Viability and Adhesion of Periodontal Ligament Fibroblasts on a Hydroxyapatite Scaffold Combined with Collagen, Polylactic Acid–Polyglycolic Acid Copolymer and Platelet-Rich Fibrin: A Preclinical Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
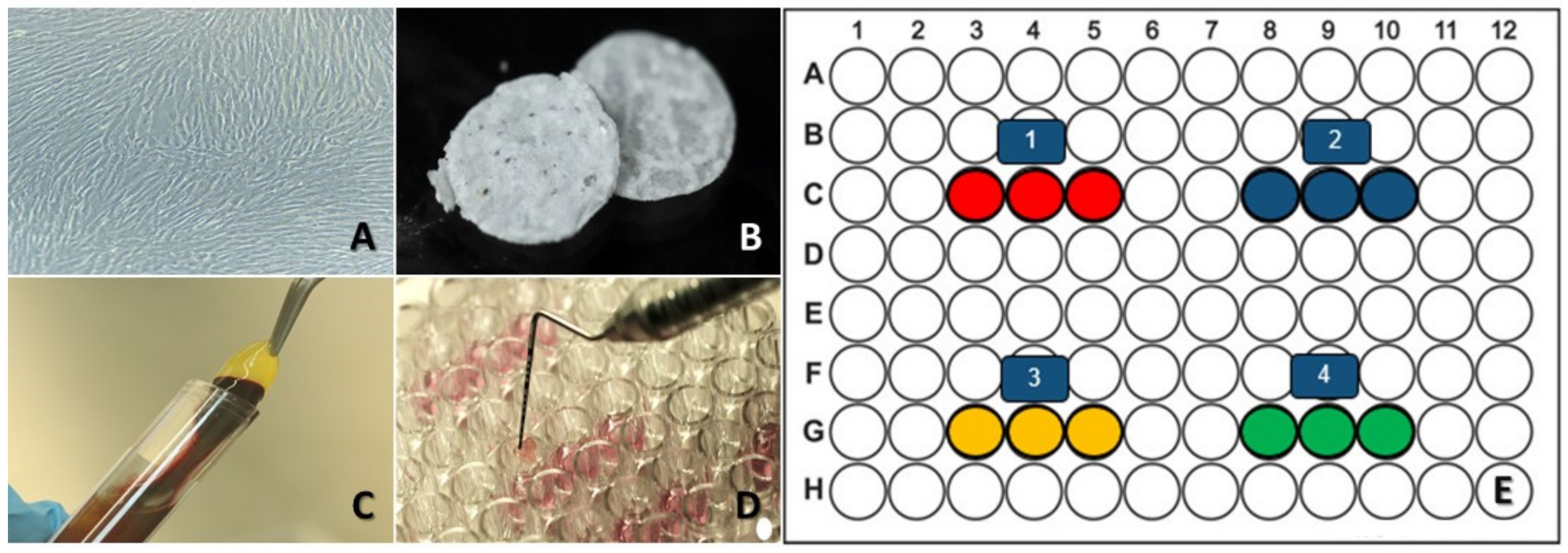
2.1. Cell Culture
2.2. HAp-Egg Shell/PLGA Scaffold Contribution
2.3. Platelet-Rich Fibrin (PRF)
2.4. Collagen
2.5. Experimental Design
2.6. Viability and Cell Adhesion Analysis
2.7. Results Analysis
2.8. Data Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.L.; Greenwell, H.; Fiorellini, J.; Giannobile, W.; Offenbacher, S.; Salkin, L.; Townsend, C.; Sheridan, P.; Genco, R.J. Periodontal regeneration. J Periodontol 2005, 76, 1601–1622. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Decker, A.M.; Nibali, L.; Pilipchuk, S.P.; Berglundh, T.; Giannobile, W.V. Regenerative Medicine for Periodontal and Peri-implant Diseases. J. Dent. Res. 2016, 95, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Ibáñez, R.; Alvarado-Estrada, K.N.; Ojeda-Gutiérrez, F. Tissue Engineering in Dentistry. Rev. ADM 2012, 69, 164–167. [Google Scholar]
- Mishra, M.; Mishra, P.; Shambharkar, V.; Raut, A. Scaffolds in Periodontal Regeneration. J. Pharm. Biomed. Sc. 2016, 6, 10–17. [Google Scholar]
- Parra, M.; Haidar, Z.S.; Olate, S. Use of PRF in Combination with Synthetic Filling Materials (HA and ß-TCP) for Bone Reconstructions. Avances en Odontoestomatologí-a 2018, 34, 79–86. [Google Scholar]
- Camacho-Díaz, I.; Vela-Rodríguez, M.; Villanueva-Aburto, L.; Borja-Villanueva, A.; Montalvo-Amanca, F.; Quispe-Marcatoma, J. Hydroxyapatite synthesized from the eggshell as a potential bone substitute in periodontal and peri-implant defects. Odontol. Sanmarquina 2018, 21, 296–301. [Google Scholar] [CrossRef]
- Pan, Z.; Ding, J. Poly(lactide-co-glycolide) porous scaffolds for tissue engineering and regenerative medicine. Interface Focus 2012, 2, 366–377. [Google Scholar] [CrossRef]
- Miron, R.J.; Zucchelli, G.; Pikos, M.A.; Salama, M.; Lee, S.; Guillemette, V.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Wang, H.L.; et al. Use of platelet-rich fibrin in regenerative dentistry: A systematic review. Clin. Oral Investig. 2017, 21, 1913–1927. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.; Guo, J.; Yang, L.; Liu, Y.; Sun, Q.; Li, R.; Yu, W. Hybrid composites of mesenchymal stem cell sheets, hydroxyapatite, and platelet-rich fibrin granules for bone regeneration in a rabbit calvarial critical-size defect model. Exp. Ther. Med. 2017, 13, 1891–1899. [Google Scholar] [CrossRef]
