Clinical and Radiographic Evaluation of a Novel Triangular Implant Neck Design: A Case Series
Abstract
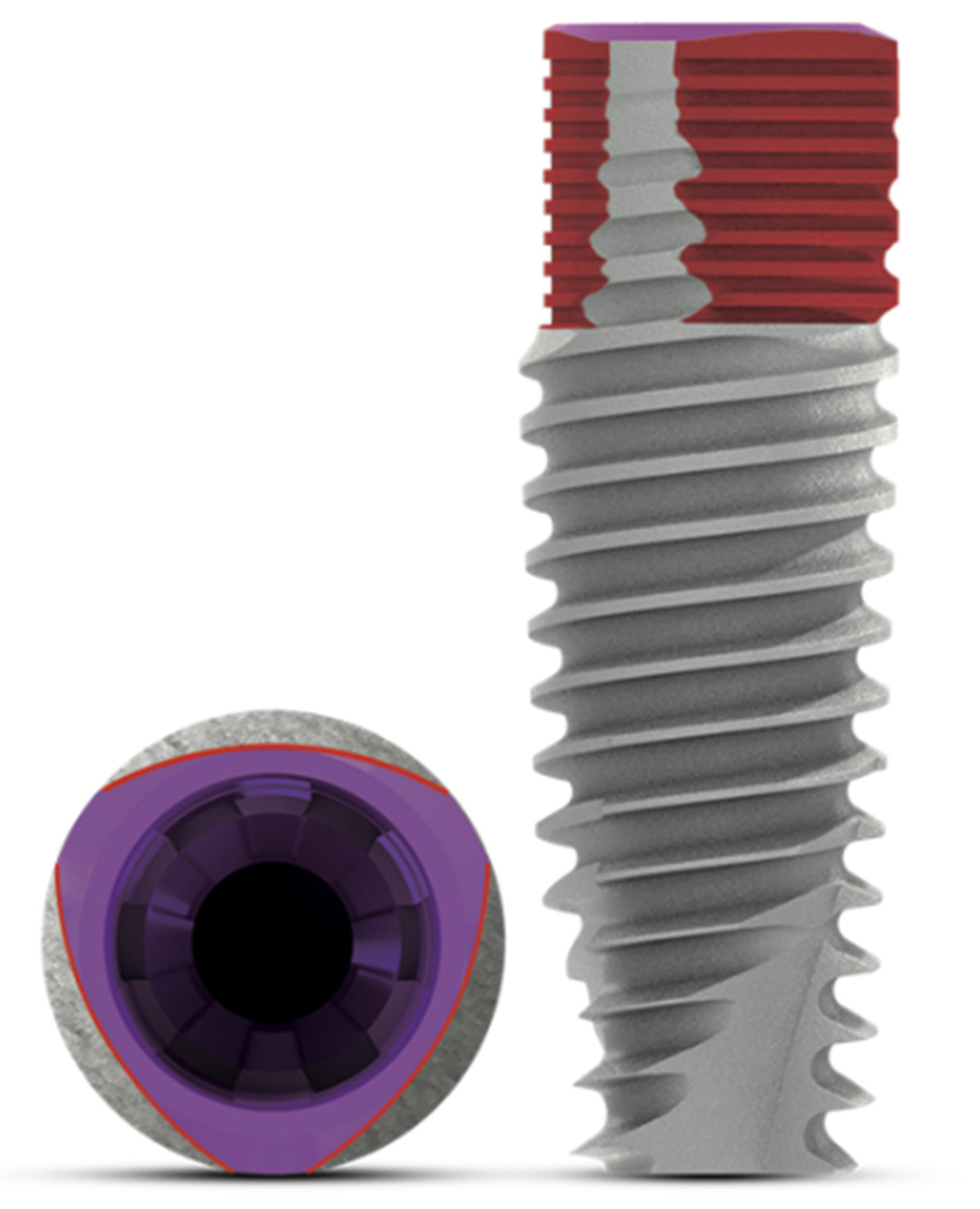
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Surgical Procedures
2.3. Prosthetic Procedures
2.4. Outcomes of the Study
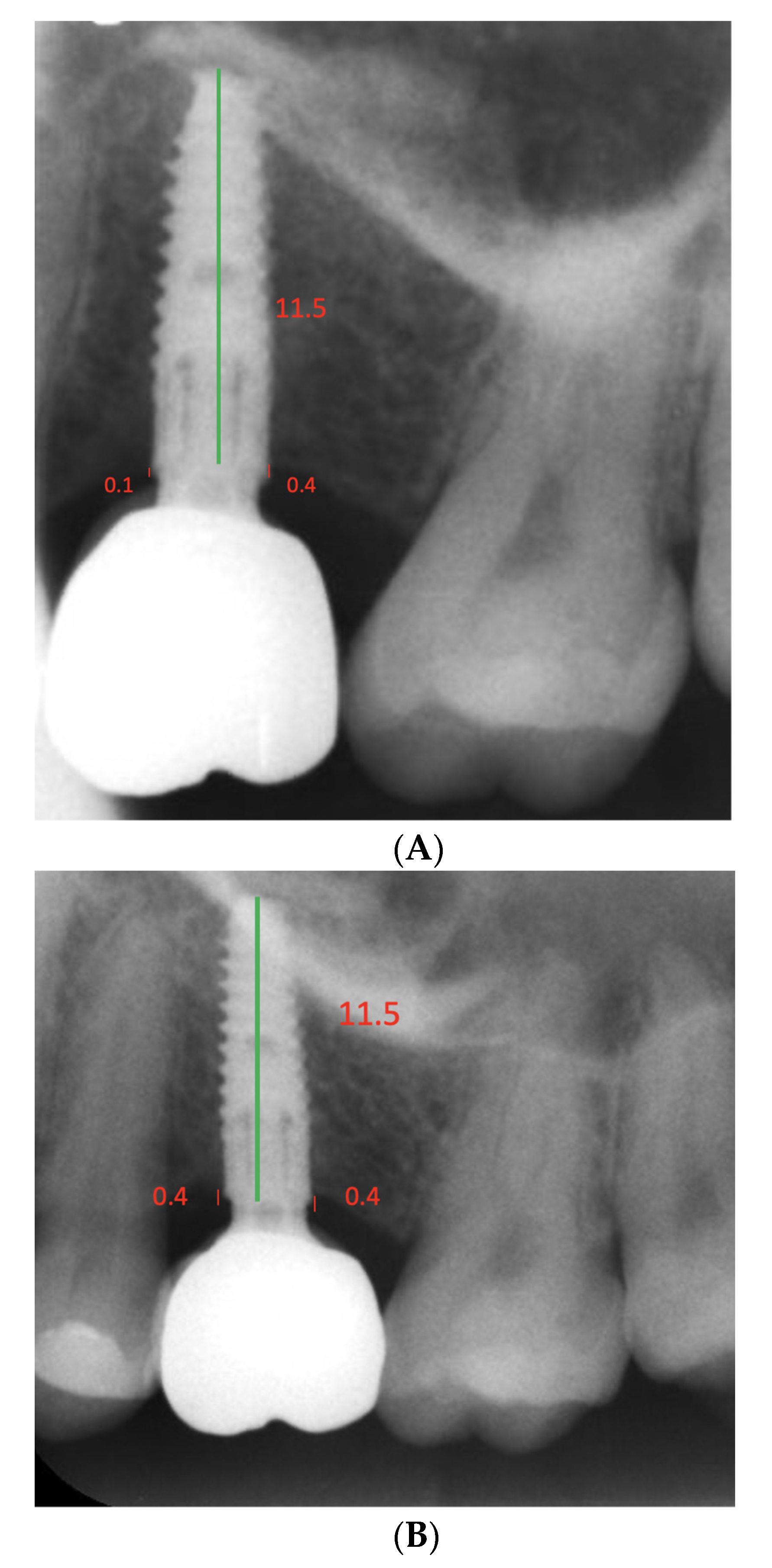
2.5. Radiographic Examinations
2.6. Clinical Measurements and Examinations
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buser, D.; Sennerby, L.; De Bruyn, H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontol 2000 2016, 73, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T. Hard tissue implant interface. Aust. Dent. J. 2008, 53, S34–S38. [Google Scholar] [CrossRef] [PubMed]
- De Bruyn, H.; Raes, S.; Matthys, C.; Cosyn, J. The current use of patient-centered/reported outcomes in implant dentistry: A systematic review. Clin. Oral Implant. Res. 2015, 26 (Suppl. 11), 45–56. [Google Scholar] [CrossRef] [PubMed]
- Vandeweghe, S.; Ferreira, D.; Vermeersch, L.; Mariën, M.; De Bruyn, H. Long-term retrospective follow-up of turned and moderately rough implants in the edentulous jaw. Clin. Oral Implant. Res. 2016, 27, 421–426. [Google Scholar] [CrossRef]
- Gallucci, G.O.; Hamilton, A.; Zhou, W.; Buser, D.; Chen, S. Implant placement and loading protocols in partially edentulous patients: A systematic review. Clin. Oral Implant. Res. 2018, 29 (Suppl. 16), 106–134. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.T.; Buser, D. Esthetic outcomes following immediate and early implant placement in the anterior maxilla--a systematic review. Int. J. Oral Maxillofac. Implant. 2014, 29, 186–215. [Google Scholar] [CrossRef] [Green Version]
- Ragucci, G.M.; Elnayef, B.; Criado-Cámara, E.; Del Amo, F.S.; Hernández-Alfaro, F. Immediate implant placement in molar extraction sockets: A systematic review and meta-analysis. Int. J. Implant. Dent. 2020, 6, 40. [Google Scholar] [CrossRef]
- Ernst, S.; Stübinger, S.; Schupbach, P.; Sidler, M.; Klein, K.; Ferguson, S.; Von Rechenberg, B. Comparison of two dental implant surface modifications on implants with same macrodesign: An experimental study in the pelvic sheep model. Clin. Oral Implant. Res. 2014, 26, 898–908. [Google Scholar] [CrossRef]
