Immunomodulatory Effects of Endodontic Sealers: A Systematic Review
Abstract
:1. Introduction
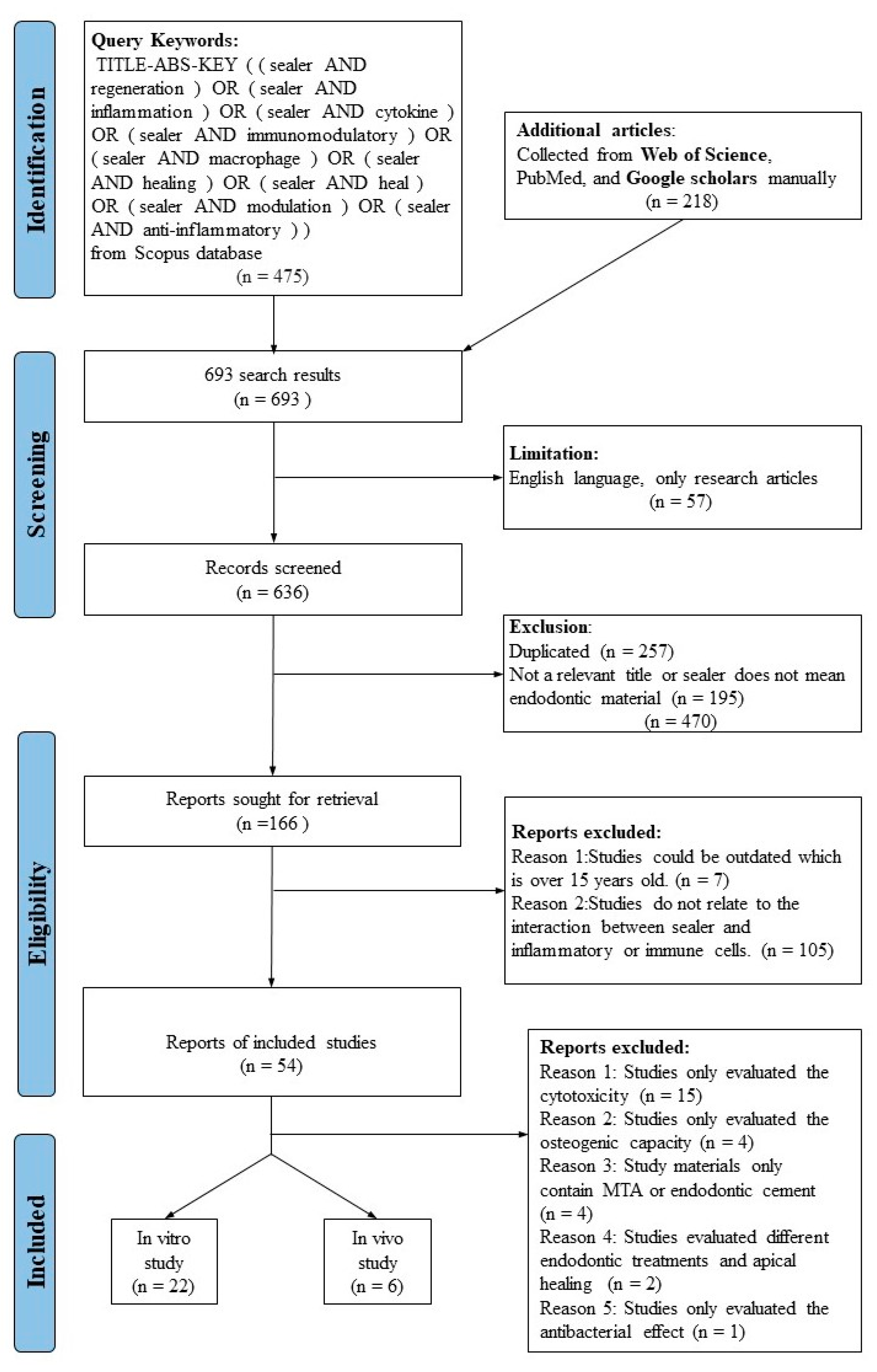
2. Materials and Methods
2.1. Study Selection
- (1)
- Studies must relate to “immunomodulatory effect of sealer” or “pro-inflammatory effect of sealer” or “anti-inflammatory effect of sealer” or “tissue regenerative ability”.
- (2)
- Studies must use proper and quantitative methods, such RT-PCR and ELISA to investigate the potential immunomodulatory effects of sealers.
- (3)
- The main results of studies must relate to at least one of these keywords: “macrophages”, “cytokines”, “immune cells”, “inflammatory cells”, “immunomodulation”, and “inflammation” or “anti-inflammation”.
- (4)
- Studies published in press within the last 15 years were included to obtain the most recent evidence.
- (5)
- Studies do not investigate the interaction between sealers and immune cells in the inflammation process or tissue repairment process.
2.2. Search Strategy and Information Sources
2.3. Selection Procedure
2.4. Data Items
2.5. Risk of Bias Assessment
3. Results
3.1. In Vitro Studies
3.2. In Vivo Studies
3.3. In Vitro Studies
4. Discussion
4.1. Inflammatory Pathway and Signaling Mechanisms Related to Endodontic Sealers
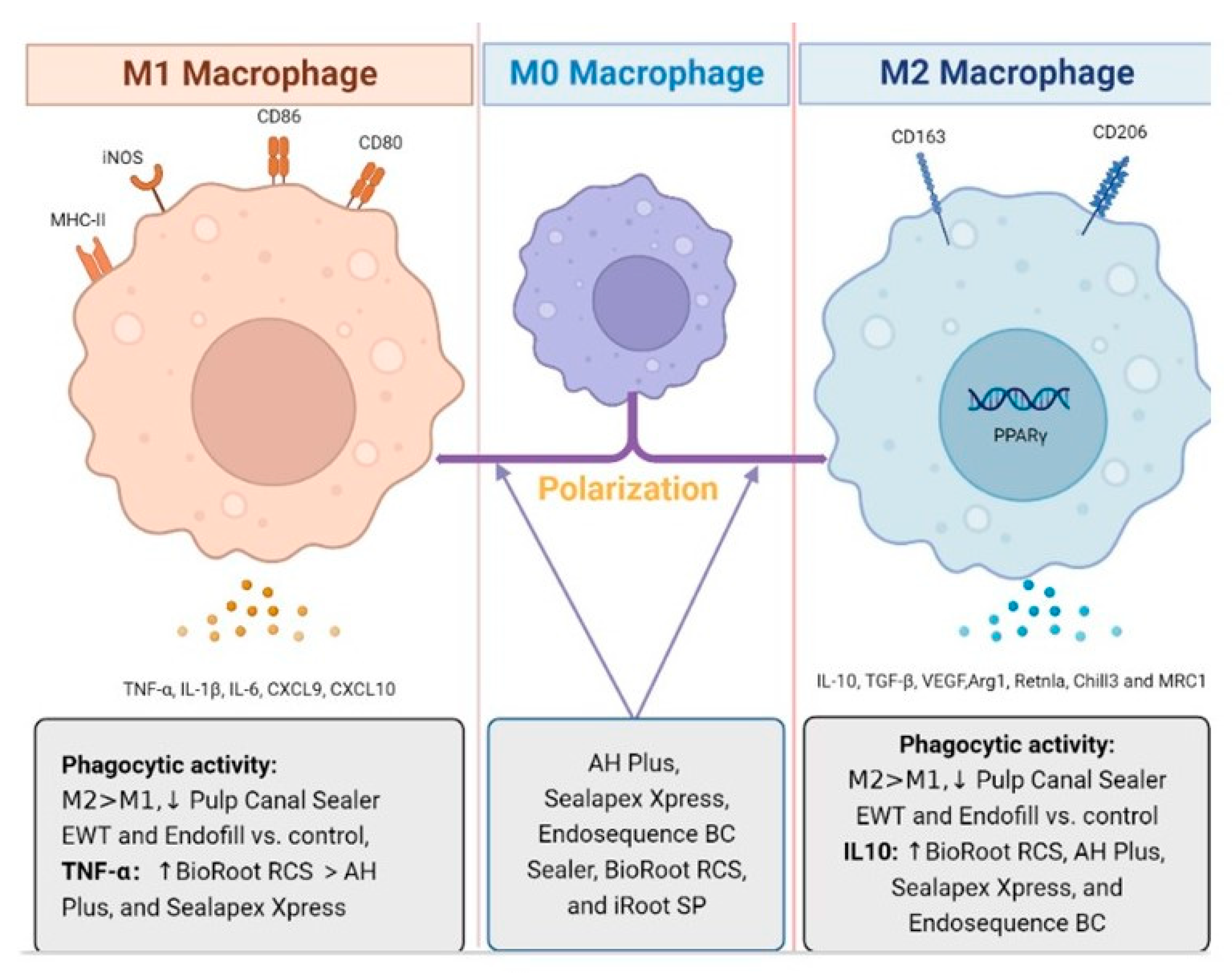
4.2. Modulatory Effects of Endodontic Sealers on Macrophages
4.3. Methodology in the Immunomodulatory Endodontic Sealers
4.4. Future Persective and Clinical Relevance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tribble, G.D.; Lamont, R.J. Bacterial invasion of epithelial cells and spreading in periodontal tissue. Periodontol. 2000 2010, 52, 68. [Google Scholar] [CrossRef] [PubMed]
- Boehm, B.J.; Colopy, S.A.; Jerde, T.J.; Loftus, C.J.; Bushman, W. Acute bacterial inflammation of the mouse prostate. Prostate 2012, 72, 307–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.H.; Kim, N.R.; Lim, B.S.; Lee, Y.K.; Hwang, K.K.; Yang, H.C. Effects of Root Canal Sealers on Lipopolysaccharide-induced Expression of Cyclooxygenase-2 mRNA in Murine Macrophage Cells. J. Endod. 2007, 33, 1329–1333. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Lim, B.S.; Lee, Y.K.; Kim, N.R.; Yang, H.C. Inhibitory effects of root canal sealers on the expression of inducible nitric oxide synthase in lipopolysaccharide-stimulated murine macrophage cells. J. Biomed. Mater. Res. Part B-Appl. Biomater. 2007, 83B, 91–96. [Google Scholar] [CrossRef]
- Thein, H.S.S.; Hashimoto, K.; Kawashima, N.; Noda, S.; Okiji, T. Evaluation of the anti-inflammatory effects of surface-reaction-type pre-reacted glass-ionomer filler containing root canal sealer in lipopolysaccharide-stimulated RAW264.7 macrophages. Dent. Mater. J. 2022, 41, 150–158. [Google Scholar] [CrossRef]
- Kharouf, N.; Sauro, S.; Eid, A.; Zghal, J.; Jmal, H.; Seck, A.; Maculuso, V.; Addiego, F.; Inchingolo, F.; Affolter-Zbaraszczuk, C. Physicochemical and Mechanical Properties of Premixed Calcium Silicate and Resin Sealers. J. Funct. Biomater. 2022, 14, 9. [Google Scholar] [CrossRef]
- Lee, B.-N.; Hong, J.-U.; Kim, S.-M.; Jang, J.-H.; Chang, H.-S.; Hwang, Y.-C.; Hwang, I.-N.; Oh, W.-M. Anti-inflammatory and Osteogenic Effects of Calcium Silicate–based Root Canal Sealers. J. Endod. 2019, 45, 73–78. [Google Scholar] [CrossRef]
- Cooper, P.R.; Takahashi, Y.; Graham, L.W.; Simon, S.; Imazato, S.; Smith, A.J. Inflammation–regeneration interplay in the dentine–pulp complex. J. Dent. 2010, 38, 687–697. [Google Scholar] [CrossRef]
- Miao, Y.; He, L.; Qi, X.; Lin, X. Injecting immunosuppressive M2 macrophages alleviates the symptoms of periodontitis in mice. Front. Mol. Biosci. 2020, 7, 603817. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Rodero, M.P.; Khosrotehrani, K. Skin wound healing modulation by macrophages. Int. J. Clin. Exp. Pathol. 2010, 3, 643. [Google Scholar] [PubMed]
- Golebiewska, E.M.; Poole, A.W. Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev. 2015, 29, 153–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha, M.; Sen, C.K.; Singh, K.; Das, A.; Ghatak, S.; Rhea, B.; Blackstone, B.; Powell, H.M.; Khanna, S.; Roy, S. Direct conversion of injury-site myeloid cells to fibroblast-like cells of granulation tissue. Nat. Commun. 2018, 9, 936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotwal, G.J.; Chien, S. Macrophage differentiation in normal and accelerated wound healing. Macrophages 2017, 62, 353–364. [Google Scholar]
