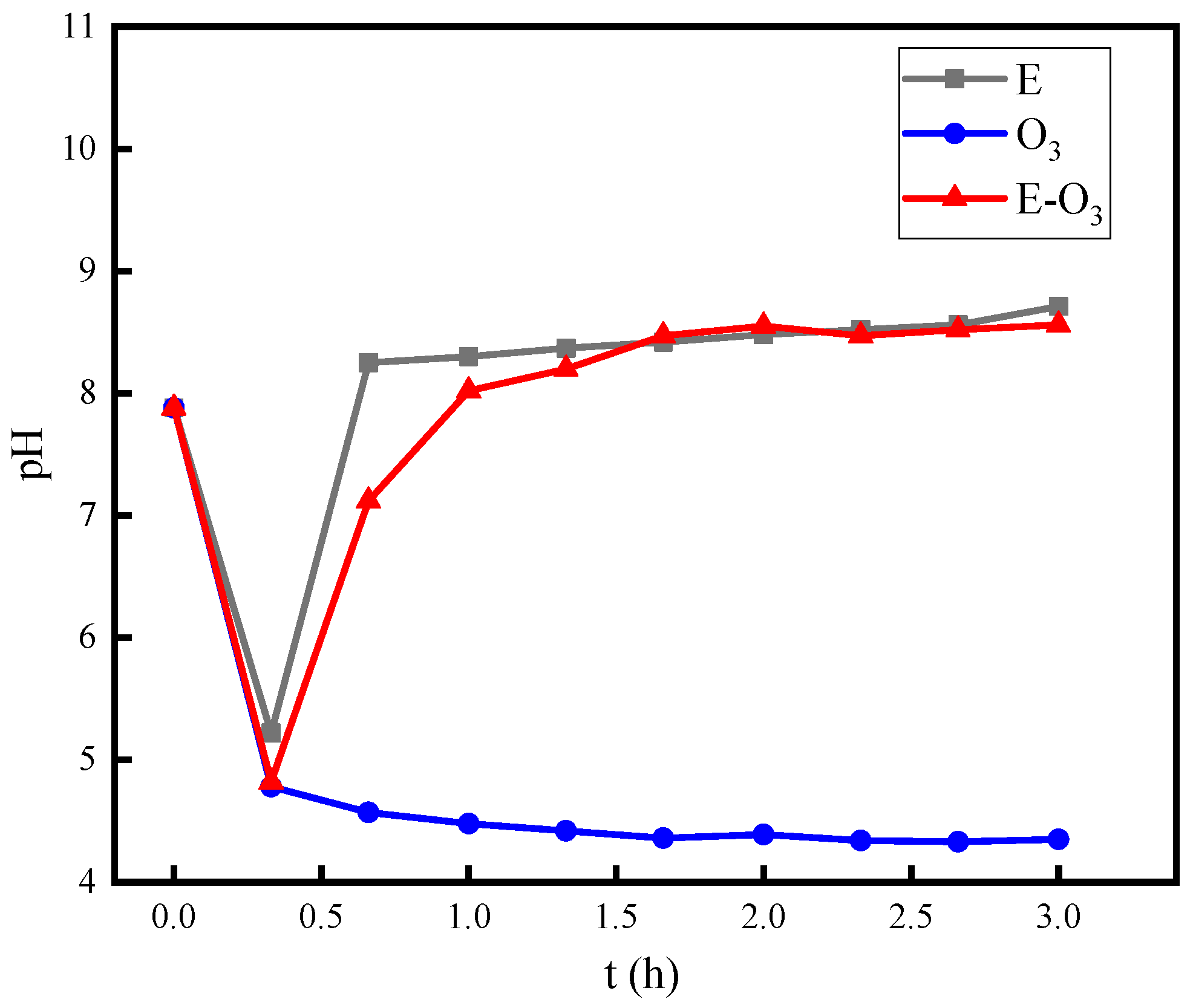
3.5. Mechanism Investigation
4. Conclusions
- It is necessary to remove carbonate ions through pretreatment in the application process of E-O3; the advantage of E-O3 can only be obtained after carbonate ion removal.
- The combination of electrolysis and ozonation not only exhibits synergy in the generation of hydroxyl radicals but also in the degradation pathways of substances.
- The combination of electrolysis and ozonation has an inhibitory effect on the decrease in pH, which is an important factor in the synergistic mechanism of hydroxyl radicals.