- Xuereb, M.; Camilleri, J.; Attard, N.J. Systematic review of current dental implant coating materials and novel coating techniques. Int. J. Prosthodont. 2015, 28, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Allarico, M.; Baldini, N.; Martinolli, M.; Xhanari, E.; Kim, Y.-J.; Cervino, G.; Meloni, S.M. Do the New Hydrophilic Surface Have Any Influence on Early Success Rate and Implant Stability during Osseointegration Period? Four-Month Preliminary Results from a Split-Mouth, Randomized Controlled Trial. Eur. J. Dent. 2019, 13, 095–101. [Google Scholar]
- Tallarico, M.; Baldini, N.; Gatti, F.; Martinolli, M.; Xhanari, E.; Meloni, S.M.; Gabriele, C.; Immacolata, L.A. Role of New Hydrophilic Surfaces on Early Success Rate and Implant Stability: 1-Year Post-loading Results of a Multicenter, Split-Mouth, Randomized Controlled Trial. Eur. J. Dent. 2020, 15, 001–007. [Google Scholar] [CrossRef]
- Körmöczi, K.; Komlós, G.; Papócsi, P.; Horváth, F.; Joób-Fancsaly, A. The early loading of different surface-modified implants: A randomized clinical trial. BMC Oral Health 2021, 21, 207. [Google Scholar] [CrossRef] [PubMed]
- Abuhussein, H.; Pagni, G.; Rebaudi, A.; Wang, H.-L. The effect of thread pattern upon implant osseointegration. Clin. Oral Implant. Res. 2010, 21, 129–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gracis, S.; Llobell, A.; Bichacho, N.; Jahangiri, L.; Ferencz, J. The Influence of Implant Neck Features and Abutment Diameter on Hard and Soft Tissues Around Single Implants Placed in Healed Ridges: Clinical Criteria for Selection. Int. J. Periodontics Restor. Dent. 2020, 40, 39–48. [Google Scholar] [CrossRef]
- Huang, H.-L.; Chang, C.-H.; Hsu, J.-T.; Fallgatter, A.M.; Ko, C.-C. Comparison of implant body designs and threaded designs of dental implants: A 3-dimensional finite element analysis. Int. J. Oral Maxillofac. Implant. 2007, 22, 551–562. [Google Scholar]
- Javaid, M.; Haleem, A. Current status and applications of additive manufacturing in dentistry: A literature-based review. J. Oral Biol. Craniofacial Res. 2019, 9, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. BioMed Res. Int. 2016, 2016, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Albrektsson, T.; Wennerberg, A. On osseointegration in relation to implant surfaces. Clin. Implant. Dent. Relat. Res. 2019, 21, 4–7. [Google Scholar] [CrossRef] [Green Version]
- Hsu, Y.-T.; Lin, G.-H.; Wang, H.-L. Effects of Platform-Switching on Peri-implant Soft and Hard Tissue Outcomes: A Systematic Review and Meta-analysis. Int. J. Oral Maxillofac. Implant. 2017, 32, 9–24. [Google Scholar] [CrossRef]