- Kakehashi, S.; Stanley, H.; Fitzgerald, R. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg. Oral Med. Oral Pathol. 1965, 20, 340–349. [Google Scholar] [CrossRef]
- Nair, P.R.; Pajarola, G.; Schroeder, H.E. Types and incidence of human periapical lesions obtained with extracted teeth. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 1996, 81, 93–102. [Google Scholar] [CrossRef]
- Jeanneau, C.; Giraud, T.; Milan, J.L.; About, I. Investigating unset endodontic sealers’ eugenol and hydrocortisone roles in modulating the initial steps of inflammation. Clin. Oral Investig. 2020, 24, 639–647. [Google Scholar] [CrossRef]
- Sousa, C.J.; Montes, C.R.; Pascon, E.A.; Loyola, A.M.; Versiani, M.A. Comparison of the intraosseous biocompatibility of AH Plus, EndoREZ, and Epiphany root canal sealers. J. Endod. 2006, 32, 656–662. [Google Scholar] [CrossRef]
- Lawrence, T.; Gilroy, D.W. Chronic inflammation: A failure of resolution? Int. J. Exp. Pathol. 2007, 88, 85–94. [Google Scholar] [CrossRef]
- Lin, L.M.; Huang, G.T.-J.; Rosenberg, P.A. Proliferation of epithelial cell rests, formation of apical cysts, and regression of apical cysts after periapical wound healing. J. Endod. 2007, 33, 908–916. [Google Scholar] [CrossRef]
- Silva-Herzog, D.; Ramírez, T.; Mora, J.; Pozos, A.; Silva, L.; Silva, R.; Nelson-Filho, P. Preliminary study of the inflammatory response to subcutaneous implantation of three root canal sealers. Int. Endod. J. 2011, 44, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Hudecki, A.; Kiryczyński, G.; Łos, M.J. Biomaterials, definition, overview. In Stem Cells and Biomaterials for Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 85–98. [Google Scholar]
- Williams, D.F. Titanium for medical applications. In Titanium in Medicine; Springer: Berlin, Germany, 2001; pp. 13–24. [Google Scholar]
- Tan, L.; Yu, X.; Wan, P.; Yang, K. Biodegradable materials for bone repairs: A review. J. Mater. Sci. Technol. 2013, 29, 503–513. [Google Scholar] [CrossRef]
- Athanasiou, K.A.; Niederauer, G.G.; Agrawal, C.M. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials 1996, 17, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.; Mancino, D.; Rekab, M.S.; Haikel, Y.; Kharouf, N. Effectiveness of Three Agents in Pulpotomy Treatment of Permanent Molars with Incomplete Root Development: A Randomized Controlled Trial. Healthcare 2022, 10, 431. [Google Scholar] [CrossRef]
- Crum, R.J.; Hall, K.; Molina, C.P.; Hussey, G.S.; Graham, E.; Li, H.; Badylak, S.F. Immunomodulatory matrix-bound nanovesicles mitigate acute and chronic pristane-induced rheumatoid arthritis. NPJ Regen. Med. 2022, 7, 13. [Google Scholar] [CrossRef]
- Kouroupis, D.; Kaplan, L.D.; Best, T.M. Human infrapatellar fat pad mesenchymal stem cells show immunomodulatory exosomal signatures. Sci. Rep. 2022, 12, 3609. [Google Scholar] [CrossRef]
- Kaur, A.; Shah, N.; Logani, A.; Mishra, N. Biotoxicity of commonly used root canal sealers: A meta-analysis. J. Conserv. Dent. JCD 2015, 18, 83. [Google Scholar]
- Desai, S.; Chandler, N. Calcium hydroxide–based root canal sealers: A review. J. Endod. 2009, 35, 475–480. [Google Scholar] [CrossRef]
- Komabayashi, T.; Colmenar, D.; Cvach, N.; Bhat, A.; Primus, C.; Imai, Y. Comprehensive review of current endodontic sealers. Dent. Mater. J. 2020, 39, 703–720. [Google Scholar] [CrossRef] [Green Version]
- Wan, Q.-Q.; Sun, J.-L.; Ma, Y.-X.; Noble, L.C.; Dong, Y.; Feng, Z.-H.; Gu, J.-T.; Wang, Y.-R.; Wang, W.-R.; Bergeron, B.E. Immunomodulatory effects of tricalcium silicate-based cements on osteogenesis. Appl. Mater. Today 2021, 24, 101145. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, H.H.; Kim, H.W.; Yu, J.W.; Kim, K.N.; Kim, K.M. Immunomodulatory/anti-inflammatory effect of ZOE-based dental materials. Dent. Mater. 2017, 33, e1–e12. [Google Scholar] [CrossRef] [PubMed]
- Diomede, F.; Caputi, S.; Merciaro, I.; Frisone, S.; D’Arcangelo, C.; Piattelli, A.; Trubiani, O. Pro-inflammatory cytokine release and cell growth inhibition in primary human oral cells after exposure to endodontic sealer. Int. Endod. J. 2014, 47, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Jeanneau, C.; Giraud, T.; Laurent, P.; About, I. BioRoot RCS Extracts Modulate the Early Mechanisms of Periodontal Inflammation and Regeneration. J. Endod. 2019, 45, 1016–1023. [Google Scholar] [CrossRef]