- In engineering applications, the efficiency of the E-O3 technology can be regulated by synergistically controlling the ozone dosage and current density.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Minier-Matar, J.; Alshamari, E.; Raja, M.; Khan, F.; Al-Maas, M.; Hussain, A.; Adham, S. Detailed organic characterization of process water to evaluate reverse osmosis membrane fouling in industrial wastewater treatment. Desalination 2024, 572, 117128. [Google Scholar] [CrossRef]
- Kim, S.; Chu, K.H.; Al-Hamadani, Y.A.J.; Park, C.M.; Jang, M.; Kim, D.H.; Yu, M.; Heo, J.; Yoon, Y. Removal of contaminants of emerging concern by membranes in water and wastewater: A review. Chem. Eng. J. 2018, 335, 896–914. [Google Scholar] [CrossRef]
- Choi, M.Y.; Theerthagiri, J.; Maia, G. 2D advanced materials and technologies for industrial wastewater treatment. Chemosphere 2021, 284, 131394. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.; Herrera-León, S.; Calisaya-Azpilcueta, D.; Salazar, R.; Cisternas, L.A.; Kraslawski, A. Using Waste Brine from Desalination Plant as a Source of Industrial Water in Copper Mining Industry. Minerals 2022, 12, 1162. [Google Scholar] [CrossRef]
- Dong, X.; Liu, H.; Li, J.; Gan, R.; Liu, Q.; Zhang, X. Fenton Oxidation Combined with Iron–Carbon Micro-Electrolysis for Treating Leachate Generated from Thermally Treated Sludge. Separations 2023, 10, 568. [Google Scholar] [CrossRef]
- Walschot, M.; Luis, P.; Liégeois, M. The challenges of reverse osmosis desalination: Solutions in Jordan. Water Int. 2020, 45, 112–124. [Google Scholar] [CrossRef]
- Dilaver, M.; Çelebi, M.D.; Agtas, M.; Koyuncu, I. Brackish water RO concentrate treatment and water recovery using cost lowering integrated technology. J. Environ. 2022, 10, 108463. [Google Scholar] [CrossRef]