- Gupta, S.; Sabharwal, R.; Nazeer, J.; Taneja, L.; Choudhury, B.; Sahu, S. Platform switching technique and crestal bone loss around the dental implants: A systematic review. Ann. Afr. Med. 2019, 18, 1–6. [Google Scholar] [CrossRef]
- Macedo, J.P.; Pereira, J.; Vahey, B.R.; Henriques, B.; Benfatti, C.A.M.; Magini, R.S.; López-López, J.; Souza, J.C.M. Morse taper dental implants and platform switching: The new paradigm in oral implantology. Eur. J. Dent. 2016, 10, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Mihali, S.; Wang, H.-L.; Karancsi, O.; Bratu, E.A. Internal hexagon versus conical implant–abutment connections: Evaluation of 3-year postloading outcomes. J. Oral Implant. 2021, 47, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Caricasulo, R.; Malchiodi, L.; Ghensi, P.; Fantozzi, G.; Cucchi, A. The influence of implant-abutment connection to peri-implant bone loss: A systematic review and meta-analysis. Clin. Implant Dent. Relat. Res. 2018, 20, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, D.; Moreo, G.; Lucchese, A.; Viganoni, C.; Limongelli, L.; Carinci, F. The Impact of Implant–Abutment Connection on Clinical Outcomes and Microbial Colonization: A Narrative Review. Materials 2020, 13, 1131. [Google Scholar] [CrossRef] [Green Version]
- Romanos, G.E.; Javed, F. Platform switching minimises crestal bone loss around dental implants: Truth or myth? J. Oral Rehabil. 2014, 41, 700–708. [Google Scholar] [CrossRef]
- Pozzan, M.C.; Grande, F.; Zamperoli, E.M.; Tesini, F.; Carossa, M.; Catapano, S. Assessment of Preload Loss after Cyclic Loading in the OT Bridge System in an “All-on-Four” Rehabilitation Model in the Absence of One and Two Prosthesis Screws. Materials 2022, 15, 1582. [Google Scholar] [CrossRef]
- Sanz-Martin, I.; Vignoletti, F.; Nuñez, J.; Permuy, M.; Muñoz, F.; Sanz-Esporrín, J.; Fierravanti, L.; Shapira, L.; Sanz, M. Hard and soft tissue integration of immediate and delayed implants with a modified coronal macrodesign: Histological, micro-CT and volumetric soft tissue changes from a pre-clinical in vivo study. J. Clin. Periodontol. 2017, 44, 842–853. [Google Scholar] [CrossRef]
- Yogev, I.E.; Tandlich, M.; Shapira, L. Effect of implant neck design on primary and secondary implant stability in the posterior maxilla: A prospective randomized controlled study. Clin. Oral Implant. Res. 2019, 30, 1220–1228. [Google Scholar] [CrossRef]