- Moher, D.; Altman, D.G.; Liberati, A.; Tetzlaff, J. PRISMA statement. Epidemiology 2011, 22, 128. [Google Scholar] [CrossRef] [Green Version]
- Faggion Jr, C.M. Guidelines for reporting pre-clinical in vitro studies on dental materials. J. Evid. Based Dent. Pract. 2012, 12, 182–189. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.Y.; Hung, S.L.; Pai, S.F.; Lee, Y.H.; Yang, S.F. Eugenol Suppressed the Expression of Lipopolysaccharide-induced Proinflammatory Mediators in Human Macrophages. J. Endod. 2007, 33, 698–702. [Google Scholar] [CrossRef]
- Brackett, M.G.; Marshall, A.; Lockwood, P.E.; Lewis, J.B.; Messer, R.L.W.; Bouillaguet, S.; Wataha, J.C. Inflammatory suppression by endodontic sealers after aging 12 weeks in vitro. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2009, 91, 839–844. [Google Scholar] [CrossRef]
- de Oliveira Mendes, S.T.; de Brito, L.C.N.; Rezende, T.M.B.; de Oliveira Reis, R.; Cardoso, F.P.; Vieira, L.Q.; Ribeiro Sobrinho, A.P. A decrease in the innate immune response to infection in the presence of root canal sealers. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2010, 109, 315–323. [Google Scholar] [CrossRef]
- Brackett, M.G.; Lewis, J.B.; Messer, R.L.W.; Lei, L.; Lockwood, P.E.; Wataha, J.C. Dysregulation of monocytic cytokine secretion by endodontic sealers. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2011, 97B, 49–57. [Google Scholar] [CrossRef]
- Braga, J.M.; Oliveira, R.R.; Martins, R.C.; Ribeiro Sobrinho, A.P. The effects of a mineral trioxide aggregate-based sealer on the production of reactive oxygen species, nitrogen species and cytokines by two macrophage subtypes. Int. Endod. J. 2014, 47, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.H.; Lin, D.J.; Chang, K.W.; Hsia, S.M.; Ko, S.Y.; Lee, S.Y.; Hsue, S.S.; Wang, T.H.; Chen, Y.L.; Shieh, T.M. Evaluation Physical Characteristics and Comparison Antimicrobial and Anti-Inflammation Potentials of Dental Root Canal Sealers Containing Hinokitiol In Vitro. PLoS ONE 2014, 9, e94941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinho, F.C.; Camargo, S.E.A.; Fernandes, A.M.M.; Campos, M.S.; Prado, R.F.; Camargo, C.H.R.; Valera, M.C. Comparison of cytotoxicity, genotoxicity and immunological inflammatory biomarker activity of several endodontic sealers against immortalized human pulp cells. Int. Endod. J. 2018, 51, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.-G.; Sun, K.-T.; Wang, T.-H.; He, Y.-Z.; Hsia, S.-M.; Tsai, B.-H.; Shih, Y.-H.; Shieh, T.-M. Effects of mineral trioxide aggregate and bioceramics on macrophage differentiation and polarization in vitro. J. Formos. Med. Assoc. 2019, 118, 1458–1465. [Google Scholar] [CrossRef]
- Gaudin, A.; Tolar, M.; Peters, O.A. Cytokine Production and Cytotoxicity of Calcium Silicate–based Sealers in 2- and 3-dimensional Cell Culture Models. J. Endod. 2020, 46, 818–826. [Google Scholar] [CrossRef]
- Da Pedrosa, M.S.; Alves, T.; Nogueira, F.N.; Holzhausen, M.; Sipert, C.R. Cytotoxicity and cytokine production by calcium silicate-based materials on periodontal ligament stem cells. Braz. Dent. J. 2021, 32, 65–74. [Google Scholar] [CrossRef]
- da Silva, L.A.B.; Hidalgo, L.R.D.C.; de Sousa-Neto, M.D.; Arnez, M.F.M.; Barnett, F.; Hernández, P.M.G.; Faccioli, L.H.; Paula-Silva, F.W.G. Cytotoxicity and inflammatory mediators release by macrophages exposed to real seal xt and sealapex xpress. Braz. Dent. J. 2021, 32, 48–52. [Google Scholar] [CrossRef]
- Da Silva, R.A.B.; Da Silva, L.A.B.; Gabriel-Junior, E.A.; Sorgi, C.A.; Faccioli, L.H.; Massoni, V.V.; Nelson-Filho, P.; Pucinelli, C.M. Ml and M2 macrophages phenotypes modulation after stimuli with materials used in endodontic treatment. Braz. Dent. J. 2021, 32, 32–43. [Google Scholar] [CrossRef]
- Pérez-Serrano, R.M.; Soza-Bolaños, A.I.; Castillo-Valdés, S.N.; Hernández-Valdez, G.; Mora-Izaguirre, O.; González-Dávalos, M.L.; Dammaschke, T.; Domínguez-Pérez, R.A. Endodontic set sealer eluates promote cytokine production in human mononuclear and periodontal ligament cells. Aust. Endod. J. 2021, 47, 415–422. [Google Scholar] [CrossRef]
- Sharma, A.; Sanjeev, K.; Selvanathan, V.M.J.; Sekar, M.; Harikrishnan, N. The evaluation of cytotoxicity and cytokine IL-6 production of root canal sealers with and without the incorporation of simvastatin: An invitro study. BMC Oral Health 2022, 22, 6. [Google Scholar] [CrossRef]