- Samimi, M.; Moghadam, H. Modified evacuated tube collector basin solar still for optimal desalination of reverse osmosis concentrate. Energy 2024, 289, 129983. [Google Scholar] [CrossRef]
- Mortula, M.M.; Abdelrahman, M.; Tatan, B. Comparative Evaluation of Membrane Filtration on the Tertiary Treatment of Synthetic Secondary Effluent. Separations 2022, 9, 63. [Google Scholar] [CrossRef]
- Sun, W.Q.; Xiao, Z.Q.; Sun, Y.J.; Ding, L.; Zhou, J. Preparation of Cu-Ce@γ-Al2O3 and Study on Catalytic Ozone Oxidation for the Treatment of RO Concentrate Water. Water 2022, 14, 2881. [Google Scholar] [CrossRef]
- Zhao, D.D.; Lee, L.Y.; Ong, S.L.; Chowdhury, P.; Siah, K.B.; Ng, H.Y. Electrodialysis reversal for industrial reverse osmosis brine treatment. Sep. Purif. Technol. 2019, 213, 339–347. [Google Scholar] [CrossRef]
- Von Eiff, D.; Wong, P.W.; Gao, Y.G.; Jeong, S.; An, A.K. Technical and economic analysis of an advanced multi-stage flash crystallizer for the treatment of concentrated brine. Desalioation 2021, 503, 114925. [Google Scholar] [CrossRef]
- Hu, Z.; Xiang, F.; Mao, J.; Ding, Y.; Tong, S. Oxidative Efficiency of Ozonation Coupled with Electrolysis for Treatment of Acid Wastewater. J. Electrochem. 2022, 28, 2104191. [Google Scholar]
- Bai, G.; Chen, M.; Cai, N.; Guo, Z.; Zhang, T.; Guo, P. Advances on Determination Methods of Free Radicals in Advanced Oxidation Processes. J. Instrum. Anal. 2021, 40, 1109–1118. [Google Scholar]