- Li Manni, L.; Lecloux, G.; Rompen, E.; Aouini, W.; Shapira, L.; Lambert, F. Clinical and radiographic assessment of circular versus triangular cross-section neck Implants in the posterior maxilla: A 1-year randomized controlled trial. Clin. Oral Implant. Res. 2020, 31, 814–824. [Google Scholar] [CrossRef]
- Linkevicius, T.; Linkevicius, R.; Alkimavicius, J.; Linkeviciene, L.; Andrijauskas, P.; Puisys, A. Influence of titanium base, lithium disilicate restoration and vertical soft tissue thickness on bone stability around triangular-shaped implants: A prospective clinical trial. Clin. Oral Implant. Res. 2018, 29, 716–724. [Google Scholar] [CrossRef]
- Supplement, D.; D’Avenia, F.; Del Fabbro, M.; Karanxha, L.; Weinstein, T.; Corbella, S.; Fumagalli, D.; Francetti, L.; Taschieri, S. Hard and soft tissue changes in the rehabilitation of the anterior maxilla with triangular shape neck implants: A retrospective clinical study with a one-year follow up. J. Biol. Regul. Homeost Agents. 2019, 33, 13–21. [Google Scholar]
- Nevins, M.; Benfenati, S.; Galletti, P.; Sava, C.; Sava, C.; Trifan, M.; Muńoz, F.; Chen, C.-Y.; Kim, D. Human Histologic Evaluations of Implants with a Unique Triangular Neck Design. Int. J. Periodontics Restor. Dent. 2020, 40, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Szmukler-Moncler, S.; Trisi, P.; Benfenati, S.; Nevins, M. Osseoconduction of an Airborne Particle–Abraded and Etched Titanium Alloy Surface in Type IV Bone: A Human Histologic and Micro-CT Evaluation. Int. J. Periodontics Restor. Dent. 2022, 42, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Romanos, G.; Damouras, M.; Veis, A.A.; Hess, P.; Schwarz, F.; Brandt, S. Comparison of histomorphometry and microradiography of different implant designs to assess primary implant stability. Clin. Implant Dent. Relat. Res. 2020, 22, 373–379. [Google Scholar] [CrossRef]
- Falco, A.; Berardini, M.; Trisi, P. Correlation Between Implant Geometry, Implant Surface, Insertion Torque, and Primary Stability: In Vitro Biomechanical Analysis. Int. J. Oral Maxillofac. Implant. 2018, 33, 824–830. [Google Scholar] [CrossRef]
- Koodaryan, R.; Hafezeqoran, A. Evaluation of Implant Collar Surfaces for Marginal Bone Loss: A Systematic Review and Meta-Analysis. Biomed. Res. Int. 2016, 2016, 4987526. [Google Scholar] [CrossRef] [Green Version]