- Hosseinpour, S.; Gaudin, A.; Peters, O.A. A critical analysis of research methods and experimental models to study biocompatibility of endodontic materials. Int. Endod. J. 2022, 55, 346–369. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, Y.; Shigetani, Y.; Yoshiba, K.; Kaneko, T.; Yoshiba, N.; Okiji, T. Evaluation of the responses of MHC class II molecule-expressing cells and macrophages to epoxy resin-based and 4-META-containing, methacrylate resin-based root canal sealers in rat subcutaneous tissue. Dent. Mater. J. 2013, 32, 822–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saraiva, J.A.; Da Fonseca, T.S.; Da Silva, G.F.; Sasso-Cerri, E.; Guerreiro-Tanomaru, J.M.; Tanomaru-Filho, M.; Cerri, P.S. Reduced interleukin-6 immunoexpression and birefringent collagen formation indicate that MTA Plus and MTA Fillapex are biocompatible. Biomed. Mater. 2018, 13, 035002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delfino, M.M.; Guerreiro-Tanomaru, J.M.; Tanomaru-Filho, M.; Sasso-Cerri, E.; Cerri, P.S. Immunoinflammatory response and bioactive potential of GuttaFlow bioseal and MTA Fillapex in the rat subcutaneous tissue. Sci. Rep. 2020, 10, 7173. [Google Scholar] [CrossRef]
- Yang, X.Q.; Tian, J.; Li, M.J.; Chen, W.Y.; Liu, H.; Wang, Z.J.; Haapasalo, M.; Shen, Y.; Wei, X. Biocompatibility of a New Calcium Silicate-Based Root Canal Sealer Mediated via the Modulation of Macrophage Polarization in a Rat Model. Materials 2022, 15, 1962. [Google Scholar] [CrossRef]
- Santos, J.M.; Pereira, S.; Sequeira, D.B.; Messias, A.L.; Martins, J.B.; Cunha, H.; Palma, P.J.; Santos, A.C. Biocompatibility of a bioceramic silicone-based sealer in subcutaneous tissue. J. Oral Sci. 2019, 61, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Vinola, S.M.; Karthikeyan, K.; Sharma, A.; Sudheshna, S.; Sekar, M. Anti-inflammatory efficacy of petasin-incorporated zinc oxide eugenol sealer–An in vivo zebrafish study. J. Conserv. Dent. JCD 2021, 24, 539. [Google Scholar] [CrossRef]
- Yu, M.-K.; Lee, Y.-H.; Yoon, M.-R.; Bhattarai, G.; Lee, N.-H.; Kim, T.-G.; Jhee, E.-C.; Yi, H.-K. Attenuation of AH26-induced apoptosis by inhibition of SAPK/JNK pathway in MC-3T3 E1 cells. J. Endod. 2010, 36, 1967–1971. [Google Scholar] [CrossRef]
- Kim, T.-G.; Lee, Y.-H.; Bhattari, G.; Lee, N.-H.; Lee, K.-W.; Yi, H.-K.; Yu, M.-K. PPARγ inhibits inflammation and RANKL expression in epoxy resin-based sealer-induced osteoblast precursor cells E1 cells. Arch. Oral Biol. 2013, 58, 28–34. [Google Scholar] [CrossRef]
- Chang, S.-W.; Lee, S.-Y.; Kang, S.-K.; Kum, K.-Y.; Kim, E.-C. In vitro biocompatibility, inflammatory response, and osteogenic potential of 4 root canal sealers: Sealapex, Sankin apatite root sealer, MTA Fillapex, and iRoot SP root canal sealer. J. Endod. 2014, 40, 1642–1648. [Google Scholar] [CrossRef]
- Diomede, F.; Marconi, G.D.; Guarnieri, S.; D’Attilio, M.; Cavalcanti, M.F.; Mariggiò, M.A.; Pizzicannella, J.; Trubiani, O. A novel role of ascorbic acid in anti-inflammatory pathway and ROS generation in HEMA treated dental pulp stem cells. Materials 2019, 13, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khera, R.; Mehan, S.; Bhalla, S.; Kumar, S.; Alshammari, A.; Alharbi, M.; Sadhu, S.S. Guggulsterone mediated JAK/STAT and PPAR-gamma modulation prevents neurobehavioral and neurochemical abnormalities in propionic acid-induced experimental model of autism. Molecules 2022, 27, 889. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxidative Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, P.; Gao, Z.; Zhao, X.; Qi, G. Surfactin inducing mitochondria-dependent ROS to activate MAPKs, NF-κB and inflammasomes in macrophages for adjuvant activity. Sci. Rep. 2016, 6, 39303. [Google Scholar] [CrossRef]
- Bae, W.-J.; Chang, S.-W.; Lee, S.-I.; Kum, K.-Y.; Bae, K.-S.; Kim, E.-C. Human periodontal ligament cell response to a newly developed calcium phosphate–based root canal sealer. J. Endod. 2010, 36, 1658–1663. [Google Scholar] [CrossRef]
- Futagami, A.; Ishizaki, M.; Fukuda, Y.; Kawana, S.; Yamanaka, N. Wound healing involves induction of cyclooxygenase-2 expression in rat skin. Lab. Investig. 2002, 82, 1503–1513. [Google Scholar] [CrossRef] [Green Version]
- Nasry, W.H.S.; Rodriguez-Lecompte, J.C.; Martin, C.K. Role of COX-2/PGE2 mediated inflammation in oral squamous cell carcinoma. Cancers 2018, 10, 348. [Google Scholar] [CrossRef] [Green Version]