- Kapoor, A.; Ali, A.R.; Saini, N.; Gautam, K.; Goyal, A.; Prakash, V. Comparative evaluation of implant stability with and without autologous platelet-rich fibrin prior to prosthetic loading—A split-mouth randomized clinical trial. J. Indian Soc. Periodontol. 2022, 26, 137–142. [Google Scholar] [CrossRef]
- Orozco, A.V.; Gómez, C.; Ninin, J.L.; Celis, M. Effectiveness of platelet concentrates for bone regeneration in oral surgery and periodontal treatments. A systematic review. Rev. Venez. Invest. Odont. IADR 2016, 4, 253–272. [Google Scholar]
- Cid-Cisternas, F.A. Efficacy of Platelet Rich Plasma and Platelet Rich Fibrin in Periodontal Regeneration: Systematic Review. Int. J. Med. Surg. Sci. 2017, 4, 1196–1202. [Google Scholar]
- Acosta-Gómez, A.P.; García-Robayo, D.A.; Perdomo-Lara, S.J.; Gónzalez, O.A.; Velosa-Porras, J.; Ouellette, J.; Pereira-Ebratr, R. Effect of platelet-rich plasma on human gingival fibroblasts proliferation. Univ. Odontol. 2010, 15, 4–13. [Google Scholar]
- Al-Ahmady, H.H.; Abd Elazeem, A.F.; Bellah Ahmed, N.E.; Shawkat, W.M.; Elmasry, M.; Abdelrahman, M.A.; Abderazik, M.A. Combining autologous bone marrow mononuclear cells seeded on collagen sponge with Nano Hydroxyapatite, and platelet-rich fibrin: Reporting a novel strategy for alveolar cleft bone regeneration. J. Craniomaxillofac. Surg. 2018, 46, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Zhang, S.; Wu, P.; Liu, Y.; Su, Y. Electrospinning With Lyophilized Platelet-Rich Fibrin Has the Potential to Enhance the Proliferation and Osteogenesis of MC3T3-E1 Cells. Front. Bioeng. Biotechnol. 2020, 8, 595579. [Google Scholar] [CrossRef]
- Sequeda, L.G.; Díaz, J.M.; Gutiérrez, S.J.; Perdomo, S.J.; Gómez, O.L. Obtaining synthetic hydroxyapatite by three different methods and its characterization to be used as a bone substitute. Rev. Colomb. Cienc. Quím. Farm. 2012, 41, 50–66. [Google Scholar]
- Gutiérrez-Prieto, S.J.; Fonseca, L.F.; Sequeda-Castañeda, L.G.; Díaz, K.J.; Castañeda, L.Y.; Leyva-Rojas, J.A.; Salcedo-Reyes, J.C.; Acosta, A.P. Elaboration and Biocompatibility of an Eggshell-Derived Hydroxyapatite Material Modified with Si/PLGA for Bone Regeneration in Dentistry. Int. J. Dent. 2019, 2019, 5949232. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Prieto, S.J.; Perdomo-Lara, S.J.; Diaz-Peraza, J.M.; Sequeda-Castañeda, L.G. Analysis of In Vitro Osteoblast Culture on Scaffolds for Future Bone Regeneration Purposes in Dentistry. Adv. Pharmacol. Sci. 2019, 2019, 5420752. [Google Scholar] [CrossRef]