- Kasprzyk-Hordern, B.; Ziólek, M.; Nawrocki, J. Catalytic ozonation and methods of enhancing molecular ozone reactions in water treatment. Appl. Catal. B-Environ. 2003, 46, 639–669. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, L.J. Advanced Oxidation Processes for Wastewater Treatment: Formation of Hydroxyl Radical and Application. Crit. Rev. Environ. Sci. Technol. 2012, 42, 251–325. [Google Scholar] [CrossRef]
- Pera-Titus, M.; García-Molina, V.; Baños, M.A.; Giménez, J.; Esplugas, S. Degradation of chlorophenols by means of advanced oxidation processes: A general review. Appl. Catal. B-Environ. 2004, 47, 219–256. [Google Scholar] [CrossRef]
- Saravanan, A.; Deivayanai, V.C.; Kumar, P.S.; Rangasamy, G.; Hemavathy, R.V.; Harshana, T.; Gayathri, N.; Alagumalai, K. A detailed review on advanced oxidation process in treatment of wastewater: Mechanism, challenges and future outlook. Chemosphere 2022, 308, 136524. [Google Scholar] [CrossRef]
- Lin, W.; Liu, X.; Ding, A.; Ngo, H.H.; Zhang, R.R.; Nan, J.; Ma, J.; Li, G.B. Advanced oxidation processes (AOPs)-based sludge conditioning for enhanced sludge dewatering and micropollutants removal: A critical review. J. Water Process Eng. 2022, 45, 102468. [Google Scholar] [CrossRef]
- Tian, K.; Hu, L.M.; Li, L.T.; Zheng, Q.Z.; Xin, Y.J.; Zhang, G.S. Recent advances in persulfate-based advanced oxidation processes for organic wastewater treatment. Chin. Chem. Lett. 2022, 33, 4461–4477. [Google Scholar] [CrossRef]
- Li, X.X.; Fu, L.; Chen, F.; Zhao, S.C.; Zhu, J.W.; Yin, C.L. Application of Heterogeneous Catalytic Ozonation in Wastewater Treatment: An Overview. Catalysts 2023, 13, 342. [Google Scholar] [CrossRef]
- Wang, J.L.; Chen, H. Catalytic ozonation for water and wastewater treatment: Recent advances and perspective. Sci. Total Environ. 2020, 704, 135249. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Segura, S.; Brillas, E. Applied photoelectrocatalysis on the degradation of organic pollutants in wastewaters. J. Photochem. Photobiol. C-Photochem. Rev. 2017, 31, 1–35. [Google Scholar] [CrossRef]