- Frick, M.; Dulak, J.; Cisowski, J.; Józkowicz, A.; Zwick, R.; Alber, H.; Dichtl, W.; Schwarzacher, S.P.; Pachinger, O.; Weidinger, F. Statins differentially regulate vascular endothelial growth factor synthesis in endothelial and vascular smooth muscle cells. Atherosclerosis 2003, 170, 229–236. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Jin, L.; Pang, X.; Lu, Y.; Wang, Z.; Yu, Y.; Yu, J. Mineral trioxide aggregate enhances the osteogenic capacity of periodontal ligament stem cells via NF-κB and MAPK signaling pathways. J. Cell. Physiol. 2018, 233, 2386–2397. [Google Scholar] [CrossRef]
- Han, L.; Okiji, T. Evaluation of the ions release/incorporation of the prototype S-PRG filler-containing endodontic sealer. Dent. Mater. J. 2011, 30, 898–903. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Zhu, Y.; Duan, D.; Wang, P.; Xin, Y.; Bai, L.; Liu, Y.; Xu, Y. Enhanced activity of macrophage M1/M2 phenotypes in periodontitis. Arch. Oral Biol. 2018, 96, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, H.; Wang, Y.; Gu, Y. Akt2 affects periodontal inflammation via altering the M1/M2 ratio. J. Dent. Res. 2020, 99, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Zhu, X.; Li, Y.; Yan, P.; Jiang, H. Influence of iRoot SP and mineral trioxide aggregate on the activation and polarization of macrophages induced by lipopolysaccharide. BMC Oral Health 2018, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Kaneko, T.; Yamanaka, Y.; Shigetani, Y.; Yoshiba, K.; Okiji, T. M2 macrophages participate in the biological tissue healing reaction to mineral trioxide aggregate. J. Endod. 2014, 40, 379–383. [Google Scholar] [CrossRef]
- Takei, E.; Shigetani, Y.; Yoshiba, K.; Hinata, G.; Yoshiba, N.; Okiji, T. Initial Transient Accumulation of M2 Macrophage–associated Molecule-expressing Cells after Pulpotomy with Mineral Trioxide Aggregate in Rat Molars. J. Endod. 2014, 40, 1983–1988. [Google Scholar] [CrossRef]
- Fonseca, J.; Santos, M.; Canhao, H.; Choy, E. Interleukin-6 as a key player in systemic inflammation and joint destruction. Autoimmun. Rev. 2009, 8, 538–542. [Google Scholar] [CrossRef]
- Park, Y.-T.; Lee, S.-M.; Kou, X.; Karabucak, B. The role of interleukin 6 in osteogenic and neurogenic differentiation potentials of dental pulp stem cells. J. Endod. 2019, 45, 1342–1348. [Google Scholar] [CrossRef]
- Nebel, D.; Arvidsson, J.; Lillqvist, J.; Holm, A.; Nilsson, B.-O. Differential effects of LPS from Escherichia coli and Porphyromonas gingivalis on IL-6 production in human periodontal ligament cells. Acta Odontol. Scand. 2013, 71, 892–898. [Google Scholar] [CrossRef] [Green Version]
- Whitaker, R.; Hernaez-Estrada, B.; Hernandez, R.M.; Santos-Vizcaino, E.; Spiller, K.L. Immunomodulatory biomaterials for tissue repair. Chem. Rev. 2021, 121, 11305–11335. [Google Scholar] [CrossRef]
- Chen, H.; Sun, J.; Wang, Z.; Zhou, Y.; Lou, Z.; Chen, B.; Wang, P.; Guo, Z.; Tang, H.; Ma, J. Magnetic cell–scaffold interface constructed by superparamagnetic IONP enhanced osteogenesis of adipose-derived stem cells. ACS Appl. Mater. Interfaces 2018, 10, 44279–44289. [Google Scholar] [CrossRef]
- Guo, X.; Sun, Y.; Wang, Z.; Ren, B.; Xu, H.H.; Peng, X.; Li, M.; Wang, S.; Wang, H.; Wu, Y. The Preventive Effect of A Magnetic Nanoparticle-Modified Root Canal Sealer on Persistent Apical Periodontitis. Int. J. Mol. Sci. 2022, 23, 13137. [Google Scholar] [CrossRef] [PubMed]
- Vinola, S.M.; Karthikeyan, K.; Mahalaxmi, S. A novel petasin-modified zinc oxide eugenol sealer. J. Conserv. Dent. JCD 2019, 22, 490. [Google Scholar] [CrossRef] [PubMed]

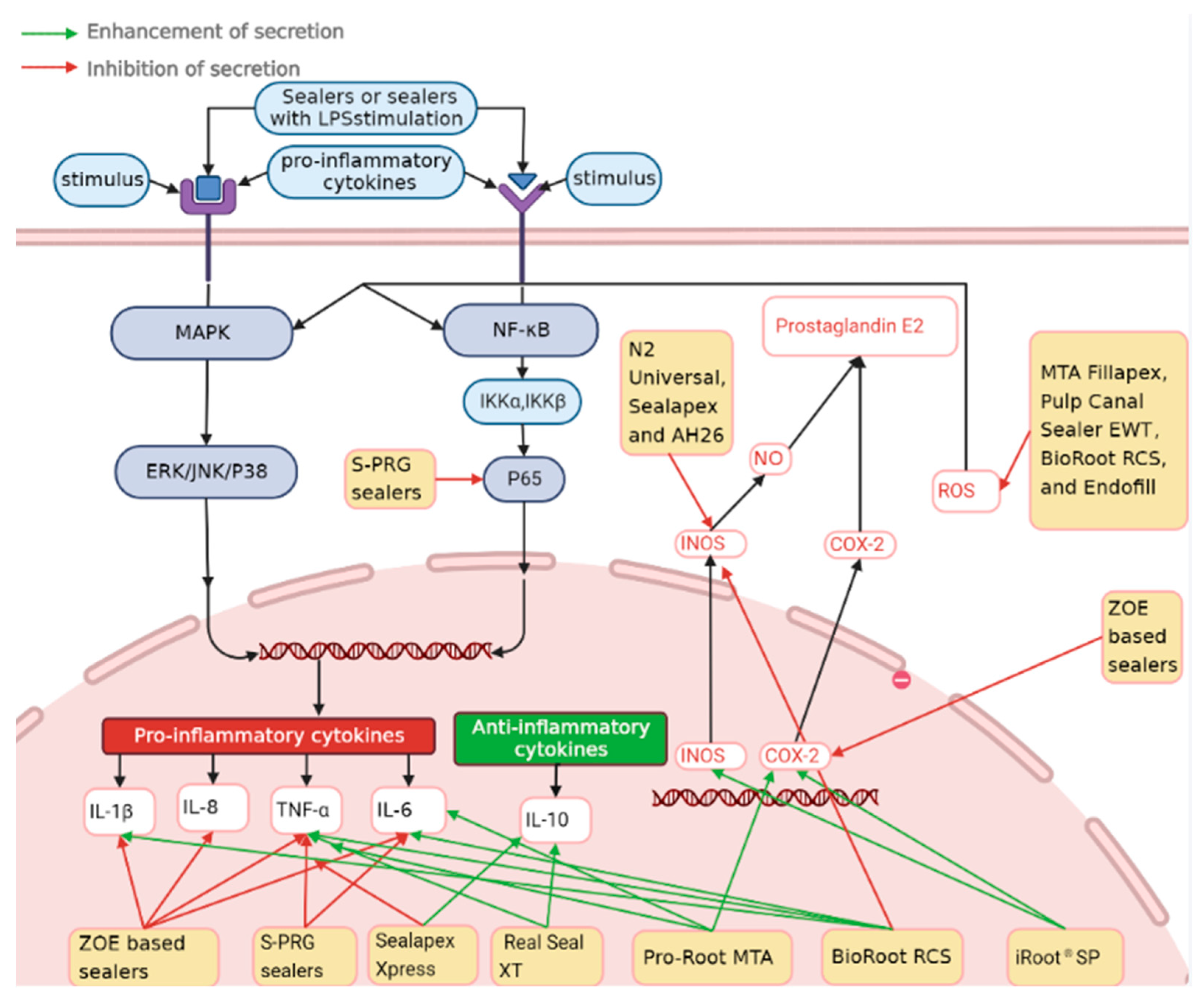
| Cell Type | Material | Marker | Method | Immunomodulatory Effect | Ref. |
|---|---|---|---|---|---|
| RAW 264.7 macrophages | AH26, Sealapex, and N2 Universal | COX-2 | RT-PCR, agarose gel electrophoresis, Cell Counting Kit-8 assay | ↑ COX-2 mRNA vs. control | [3] |
| RAW 264.7 macrophages | AH26, Sealapex, and N2 Universal | iNOS | MTT assay, RT-PCR, SDS-polyacrylamide gel electrophoresis, Colorimetric analysis | ↓ iNOS mRNA vs. control | [4] |
| U937 macrophages | Zinc oxide eugenol sealers | IL-1β, TNF-α, PGE2, COX-2 | ELISA, RT-PCR | ↓ IL-1β, ↓TNF-α, ↓ PGE2, ↓ COX-2 mRNA vs. LPS | [39] |