- Acosta, A.P.; García, D.; Gutiérrez, S.J.; Sequeda, L.G. Biocompatibility, Bioengineering and Biologic Effects of Materials. In Proceedings of the Novel Egg-Shell Hydroxyapatite and Platelet-Rich Fibrin Biomaterial for Tissue Engineering, IADR/PER General Session, London, UK, 28 July 2018; p. 2. [Google Scholar]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef]
- Hu, K.; Cui, F.; Lv, Q.; Ma, J.; Feng, Q.; Xu, L.; Fan, D. Preparation of fibroin/recombinant human-like collagen scaffold to promote fibroblasts compatibility. J. Biomed. Mater. Res. A 2008, 84, 483–490. [Google Scholar] [CrossRef]
- Jin, S.; Sun, F.; Zou, Q.; Huang, J.; Zuo, Y.; Li, Y.; Wang, S.; Cheng, L.; Man, Y.; Yang, F.; et al. Fish Collagen and Hydroxyapatite Reinforced Poly(lactide-co-glycolide) Fibrous Membrane for Guided Bone Regeneration. Biomacromolecules 2019, 20, 2058–2067. [Google Scholar] [CrossRef]
- Chang, Y.C.; Zhao, J.H. Effects of platelet-rich fibrin on human periodontal ligament fibroblasts and application for periodontal infrabony defects. Aust. Dent. J. 2011, 56, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yin, X.; Ye, Q.; He, W.; Ge, M.; Zhou, X.; Hu, J.; Zou, S. Periodontal regeneration with stem cells-seeded collagen-hydroxyapatite scaffold. J. Biomater. Appl. 2016, 31, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H. Platelet Rich Fibrin and Nanocrystalline Hydroxyapatite with Collagen Combination in Treatment of Periapical Lesion: A Novel Clinical Approach. Br. J. Med. Med. Res. 2015, 5, 275–282. [Google Scholar] [CrossRef]
- Bacakova, L.; Novotna, K.; Hadraba, D.; Musilkova, J.; Slepicka, P.; Beran, M. Influence of Biomimetically Mineralized Collagen Scaffolds on Bone Cell Proliferation and Immune Activation. Polymers 2022, 14, 602. [Google Scholar] [CrossRef] [PubMed]
- Kanemoto, Y.; Miyaji, H.; Nishida, E.; Miyata, S.; Mayumi, K.; Yoshino, Y.; Kato, A.; Sugaya, T.; Akasaka, T.; Nathanael, A.J.; et al. Periodontal tissue engineering using an apatite/collagen scaffold obtained by a plasma- and precursor-assisted biomimetic process. J. Periodontal. Res. 2022, 57, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Parisi, J.R.; Fernandes, K.R.; de Almeida Cruz, M.; Avanzi, I.R.; de França Santana, A.; do Vale, G.C.A.; de Andrade, A.L.M.; de Góes, C.P.; Fortulan, C.A.; de Sousa Trichês, E.; et al. Evaluation of the In Vivo Biological Effects of Marine Collagen and Hydroxyapatite Composite in a Tibial Bone Defect Model in Rats. Mar. Biotechnol. 2020, 22, 357–366. [Google Scholar] [CrossRef]