- De Bruyn, H.; Vandeweghe, S.; Ruyffelaert, C.; Cosyn, J.; Sennerby, L. Radiographic evaluation of modern oral implants with emphasis on crestal bone level and relevance to peri-implant health. Periodontol 2000 2013, 62, 256–270. [Google Scholar] [CrossRef]
- Doornewaard, R.; Christiaens, V.; De Bruyn, H.; Jacobsson, M.; Cosyn, J.; Vervaeke, S.; Jacquet, W. Long-term effect of surface roughness and patients’ factors on crestal bone loss at dental implants. A systematic review and meta-analysis. Clin. Implant. Dent. Relat. Res. 2017, 19, 372–399. [Google Scholar] [CrossRef]
- Penarrocha-Diago, M.A.; Flichy-Fernández, A.J.; Alonso-González, R.; Penarrocha-Oltra, D.; Balaguer-Martínez, J.; Peñarrocha-Diago, M. Influence of implant neck design and implant-abutment connection type on peri-implant health. Radiological study. Clin. Oral Implant. Res. 2013, 24, 1192–1200. [Google Scholar] [CrossRef]
- Lang, N.P.; Tan, W.C.; Schmidlin, K.; Pjetursson, B.E.; Zwahlen, M. The effect of different implant neck configurations on soft and hard tissue healing: A randomized-controlled clinical trial. Clin. Oral Implant. Res. 2011, 22, 14–19. [Google Scholar]
- Vivan Cardoso, M.; Vandamme, K.; Chaudhari, A.; De Rycker, J.; Van Meerbeek, B.; Naert, I.; Duyck, J. Dental Implant Macro-Design Features Can Impact the Dynamics of Osseointegration. Clin. Implant. Dent. Relat. Res. 2015, 17, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Kholy, K.E.; Ebenezer, S.; Wittneben, J.G.; Lazarin, R.; Rousson, D.; Buser, D. Influence of implant macrodesign and insertion connection technology on the accuracy of static computer-assisted implant surgery. Clin. Implant. Dent. Relat. Res. 2019, 21, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Degidi, M.; Perrotti, V.; Shibli, J.A.; Mortellaro, C.; Piattelli, A.; Iezzi, G. Evaluation of the peri-implant bone around parallel-walled dental implants with a condensing thread macrodesign and a self-tapping apex: A 10-year retrospective histological analysis. J. Craniofac. Surg. 2014, 25, 840–842. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, C.L.; Carvalho, M.A.; Bordin, D.; da Silva, W.J.; Del Bel Cury, A.A.; Sotto-Maior, B.S. Biomechanical Behavior of the Dental Implant Macrodesign. Int. J. Oral Maxillofac. Implant. 2017, 32, 264–270. [Google Scholar] [CrossRef] [Green Version]
- Montemezzi, P.; Ferrini, F.; Pantaleo, G.; Gherlone, E.; Capparè, P. Dental Implants with Different Neck Design: A Prospective Clinical Comparative Study with 2-Year Follow-Up. Materials 2020, 13, 1029. [Google Scholar] [CrossRef] [Green Version]