| Human THP-l monocytes (ATCC TIB 202) | AH-Plus, Pulp Canal Sealer, Epiphany, Endo-Rez, and an experimental Endo-Rez | TNF-α, IL-1β, IL-6 | ELISA, MTT assay | ↓ IL-1β vs. control Inhibition: PCS > EPH > ER (α = 0.05) ↓ IL-6 vs. Control EPH > PCS (α = 0.05) ↓TNF-α vs. control EPH > PCS (α = 0.05) | [40] |
| Morphologic characteristics of macrophages from C57BL/6 mice | Pulp Canal Sealer EWT and Endofill | NO, ROS, TNF-α IL-10 IL-12 | ELISA, Nitric Oxide Assay(colorimetric), ROS assay (Spectrophotometric assays) | ↓ Phagocytic activity of macrophages ↓ ROS vs. control ↓ TNF-α when M2 cells + F. nucleatum + IFN-γ TNF-α: P. anaerobius + M1 cells + IFN-γ > M2 cells (p < 0.05) | [41] |
| THP1 human monocytic cells (ATCC TIB 202) | AH-Plus-Jet, Pulp Canal Sealer, MTA-type sealers, ProRoot White MTA, and an experimental calcium silicate-based sealer | IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-15, IFN-γ, TGF-β1, TNF-α, TNF-β, VEGF | Cytokine Array based on ELISA | ↓ IL-2, IL-6, IL-10, and IL-15 ↑ IL-1α and IL-1β | [42] |
| M1 (from C57BL/6 mice) and M2 (from BALB/c mice) peritoneal inflammatory macrophages | MTA and MTA Fillapex (FLPX) | ROS, IL-12, IL-10, TNF-α, NO | Reactive Oxygen intermediates assay, MTT assay, Nitric Oxide Assay | ↓ ROS and NO vs. control in both M1 and M2 cells. (p < 0.05) ↑ TNF-α: IFN-γ + FLPX + P. anaerobius stimulated M1 cells vs. control (p < 0.05) ↓ TNF-α: F. nucleatum-stimulated M2 cells vs. control (p < 0.05) IL-10: M1 > M2 cells | [43] |
| Human dental pulp and periodontal ligament stem cells, osteoblasts, and fibroblasts | EndoRez | IL-6, IL-8, IL-12, TNF-α | MTT assay, Fluorescence staining, confocal laser scanning microscope analysis, ELISA | ↑ IL-6, IL-8, IL-12, and TNF-α: EndoRez vs. control | [34] |
| Human gingival fibroblasts, and Human osteosarcoma cell lines MG-63 | AH Plus, Apexit Plus, and Canals | COX-2, HIF-1α, LOX | MTT assay, RT-PCR | ↓ COX-2 and HIF-1α: Apexit Plus + 1% Hinokitiol vs. control in MG-63 (p < 0.05) ↓ COX-2, HIF-1α, and LOX: Apexit Plus + 1% Hinokitiol vs. control in HGF (p < 0.01, p < 0.001, p < 0.001, respectively) | [44] |
| Immortalized human dental pulp stem cells (IHDPSCs) and mouse bone marrow monocytes (IMBMMs) | Zinc oxide–eugenol (ZOE)-based endodontic sealers and cement (IRM and Tubli-Seal) | IL-1β, IL-6, IL-8, TNF-α | RT-PCR, WST assay, Live and Dead Cell Assay | ↓ IL-1β, IL-6, and IL-8 vs. LPS control (p < 0.05) in HDPC ↓ IL-1β, IL-6, and IL-8 vs. control (p < 0.05) in HDPC ↑ IL-1β and IL-6: Zn 2+ vs. media-exchange condition (p < 0.05) in IMBMM ↓ TNF-α eugenol vs. control (p < 0.05) in IMBMM | [33] |
| SV40 T-Ag-transfected cell line of human pulp-derived cells | Apexit Plus, Real Seal, AH Plus, and EndoREZ | IL-6, IL-8, TNF-α | MTT assay, MTN assay, RT-PCR, Immunohistochemistry, ELISA | ↑ IL6, IL8, and TNF-α vs. control (p < 0.05) | [45] |
| Primary human periodontal ligament (PDL) cells, human umbilical vein endothelial cells, and Inflammatory (THP-1) Cell | BioRoot RCS and Pulp Canal Sealer | IL-6, TGF-β1 | Immunofluorescence, RT-PCR, ELISA, MTT assay | ↓ IL-6 BioRoot RCS vs. control (p < 0.05) ↑ IL-6 PCS vs. control (p < 0.05) ↑ TGF-β1 BioRoot RCS vs. control (p < 0.05) ↓ TGF-β1 PCS vs. BioRoot RCS (p < 0.05) ↓ IL-6 BioRoot RCS vs. PCS (p < 0.05) | [35] |
| MC3T3-E1 cells | AH Plus, MTA Fillapex, and EndoSequence BC | IL-6, TNF-α, ALP, OCN | WST assay, RT-PCR, Alkaline Phosphatase Staining, Alizarin Red Staining | ↓ IL6 and TNF-α vs. control (p < 0.05) | [7] |
| RAW 264.7 macrophages | Mineral trioxide aggregate (Pro-Root MTA, PR-MTA) and other calcium silicate-based materials (iRoot® SP Injectable Root Canal Sealer, IR-BC) | TNF-α, IL-6, IL-1β, COX-2, iNOS | MTT assay, Western Blotting, RT-PCR | ↑ iNOS iR-BC vs. control ↑ COX-2 iR-BC and PR-MTA vs. control ↑ TNF-α iR-BC and PR-MTA vs. control ↑ IL-1β iR-BC vs. control ↑ IL-6 R-BC and PR-MTA vs. control | [46] |
| Primary human periodontal ligament stem cells (PDLSCs) | BioRoot RCS, ProRoot ES, MTA Fillapex | IL-6, IL-8, GRO, IL-4, IL-10 | Flow cytometry, MTT assay, Multiplex bead-based cytokine assay | ↑ IL-6, IL-8, and GRO MTA Fillapex and AH Plus vs. control (p < 0.05) ↑ IL-4 and IL-10 BioRoot RCS vs. control (p < 0.05) | [47] |
| Primary human periodontal ligament cell | Endomethasone N (EN) and Pulp Canal Sealer (PCS) | IL-6, TNF-α | ELISA, spectrofluorimetry | ↓ IL6 EN vs. control ↑ IL-6 PCS vs. control ↓ TNF-α EN vs. control ↑ TNF-α PCS vs. control ↑ IL-6 EN vs. PCS ↑ TNF-α EN vs. PCS (p < 0.05) | [17] |