- Zhong, Z.; Wu, X.; Wang, Y.; Li, M.; Li, Y.; Liu, X.; Zhang, X.; Lan, Z.; Wang, J.; Du, Y.; et al. Zn/Sr dual ions-collagen co-assembly hydroxyapatite enhances bone regeneration through procedural osteo-immunomodulation and osteogenesis. Bioact. Mater. 2022, 10, 195–206. [Google Scholar] [CrossRef]
- Pietruszka, P.; Chruścicka, I.; Duś-Ilnicka, I.; Paradowska-Stolarz, A. PRP and PRF-Subgroups and Divisions When Used in Dentistry. J. Pers. Med. 2021, 11, 944. [Google Scholar] [CrossRef]
- Patino, M.G.; Neiders, M.E.; Andreana, S.; Noble, B.; Cohen, R.E. Collagen as an Implantable Material in Medicine and Dentistry. J. Oral Implantol. 2002, 28, 220–225. [Google Scholar] [CrossRef]
- Lesaffre, E.; Feine, J.; Leroux, B.; Declerck, D. Statistical and Methodological Aspects of Oral Health Research; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Meena, A.; Bisht, D.; Yadav, R.; Saini, S.; Dangayach, G.; Patnaik, A.; Meena, M.L. Fabrication and characterization of micro alumina zirconia particulate filled dental restorative composite materials. Polym. Compos. 2021, 43, 1526–1535. [Google Scholar] [CrossRef]
- Yadav, R. Fabrication, characterization, and optimization selection of ceramic particulate reinforced dental restorative composite materials. Polym. Polym. Compos. 2022, 30, 09673911211062755. [Google Scholar] [CrossRef]
- Lardani, L.; Derchi, G.; Marchio, V.; Carli, E. One-Year Clinical Performance of Activa™ Bioactive-Restorative Composite in Primary Molars. Children 2022, 9, 433. [Google Scholar] [CrossRef] [PubMed]
- Abou Neel, E.A.; Pickup, D.M.; Valappil, S.P.; Newport, R.J.; Knowles, J.C. Bioactive functional materials: A perspective on phosphate-based glasses. J. Mater. Chem. 2009, 19, 690–701. [Google Scholar] [CrossRef]
- Swetha, M.; Sahithi, K.; Moorthi, A.; Srinivasan, N.; Ramasamy, K.; Selvamurugan, N. Biocomposites containing natural polymers and hydroxyapatite for bone tissue engineering. Int. J. Biol. Macromol. 2010, 47, 1–4. [Google Scholar] [CrossRef]