- Szmukler-Moncler, S.; Troiano, M.; Kotsakis, G.A. Exclusion from oral environment enables bony integration of subcrestal implant-abutment connection. Clin. Oral Implant. Res. 2021, 32, 77. [Google Scholar]
- Collins, J.R.; Berg, R.W.; Rodríguez, M.; Rodríguez, I.; Coelho, P.G.; Tovar, N. Evaluation of Human Periimplant Soft Tissues Around Nonsubmerged Machined Standard and Platform-Switched Abutments. Implant. Dent. 2015, 24, 57–61. [Google Scholar] [CrossRef]
- Collins, J.R.; Sued, M.R.; Rodríguez, I.J.; Berg, R.; Coehlo, P.G. Evaluation of human peri-implant soft tissues around alumina-blasted/acid-etched standard and platform-switched abutments. Int. J. Periodontics Restor. Dent. 2013, 33, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Atieh, M.A.; Ibrahim, H.M.; Atieh, A.H. Platform Switching for Marginal Bone Preservation Around Dental Implants: A Systematic Review and Meta-Analysis. J. Periodontol. 2010, 81, 1350–1366. [Google Scholar] [CrossRef]


| N (%) | ||||||
| Female | 12 (75) | |||||
| Male | 4 (25) | |||||
| Patient number and number of implants | ||||||
| Patient Number | Number of Implants | |||||
| 1 | 1 | |||||
| 2 | 1 | |||||
| 3 | 1 | |||||
| 4 | 2 | |||||
| 5 | 1 | |||||
| 6 | 1 | |||||
| 7 | 5 | |||||
| 8 | 2 | |||||
| 9 | 1 | |||||
| 10 | 1 | |||||
| 11 | 2 | |||||
| 12 | 1 | |||||
| 13 | 3 | |||||
| 14 | 1 | |||||
| 15 | 1 | |||||
| 16 | 1 | |||||
| Age 45.82 (13.15) years | ||||||
| Length and diameter | ||||||
| Diameter | N (%) | |||||
| 3.3 | 7 (28) | |||||
| 3.9 | 18 (72) | |||||
| Length | N (%) | |||||
| 8 | 4 (16) | |||||
| 10 | 8 (32) | |||||
| 11.5 | 6 (24) | |||||
| 13 | 6 (24) | |||||
| 16 | 1 (4) | |||||
| Patient implant details | ||||||
| Patient Number | Age | Sex | Implant Tooth | Location | Diameter Length | Follow-Up Length (Mo.) |
| 1 | 35 | F | 13 | Maxilla | 3.90 × 11.5 | 12 |
| 2 | 60 | F | 4 | Maxilla | 3.90 × 10 | 12 |
| 3 | 50 | F | 20 | Mandible | 3.90 × 11.5 | 8 |
| 4 | 57 | F | 3 | Maxilla | 3.30 × 10 | 12 |
| 4 | 57 | F | 4 | Maxilla | 3.90 × 8 | 12 |
| 5 | 38 | F | 19 | Mandible | 3.90 × 10 | 17 |
| 6 | 57 | F | 5 | Maxilla | 3.30 × 10 | 9 |
| 7 | 65 | F | 18 | Mandible | 3.90 × 8 | 24 |
| 7 | 65 | F | 17 | Mandible | 3.90 × 10 | 24 |
| 7 | 65 | F | 28 | Mandible | 3.90 × 11.5 | 13 |
| 7 | 65 | F | 29 | Mandible | 3.90 × 11.5 | 13 |