| Primary hPDLSCs | Bio-C Sealer, MTA Fillapex, and PBS Cimmo HP | IL-10, TNF-α | ELISA, MTT assay, immunostaining, flow cytometry | ↑ TNF-α Bio-C Sealer, Cimmo HP and MTA Fillapex vs. control (p < 0.05) | [48] |
| The J774.1 murine macrophage cell line | Sealapex Xpress and Seal Real XT | IL-4, IL-6, IL-10, TNF-α | ELISA, MTT assay | ↑ TNF-α Real Seal XT vs. control (p < 0.05) ↑ IL-6 Real Seal XT vs. control (p < 0.05) ↓ TNF-α Sealapex Xpress vs. control (p < 0.05) ↑ IL-10 Sealapex Xpress vs. Real Seal XT (p < 0.05) | [49] |
| Bone marrow-derived macrophages (BMDM) | AH Plus, Sealapex Xpress, Endosequence BC Sealer, BioRoot RCS and Calen | GM-CSF, IL-10, IL-6, IL-1β, TNF-α M1 markers: Cxcl10, CxCL9 and iNOS M2 markers: Arg1, Retnla, Chill3 and MRC1 | RT-PCR, MTT assay, Multiplex bead-based cytokine assay | Markers of M1 phenotype: ↓ iNOS BioRoot RCS and Sealapex Xpress vs. control (p < 0.001) Markers of M2 phenotype: ↓ Arg1 Sealapex Xpress vs. control (p < 0.05) ↓Retnla EndoSequence BC Seale vs. control (p < 0.001) Sealapex Xpress vs. control (p < 0.01) ↑ IL-1β, TNF-α, and IL-6 | [50] |
| Human peripheral blood mononuclear cells (hPBMC), hPDLSCs | MTA Fillapex, BioRoot RCS, AH Plus, and Pulp Canal Sealer | IL-6, TNF-α, IL-8, IL-10 | ELISA | ↑ IL-6 MTA Fillapex > BioRoot RCS(p < 0.001) AH Plus > BioRoot RCS(p < 0.05) in hPBMC afer 12 h ↓ TNF-α BioRoot RCS > MTA Fillapex BioRoot RCS > PCS (p < 0.05) in hPBMC after 6 h ↓ IL-6 BioRoot RCS > AH plus (p < 0.05) PCS > MTA Fillapex (p < 0.05) in hPDLSCs after 12 h | [51] |
| L929 mouse fibroblast cells | Zinc oxide eugenol and methacrylate based EndoREZ sealers (ZE and ER and simvastatin incorporated sealers (ZES and ERS) | IL-6 | MTT assay, Live and dead cell assay, Flow cytometry analysis | ↑ IL-6 ZE > ER > ERS > ZES > Control | [52] |
| RAW264.7 macrophages | surface-reaction-type pre-reacted glass-ionomer (S-PRG) filler containing root canal sealer (S-PRG sealer) and Canals N | IL-1α, IL-6, TNF-α, PPARα, IL-10, p-NF-kB | MTT assay, RT-PCR, Western blotting, ELISA | ↑ IL-10 LPS + S-PRG vs. LPS control (p < 0.05) ↑ PPARα LPS + S-PRG vs. LPS control (p < 0.05) | [5] |
| Animal | Material | Marker | Method | Outcomes | Ref. |
|---|---|---|---|---|---|
| 18 4-week-old male Wistar rats were randomly divided into three groups | MetaSEAL and AH Plus | MHC class II, CD68, CD43. | Histology | Epoxy resin-based sealer induced the infiltration of MHC class II molecule-expressing cells, whereas 4-META-containing, methacrylate resin-based sealer elicited macrophage infiltration. | [54] |
| 100 adult male Holtzman rats (Rattus norvegicus albinus) weighing 220 g–250 g were distributed into five groups | Root Canal sealer, MTA Plus, MTA Fillapex, AH Plus, and Endofill | IL-6, collagen | Histology and immunohistochemical analysis | The reduction in VvIC (volume density of inflammatory cells) increased with the increasing collagen in all the groups, except Endofill. MTA Plus, MTA Fillapex, and AH Plus induce regression of inflammation and formation of a fibrous capsule. MTA Plus and MTA Fillapex showed lower IL-6. | [55] |
| Sixteen young adult (8–10 weeks) Wistar rats, weighing 120-260 g | GuttaFlow Bioseal, GuttaFlow2 and AH Plus | \ | Histology | All the sealers induced macrophage infiltrate, and GuttaFlow Bioseal had the most macrophage infiltrate. The resolution of inflammation was observed after 30 days. | [58] |
| Eighty Holtzman adult male rats (Rattus norvegicus albinus) were distributed into four groups containing 20 animals each | GuttaFlow Bioseal (GFB) and MTA Fillapex (MTAF) | IL-6, VEGF | Histology and immunohistochemical analysis | Up-regulation of inflammation: pro-inflammatory cytokine IL-6 increased, and VEGF increased with the tissue repair process. | [56] |
| Fifty adult zebrafishes (Pentagrit Research Lab, Chennai, India.) | ZnOE sealers | \ | Histopathological analysis | Down-regulation of inflammation with the addition of petasin extract to ZnOE sealers. | [59] |
| Twenty-four young adult male Sprague–Dawley (SD) rats, aged 2–4 months and weighing 180–250 g | MTA, iRoot SP, BC Sealer HiFlow | CD163, CD206, CD86, mRNA of IL-1β, IL-6, TNF-α, IL-10 | RT-PCR, flow-cytometry, immunofluorescence, and histology | Down-regulation of inflammation of BC Sealer HiFlow and iRoot SP was observed, and BC Sealer HiFlow promoted M2-like macrophage polarization in vivo. | [57] |