- Radjenovic, J.; Sedlak, D.L. Challenges and Opportunities for Electrochemical Processes as Next-Generation Technologies for the Treatment of Contaminated Water. Environ. Sci. Technol. 2015, 49, 11292–11302. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.K.; Wang, J.; Ren, M.Z.; Yang, X.; Zhang, J.B.; Zhang, X.L.; Cao, H.B.; Xie, Y.B. Comprehensive effect of water matrix on catalytic ozonation of chloride contained saline wastewater. Water Res. 2023, 234, 119827. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.M.; Chen, J.J.; Shao, G.Y.; Qu, Y.X.; Zhang, F.; Tian, S.C.; Zhou, Z.Y.; Ren, Z.Q. Preparation and application of green calcium-based catalyst for advanced treatment of salty wastewater with ozone. J. Clean. Prod. 2022, 362, 132464. [Google Scholar] [CrossRef]
- Guo, H.; Li, X.K.; Li, G.H.; Liu, Y.; Rao, P.H. Preparation of SnOx-MnOx@Al2O3 for Catalytic Ozonation of Phenol in Hypersaline Wastewater. Ozone-Sci. Eng. 2023, 45, 262–275. [Google Scholar] [CrossRef]
- Hu, Z.Z.; Cai, J.J.; Song, G.; Tian, Y.S.; Zhou, M.H. Anodic oxidation of organic pollutants: Anode fabrication, process hybrid and environmental applications. Curr. Opin. Electrochem. 2021, 26, 100659. [Google Scholar] [CrossRef]
- Milh, H.; Yu, X.Y.; Cabooter, D.; Dewil, R. Degradation of ciprofloxacin using UV-based advanced removal processes: Comparison of persulfate-based advanced oxidation and sulfite-based advanced reduction processes. Sci. Total Environ. 2021, 764, 144510. [Google Scholar] [CrossRef]