| 7 | 65 | F | 30 | Mandible | 3.90 × 10 | 13 |
| 8 | 65 | F | 27 | Mandible | 3.90 × 13 | 11 |
| 8 | 65 | F | 28 | Mandible | 3.90 × 13 | 11 |
| 9 | 33 | M | 29 | Mandible | 3.30 × 10 | 16 |
| 10 | 55 | F | 4 | Maxilla | 3.30 × 11.5 | 15 |
| 11 | 50 | F | 5 | Maxilla | 3.30 × 13 | 21 |
| 11 | 50 | F | 4 | Maxilla | 3.30 × 13 | 21 |
| 12 | 32 | M | 5 | Maxilla | 3.90 × 13 | 16 |
| 13 | 45 | F | 18 | Mandible | 3.90 × 8 | 24 |
| 13 | 45 | F | 19 | Mandible | 3.90 × 10 | 24 |
| 13 | 45 | F | 30 | Mandible | 3.90 × 8 | 24 |
| 14 | 35 | F | 28 | Mandible | 3.90 × 13 | 20 |
| 15 | 28 | M | 8 | Maxilla | 3.30 × 16 | 12 |
| 16 | 28 | M | 9 | Maxilla | 3.90 × 11.5 | 16 |
| Mean follow up 15.6 months | ||||||
| Bone Levels | Baseline Mean (SD) | Follow-Up Mean (SD) | p-Value |
|---|---|---|---|
| Mesial | 0.45 (0.47) | 0.59 (0.42) | 0.30 |
| Distal | 0.57 (0.69) | 0.78 (0.59) | 0.17 |
| Baseline Mean (SD) | Follow-Up Mean (SD) | p-Value | ||
|---|---|---|---|---|
| Maxilla (N = 11) | Mesial | 0.39 (0.31) | 0.65 (0.30) | 0.046 |
| Distal | 0.44 (0.29) | 0.78 (0.39) | 0.09 | |
| Mandible (N = 14) | Mesial | 0.5 (0.57) | 0.54 (0.50) | 0.87 |
| Distal | 0.68 (0.88) | 0.79 (0.73) | 0.65 |
| Baseline | Follow-Up | ||||
|---|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | p-Value | |
| Maxilla PPD | 11 | 1.70 (0.37) | 11 | 2.20 (0.59) | 0.006 |
| Mandible PPD | 14 | 1.46 (0.35) | 14 | 1.57 (0.60) | 0.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collins, J.R.; Ogando, B.P.; Hong, H.; Hou, W.; Romanos, G.E. Clinical and Radiographic Evaluation of a Novel Triangular Implant Neck Design: A Case Series. Dent. J. 2022, 10, 113. https://doi.org/10.3390/dj10060113
Collins JR, Ogando BP, Hong H, Hou W, Romanos GE. Clinical and Radiographic Evaluation of a Novel Triangular Implant Neck Design: A Case Series. Dentistry Journal. 2022; 10(6):113. https://doi.org/10.3390/dj10060113
Chicago/Turabian StyleCollins, James Rudolph, Brendha P. Ogando, Houlin Hong, Wei Hou, and Georgios E. Romanos. 2022. "Clinical and Radiographic Evaluation of a Novel Triangular Implant Neck Design: A Case Series" Dentistry Journal 10, no. 6: 113. https://doi.org/10.3390/dj10060113
APA StyleCollins, J. R., Ogando, B. P., Hong, H., Hou, W., & Romanos, G. E. (2022). Clinical and Radiographic Evaluation of a Novel Triangular Implant Neck Design: A Case Series. Dentistry Journal, 10(6), 113. https://doi.org/10.3390/dj10060113