- Fragoso-Angeles, S.; Vera-Graziano, R.; Pérez-González, G.L.; Iglesias, A.L.; Gómez-Pineda, L.E.; Villarreal-Gómez, L.J. Synthesis and Characterization of Synthetic Hydroxyapatite for the Preparation of PLGA/HAp Films with a Potential Use in Biomedical Applications. ReCIBE—Rev. Electrónica Comput. Inf. Biomédica Electrónica 2018, 7, 93–116. [Google Scholar]
- Huang, S.; He, P.; Xu, D.; Li, J.; Peng, X.; Tang, Y. Acidic stress induces apoptosis and inhibits angiogenesis in human bone marrow-derived endothelial progenitor cells. Oncol. Lett. 2017, 14, 5695–5702. [Google Scholar] [CrossRef]
- Gantenbein-Ritter, B.; Potier, E.; Zeiter, S.; van der Werf, M.; Sprecher, C.M.; Ito, K. Accuracy of three techniques to determine cell viability in 3D tissues or scaffolds. Tissue Eng. Part C Methods 2008, 14, 353–358. [Google Scholar] [CrossRef]
- Soltan, N.; Ning, L.; Mohabatpour, F.; Papagerakis, P.; Chen, X. Printability and Cell Viability in Bioprinting Alginate Dialdehyde-Gelatin Scaffolds. ACS Biomater. Sci. Eng. 2019, 5, 2976–2987. [Google Scholar] [CrossRef]
- Uzarski, J.S.; DiVito, M.D.; Wertheim, J.A.; Miller, W.M. Essential design considerations for the resazurin reduction assay to noninvasively quantify cell expansion within perfused extracellular matrix scaffolds. Biomaterials 2017, 129, 163–175. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, N.; Xue, Y.; Ding, T.; Liu, X.; Mo, X.; Sun, J. Development of biomimetic tilapia collagen nanofibers for skin regeneration through inducing keratinocytes differentiation and collagen synthesis of dermal fibroblasts. ACS Appl. Mater. Interfaces 2015, 7, 3253–3262. [Google Scholar] [CrossRef]
- Sun, Y.; Han, F.; Zhang, P.; Zhi, Y.; Yang, J.; Yao, X.; Wang, H.; Lin, C.; Wen, X.; Chen, J.; et al. A synthetic bridging patch of modified co-electrospun dual nano-scaffolds for massive rotator cuff tear. J. Mater. Chem. B 2016, 4, 7259–7269. [Google Scholar] [CrossRef]
- Gómez, A.P.A.; Molina, N.S.R. Platelet concentrates and their application in tissue engineering: From platelet-rich plasma to fibrin-rich plasma. In Experiences and Results of Research in Dentistry: Contributions to the Knowledge of the Centro de Investigaciones Odontológicas de la Pontificia Universidad Javeriana; Editorial Pontificia Universidad Javeriana: Bogotá, Colombia, 2018; pp. 143–182. [Google Scholar]
- Madi, M.; Elakel, A.M. The clinical implications of platelet-rich fibrin on periodontal regeneration: A systematic review. Saudi Dent. J. 2021, 33, 55–62. [Google Scholar] [CrossRef]