- Kishimoto, N.; Arai, H. Effect of Acidification on Ozone-Electrolysis Advanced Oxidation Process. Ozone-Sci. Eng. 2022, 44, 265–273. [Google Scholar] [CrossRef]
- Ding, Y.L.; Wang, C.; Yin, F.; Zhang, X.F.; Wu, G.Q.; Tong, S.P. Quantitative Investigation of the Synergistic Effect of Electrolysis Combined with Ozonation for Degradation of Nitrobenzene. Ozone-Sci. Eng. 2019, 41, 351–357. [Google Scholar] [CrossRef]
- Tang, P.; Liu, B.; Zhang, Y.; Chang, H.; Zhou, P.; Feng, M.; Sharma, V.K. Sustainable reuse of shale gas wastewater by pre-ozonation with ultrafiltration-reverse osmosis. Chem. Eng. J. 2020, 392, 123743. [Google Scholar] [CrossRef]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence excitation—Emission matrix regional integration to quantify spectra for dissolved organic matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef]
- Bilińska, L.; Blus, K.; Gmurek, M.; Ledakowicz, S. Coupling of electrocoagulation and ozone treatment for textile wastewater reuse. Chem. Eng. J. 2019, 358, 992–1001. [Google Scholar] [CrossRef]
- de Leon-Condes, C.; Barrera-Díaz, C.; Barrios, J.; Becerril, E.; Reyes-Pérez, H. A coupled ozonation–electrooxidation treatment for removal of bisphenol A, nonylphenol and triclosan from wastewater sludge. Int. J. Environ. Sci. Technol. 2016, 14, 707–716. [Google Scholar] [CrossRef]
- Furatian, L.; Mohseni, M. Inuence of major anions on the 185 nm advanced oxidation process—Sulphate, bicarbonate, and chloride. Chemosphere 2018, 201, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Saylor, G.L.; Kupferle, M.J. The impact of chloride or bromide ions on the advanced oxidation of atrazine by combined electrolysis and ozonation. J. Environ. 2019, 7, 103105. [Google Scholar] [CrossRef]