| Author&Year/Item | 1 | 2a | 2b | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | Overall | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D. H. Lee, N. R. Kim, et al., 2007 | N | Y | Y | Y | N | N | N | N | N | N | Y | N | Y | Y | N | 40.00% | 6 | [3] |
| D. H. Lee, B. S. Lim, et al., 2007 | N | Y | Y | Y | N | N | N | N | N | N | Y | Y | Y | N | N | 40.00% | 6 | [4] |
| Y. Y. Lee et al., 2007 | N | Y | Y | Y | N | N | N | N | N | N | Y | N | N | Y | N | 26.67% | 4 | [39] |
| Brackett et al., 2009 | N | Y | Y | Y | N | N | N | N | N | N | Y | Y | N | N | N | 33.33% | 5 | [40] |
| S. T. de Oliveira Mendes et al., 2010 | N | N | Y | Y | N | N | N | N | N | N | Y | N | N | N | N | 20.00% | 3 | [41] |
| Brackett et al., 2011 | N | Y | Y | Y | N | N | N | N | N | N | Y | Y | Y | N | N | 40.00% | 6 | [42] |
| Braga et al., 2014 | Y | Y | Y | Y | N | N | N | N | N | N | Y | N | Y | N | N | 40.00% | 6 | [43] |
| Diomede et al., 2014 | Y | Y | Y | Y | N | N | N | N | N | N | Y | N | N | Y | N | 40.00% | 6 | [34] |
| Shih et al., 2014 | N | Y | Y | Y | N | N | N | N | N | N | Y | N | Y | Y | N | 40.00% | 6 | [44] |
| Lee et al., 2017 | Y | Y | Y | Y | N | N | N | N | N | N | Y | N | Y | Y | N | 46.67% | 7 | [33] |
| Martinho et al., 2018 | Y | Y | Y | Y | N | N | N | N | N | N | Y | N | N | Y | N | 40.00% | 6 | [45] |
| Jeanneau et al., 2019 | Y | Y | Y | Y | N | N | N | N | N | N | Y | N | Y | Y | N | 46.67% | 7 | [35] |
| Lee et al., 2019 | Y | Y | Y | Y | N | N | N | N | N | N | Y | N | Y | Y | N | 46.67% | 7 | [7] |
| Tu et al., 2019 | Y | Y | Y | Y | N | N | N | N | N | N | Y | N | N | Y | N | 40.00% | 6 | [46] |
| Gaudin et al., 2020 | Y | Y | Y | Y | N | N | N | N | N | N | Y | N | Y | Y | N | 46.67% | 7 | [47] |
| C. Jeanneau et al., 2020 | Y | Y | Y | Y | N | N | N | N | N | N | Y | N | Y | Y | N | 46.67% | 7 | [17] |
| Da Pedrosa et al., 2021 | N | N | Y | Y | N | N | N | N | N | N | Y | Y | Y | Y | N | 40.00% | 6 | [48] |
| L. A. B. da Silva et al., 2021 | N | N | Y | Y | N | N | N | N | N | N | Y | Y | N | Y | N | 33.33% | 5 | [49] |
| R. A. B. Da Silva et al., 2021 | N | Y | Y | Y | N | N | N | N | N | N | Y | Y | Y | Y | N | 46.67% | 7 | [50] |
| Pérez-Serrano et al., 2021 | N | Y | Y | Y | N | N | N | N | N | N | Y | N | Y | Y | N | 40.00% | 6 | [51] |
| Sharma et al., 2022 | Y | Y | Y | Y | N | N | N | N | N | N | Y | N | N | Y | N | 40.00% | 6 | [52] |
| H. S. S. Thein et al., 2022 | N | Y | Y | Y | N | N | N | N | N | N | Y | N | Y | Y | N | 40.00% | 6 | [5] |
| Item/Authour&Years | Yamanaka et al., 2013, [54] | Saraiva et al., 2018, [55] | Santos et al., 2019, [58] | Delfino et al., 2020, [56] | Vinola et al., 2021, [59] | Yang et al., 2022, [57] |
|---|---|---|---|---|---|---|
| Selection bias Sequence generation | NO | NOT | NO | NO | NO | NOT |
| Selection bias Baseline characteristics | NOT | NOT | NOT | NOT | NOT | YES |
| Selection bias Allocation concealment | NO | NO | NO | NO | NO | NO |
| Performance bias Random housing | NO | YES | NO | YES | NOT | NOT |
| Performance bias Blinding | NO | NO | NO | NO | NO | NO |
| Detection bias Random outcome assessment | NO | NO | NO | NO | NO | NO |
| Detection bias Blinding | YES | NOT | NOT | NOT | YES | NOT |
| Attrition bias Incomplete outcome data | YES | YES | YES | YES | YES | YES |
| Reporting bias Selective outcome reporting | YES | YES | YES | YES | YES | YES |
| Other Other sources of bias | YES | YES | YES | YES | YES | YES |
| Overall | 40.00% | 40.00% | 30.00% | 40.00% | 40.00% | 40.00% |
| 4 | 4 | 3 | 4 | 4 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Peters, O.A.; Hosseinpour, S. Immunomodulatory Effects of Endodontic Sealers: A Systematic Review. Dent. J. 2023, 11, 54. https://doi.org/10.3390/dj11020054
Guo J, Peters OA, Hosseinpour S. Immunomodulatory Effects of Endodontic Sealers: A Systematic Review. Dentistry Journal. 2023; 11(2):54. https://doi.org/10.3390/dj11020054
Chicago/Turabian StyleGuo, Jindong, Ove A. Peters, and Sepanta Hosseinpour. 2023. "Immunomodulatory Effects of Endodontic Sealers: A Systematic Review" Dentistry Journal 11, no. 2: 54. https://doi.org/10.3390/dj11020054
APA StyleGuo, J., Peters, O. A., & Hosseinpour, S. (2023). Immunomodulatory Effects of Endodontic Sealers: A Systematic Review. Dentistry Journal, 11(2), 54. https://doi.org/10.3390/dj11020054