- Paolantonio, M.; Di Tullio, M.; Giraudi, M.; Romano, L.; Secondi, L.; Paolantonio, G.; Graziani, F.; Pilloni, A.; De Ninis, P.; Femminella, B. Periodontal regeneration by leukocyte and platelet-rich fibrin with autogenous bone graft versus enamel matrix derivative with autogenous bone graft in the treatment of periodontal intrabony defects: A randomized non-inferiority trial. J. Periodontol. 2020, 91, 1595–1608. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; de Peppo, G.M.; Doglioli, P.; Sammartino, G. Slow release of growth factors and thrombospondin-1 in Choukroun’s platelet-rich fibrin (PRF): A gold standard to achieve for all surgical platelet concentrates technologies. Growth Factors 2009, 27, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.H.; Zhang, M.; Liu, N.X.; Lv, X.; Zhang, J.; Chen, F.M.; Chen, Y.J. The combined use of cell sheet fragments of periodontal ligament stem cells and platelet-rich fibrin granules for avulsed tooth reimplantation. Biomaterials 2013, 34, 5506–5520. [Google Scholar] [CrossRef] [PubMed]
- Ghanaati, S.; Al-Maawi, S.; Conrad, T.; Lorenz, J.; Rössler, R.; Sader, R. Biomaterial-based bone regeneration and soft tissue management of the individualized 3D-titanium mesh: An alternative concept to autologous transplantation and flap mobilization. J. Craniomaxillofac. Surg. 2019, 47, 1633–1644. [Google Scholar] [CrossRef]
- Gülşen, U.; Dereci, Ö. Evaluation of New Bone Formation in Sinus Floor Augmentation With Injectable Platelet-Rich Fibrin-Soaked Collagen Plug: A Pilot Study. Implant. Dent. 2019, 28, 220–225. [Google Scholar] [CrossRef]
- Lin, Z.; Nica, C.; Sculean, A.; Asparuhova, M.B. Enhanced Wound Healing Potential of Primary Human Oral Fibroblasts and Periodontal Ligament Cells Cultured on Four Different Porcine-Derived Collagen Matrices. Materials 2020, 13, 3819. [Google Scholar] [CrossRef] [PubMed]
- Zeltz, C.; Gullberg, D. The integrin-collagen connection—A glue for tissue repair? J. Cell Sci. 2016, 129, 653–664. [Google Scholar] [CrossRef]
- Kozlowska, J.; Jundzill, A.; Bajek, A.; Bodnar, M.; Marszalek, A.; Witmanowski, H.; Sionkowska, A. Preliminary in vitro and in vivo assessment of modified collagen/hydroxyapatite composite. Mater. Lett. 2018, 221, 74–76. [Google Scholar] [CrossRef]

| Scale | Area | Percentage |
|---|---|---|
| Null | 0 µm2 | 0% |
| Low | 1 µm2–46,800 µm2 | 1–25% |
| Moderate | 46,801µm2–93,600 µm2 | 26–50% |
| High | 93,601 µm2–140,400 µm2 | 51–75% |
| Very high | 140,401 µm2–187,200 µm2 | 76–100% |