- Nemes, A.; Fábián, I.; van Eldik, R. Kinetics and Mechanism of the Carbonate Ion Inhibited Aqueous Ozone Decomposition. J. Phys. Chem. A 2000, 104, 7995–8000. [Google Scholar] [CrossRef]
- Merkus, V.I.; Leupold, M.S.; Rockel, S.P.; Lutze, H.V.; Schmidt, T.C. Effects of organic matter and alkalinity on the ozonation of antiviral purine derivatives as exemplary micropollutant motif. Water Res. 2023, 243, 120387. [Google Scholar] [CrossRef]
- Qi, H.; Lin, Q.; Chen, M.; Liao, X.; Chen, J.; Li, F.; Yuan, B. Humic acid’s (HA) role in NDMA formation from daminozide (DMNZD) during ozonation. Environ. Sci. Water Res. Technol. 2020, 6, 2766–2775. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Canonica, S.; von Gunten, U. Efficiency and energy requirements for the transformation of organic micropollutants by ozone, O3/H2O2 and UV/H2O2. Water Res. 2011, 45, 3811–3822. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.; Taurozzi, J.S.; Alpatova, A.L.; Wang, F.; Tarabara, V.V. Performance of polymeric membranes treating ozonated surface water: Effect of ozone dosage. Sep. Purif. Technol. 2011, 81, 270–278. [Google Scholar] [CrossRef]
- Cheng, C.; Huang, X.; Cheng, W.; Quan, X.; Cheng, Z.; Jiang, L.; Yang, L. Ozonation of biologically-treated municipal solid waste leachate using an integrated process of O3/Ca(OH)2 and microbubble reactor. Environ. Technol. 2019, 42, 2402–2412. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Liu, M.; Tang, T.; Du, Y.; Yao, B.; Yang, C.; Yuan, C.; Chen, Y. Insights into the efficient ozonation process focusing on 2,4-di-tert-butylphenol—A notable micropollutant of typical bamboo papermaking wastewater: Performance and mechanism. J. Hazard. Mater. 2023, 443, 130346. [Google Scholar] [CrossRef] [PubMed]
- Akbari, S.; Ghanbari, F.; Moradi, M. Bisphenol A degradation in aqueous solutions by electrogenerated ferrous ion activated ozone, hydrogen peroxide and persulfate: Applying low current density for oxidation mechanism. Chem. Eng. J. 2016, 294, 298–307. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Yuan, S.; Li, Z.; Wang, B.; Huang, J.; Deng, S.; Yu, G. Degradation of the anti-inflammatory drug ibuprofen by electro-peroxone process. Water Res. 2014, 63, 81–93. [Google Scholar] [CrossRef]

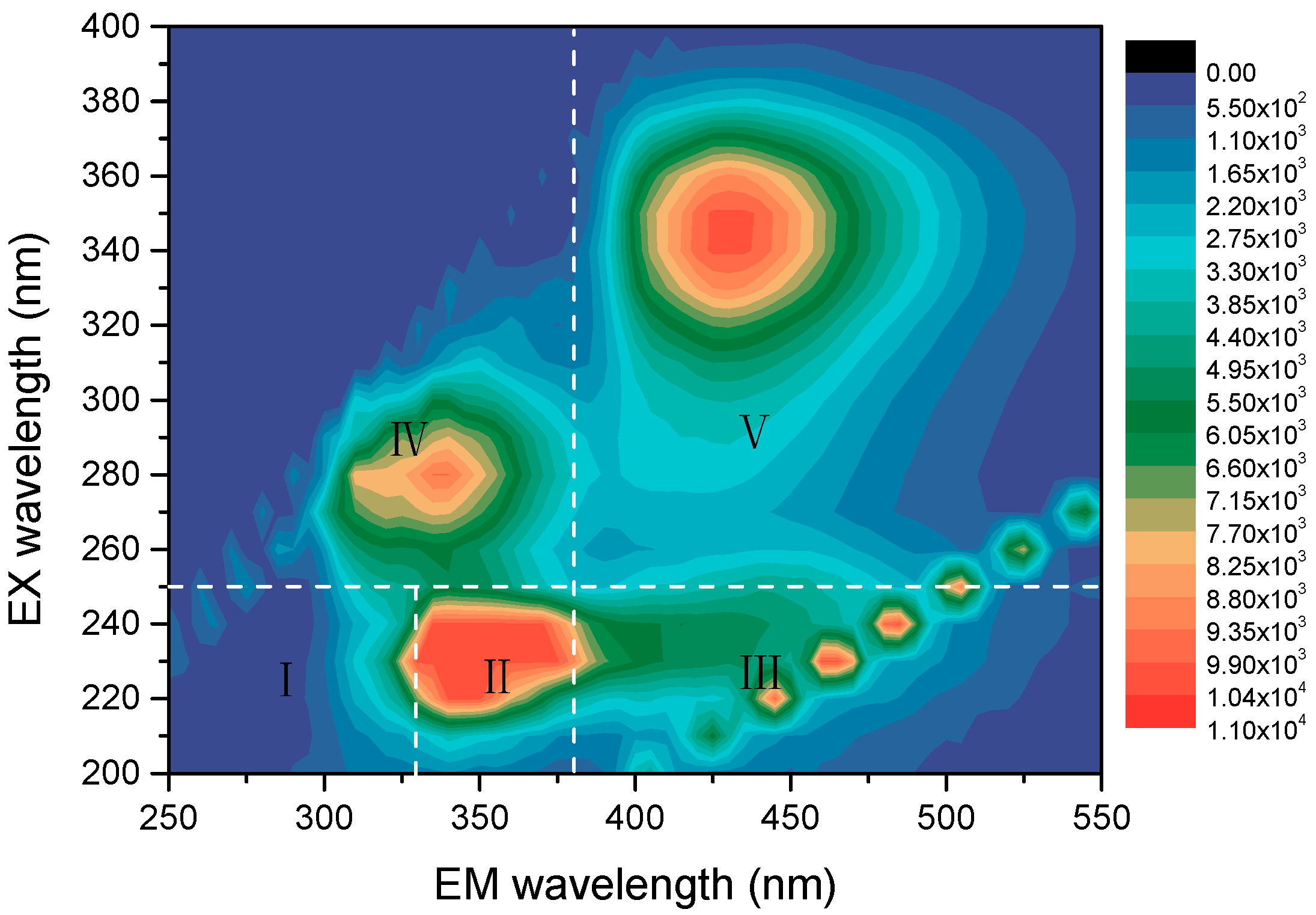
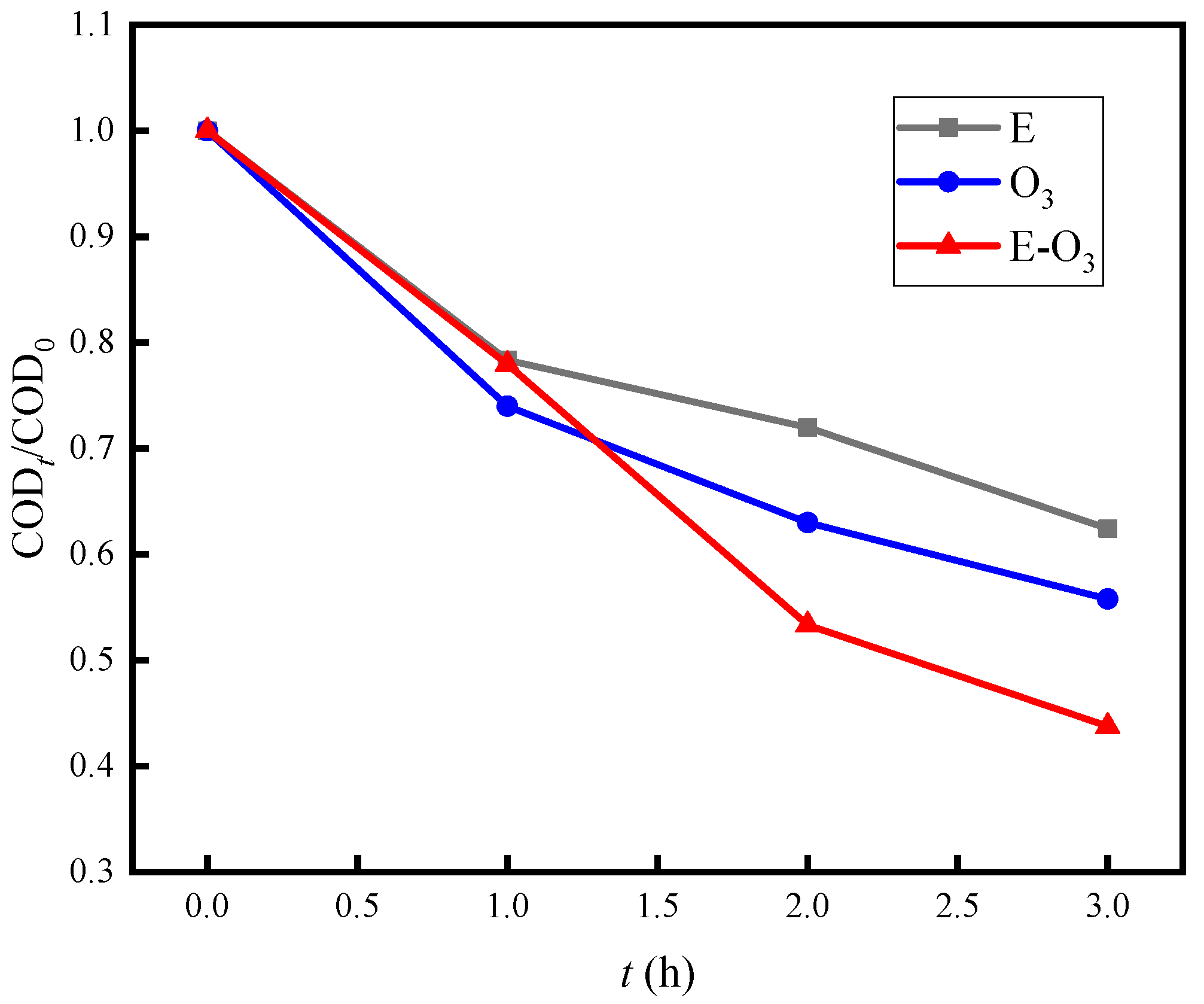
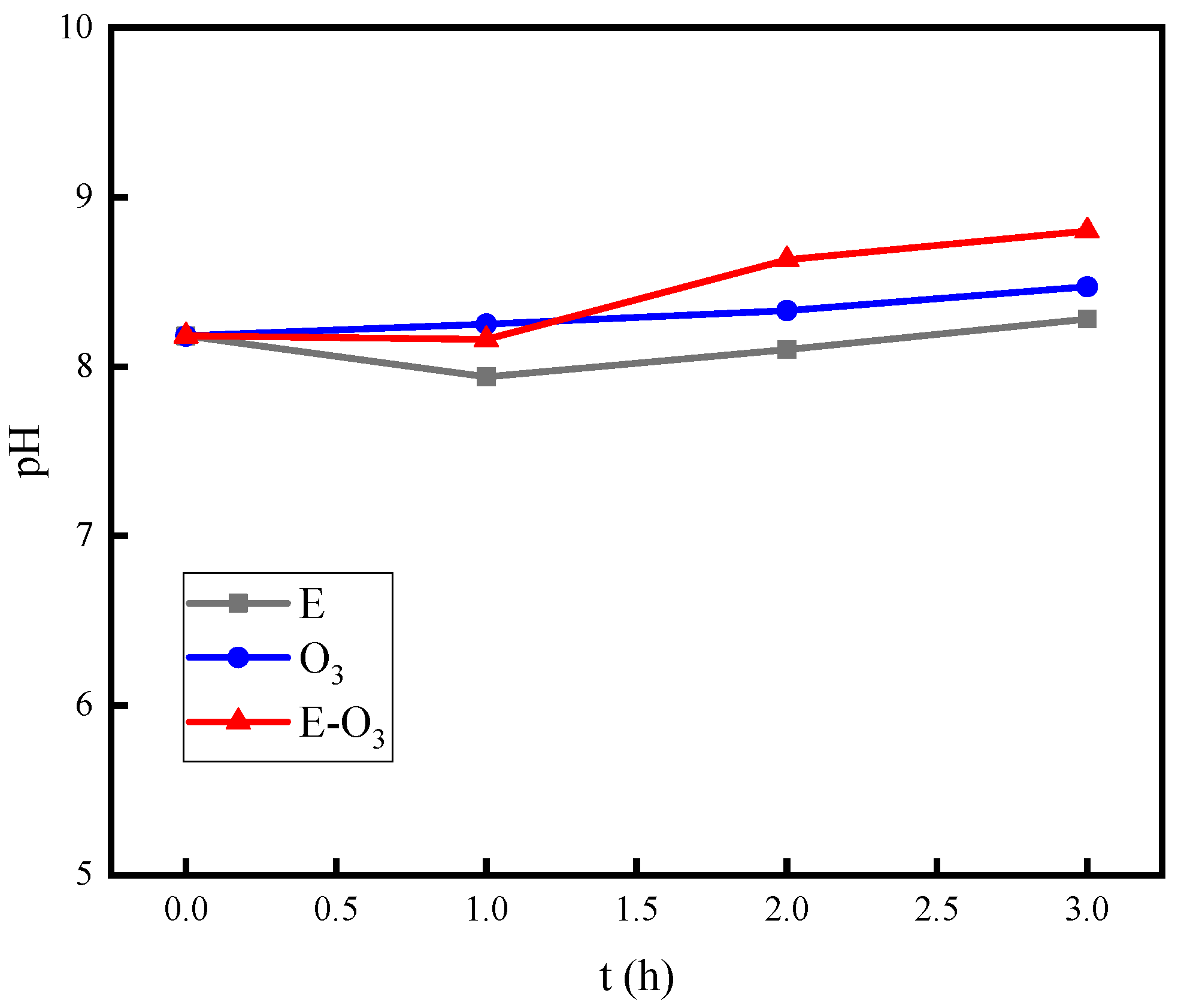
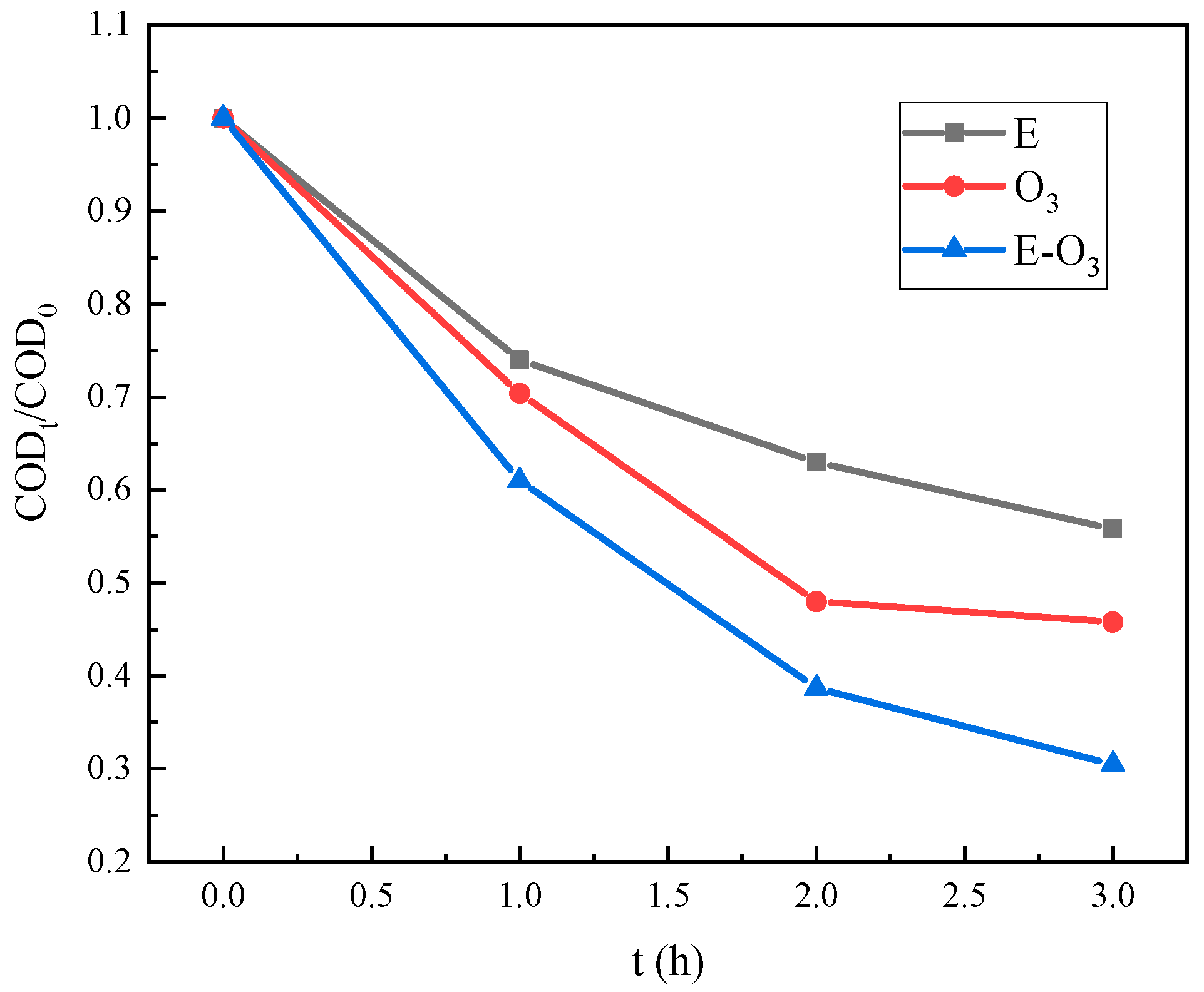
| No. | Project | Value | Unit |
|---|---|---|---|
| 1 | pH | 7.88 | - |
| 2 | CODCr | 630 | mg/L |
| 3 | TP | 1.3 | mg/L |
| 4 | Chloride ion | 3200 | mg/L |
| 5 | Sulfate ion | 3600 | mg/L |
| 6 | Fluoride ion | - | mg/L |
| 7 | Bromide ion | - | mg/L |
| 8 | Carbonate ion | 2100 | mg/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Wang, J.; Tan, B. The Removal of Organic Contaminants from Condensed Wastewater Using Electrolysis Combined with Ozonation: A Pilot-Scale Study. Separations 2024, 11, 281. https://doi.org/10.3390/separations11100281
Ding Y, Wang J, Tan B. The Removal of Organic Contaminants from Condensed Wastewater Using Electrolysis Combined with Ozonation: A Pilot-Scale Study. Separations. 2024; 11(10):281. https://doi.org/10.3390/separations11100281
Chicago/Turabian StyleDing, Yalei, Jie Wang, and Bin Tan. 2024. "The Removal of Organic Contaminants from Condensed Wastewater Using Electrolysis Combined with Ozonation: A Pilot-Scale Study" Separations 11, no. 10: 281. https://doi.org/10.3390/separations11100281