| Group | Adhesion | Viability | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | Average | p-Value | 1 | 2 | 3 | Average | p-Value | |
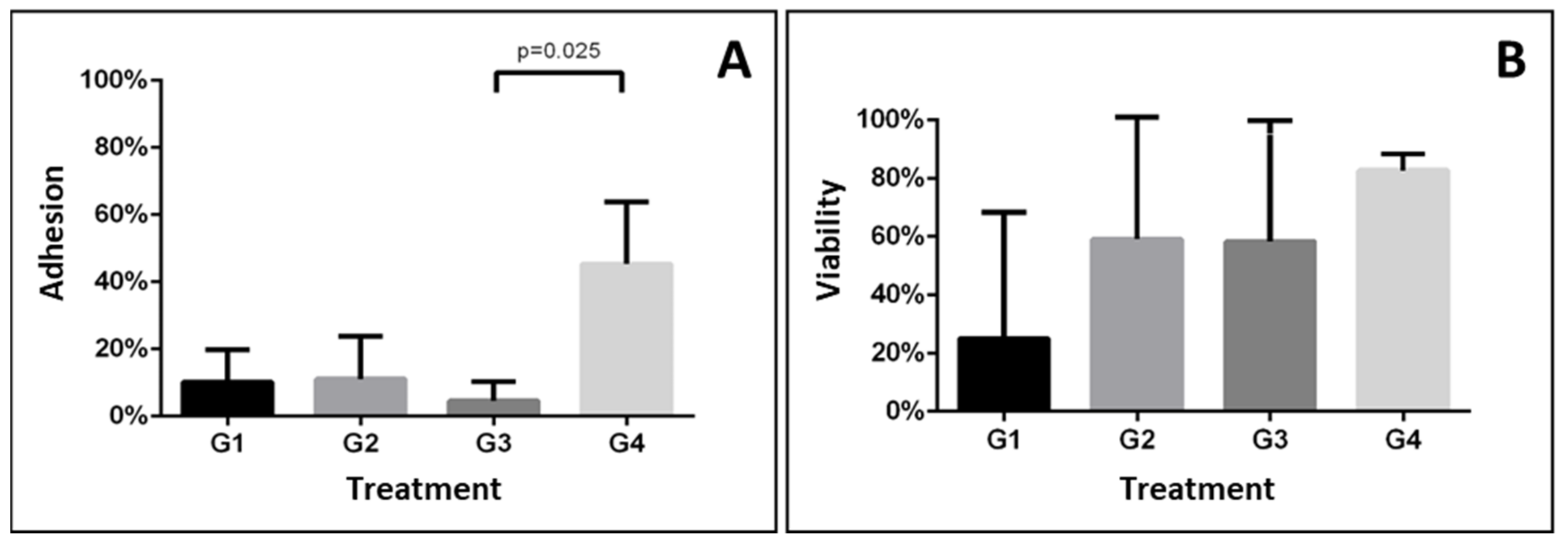
| 1 | 0.0 | 11.1 | 19.4 | 10.2 ± 9.7 | 0.015 | 0.0 | 75.0 | 0.0 | 25.0 ± 43.3 | 0.474 |
| 2 | 0.0 | 25.0 | 8.3 | 11.1 ± 12.7 | 0.0 | 77.7 | 100.0 | 59.2 ± 52.5 | ||
| 3 | 0.0 | 2.7 | 11.1 | 4.6 ± 5.8 a | 0.0 | 100.0 | 75.0 | 58.3 ± 52.0 | ||
| 4 | 25.0 | 50.0 | 61.1 | 45.4 ± 18.5 b | 88.8 | 77.7 | 81.8 | 82.7 ± 5.6 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espitia-Quiroz, L.C.; Fernández-Orjuela, A.L.; Anaya-Sampayo, L.M.; Acosta-Gómez, A.P.; Sequeda-Castañeda, L.G.; Gutiérrez-Prieto, S.J.; Roa-Molina, N.S.; García-Robayo, D.A. Viability and Adhesion of Periodontal Ligament Fibroblasts on a Hydroxyapatite Scaffold Combined with Collagen, Polylactic Acid–Polyglycolic Acid Copolymer and Platelet-Rich Fibrin: A Preclinical Pilot Study. Dent. J. 2022, 10, 167. https://doi.org/10.3390/dj10090167
Espitia-Quiroz LC, Fernández-Orjuela AL, Anaya-Sampayo LM, Acosta-Gómez AP, Sequeda-Castañeda LG, Gutiérrez-Prieto SJ, Roa-Molina NS, García-Robayo DA. Viability and Adhesion of Periodontal Ligament Fibroblasts on a Hydroxyapatite Scaffold Combined with Collagen, Polylactic Acid–Polyglycolic Acid Copolymer and Platelet-Rich Fibrin: A Preclinical Pilot Study. Dentistry Journal. 2022; 10(9):167. https://doi.org/10.3390/dj10090167
Chicago/Turabian StyleEspitia-Quiroz, Leonor C., Andrés L. Fernández-Orjuela, Lina M. Anaya-Sampayo, Adriana P. Acosta-Gómez, Luis Gonzalo Sequeda-Castañeda, Sandra Janeth Gutiérrez-Prieto, Nelly S. Roa-Molina, and Dabeiba A. García-Robayo. 2022. "Viability and Adhesion of Periodontal Ligament Fibroblasts on a Hydroxyapatite Scaffold Combined with Collagen, Polylactic Acid–Polyglycolic Acid Copolymer and Platelet-Rich Fibrin: A Preclinical Pilot Study" Dentistry Journal 10, no. 9: 167. https://doi.org/10.3390/dj10090167
APA StyleEspitia-Quiroz, L. C., Fernández-Orjuela, A. L., Anaya-Sampayo, L. M., Acosta-Gómez, A. P., Sequeda-Castañeda, L. G., Gutiérrez-Prieto, S. J., Roa-Molina, N. S., & García-Robayo, D. A. (2022). Viability and Adhesion of Periodontal Ligament Fibroblasts on a Hydroxyapatite Scaffold Combined with Collagen, Polylactic Acid–Polyglycolic Acid Copolymer and Platelet-Rich Fibrin: A Preclinical Pilot Study. Dentistry Journal, 10(9), 167. https://doi.org/10.3390/dj10090167








