Turmeric-Fortified Cow and Soya Milk: Golden Milk as a Street Food to Support Consumer Health
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
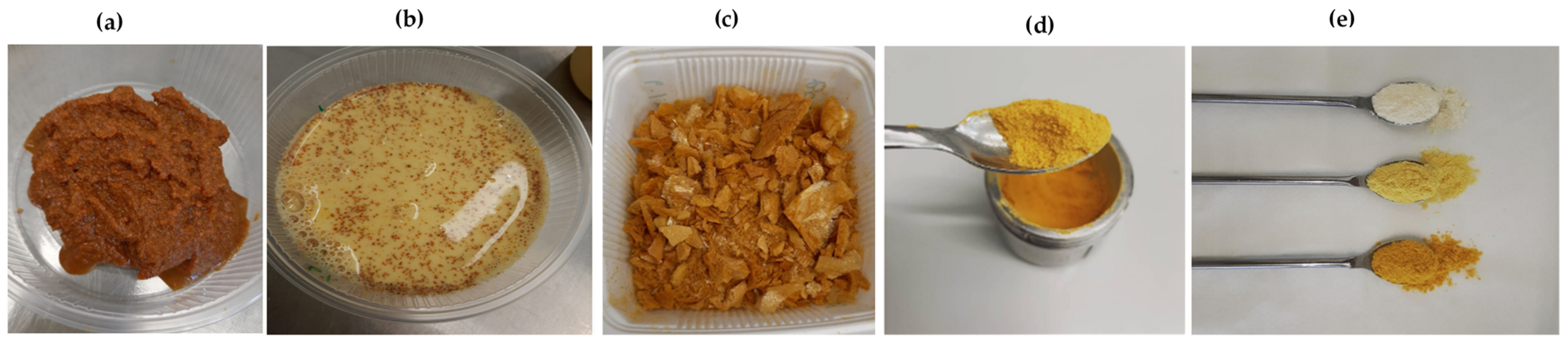
2.2. Preparation of Turmeric-Fortified Milk
2.3. Measurement of pH
2.4. Proximate Analysis
2.4.1. Moisture and Ash Content
2.4.2. Fat Content
2.4.3. Protein Content
2.5. Mineral Content
2.6. Total Phenol Content
2.7. Total Antioxidant Activity
2.8. Data Analysis
3. Results and Discussion
3.1. Proximate and Physicochemical Composition
3.2. Mineral Content
3.3. Total Phenol Content
3.4. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nwaekpe, J.; Anyaegbunam, H.; Okoye, B.; Asumugha, G. Promotion of turmeric for the food/pharmaceutical industry in Nigeria. J. Exp. Agric. Int. 2015, 8, 335–341. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Idowu-Adebayo, F.; Fogliano, V.; Oluwamukomi, M.O.; Oladimeji, S.; Linnemann, A.R. Food neophobia among Nigerian consumers: A study on attitudes towards novel turmeric-fortified drinks. J. Sci. Food Agric. 2021, 101, 3246–3256. [Google Scholar] [CrossRef]
- Idowu-Adebayo, F.; Toohey, M.J.; Fogliano, V.; Linnemann, A.R. Enriching street-vended zobo (Hibiscus sabdariffa) drink with turmeric (Curcuma longa) to increase its health-supporting properties. Food Funct. 2021, 12, 761–770. [Google Scholar] [CrossRef]
- Araujo, C.; Leon, L. Biological activities of Curcuma longa L. Memórias Inst. Oswaldo Cruz 2001, 96, 723–728. [Google Scholar] [CrossRef]
- Velayudhan, K.; Dikshit, N.; Nizar, M.A. Ethnobotany of turmeric (Curcuma longa L.). Indian J. Tradit. Knowl. 2012, 11, 607–614. [Google Scholar]
- Čosić, A.; Karić, A.; Šabanović, K.; Šutković, J.; Yildirim, A. Determination of GMO soy products in processed food from Bosnian market. Bioeng. Stud. 2020, 1, 14–20. [Google Scholar] [CrossRef]
- Mazumder, M.A.R.; Begum, A.A. Soy milk as source of nutrient for malnourished population of developing country: A review. Int. J. Adv. Sci. Tech. Res. 2016, 5, 192–203. [Google Scholar]
- Ezeigbo, O.; Ekaiko, M.; Kalu, T.; Nwodu, J. Quality Assessment of Soymilk Sold in Aba, Southeastern Nigeria. Int. J. Epidemiol. Infect. 2014, 2, 88–91. [Google Scholar] [CrossRef]
- Osuntogun, B.; Aboaba, O. Microbiological and physico-chemical evaluation of some non-alcoholic beverages. Pak. J. Nutr. 2004, 3, 188–192. [Google Scholar]
- Sęczyk, Ł.; Świeca, M.; Gawlik-Dziki, U. Soymilk enriched with green coffee phenolics–Antioxidant and nutritional properties in the light of phenolics-food matrix interactions. Food Chem. 2017, 223, 1–7. [Google Scholar] [CrossRef]
- Paul, A.A.; Kumar, S.; Kumar, V.; Sharma, R. Milk Analog: Plant based alternatives to conventional milk, production, potential and health concerns. Crit. Rev. Food Sci. Nutr. 2020, 60, 3005–3023. [Google Scholar] [CrossRef]
- Silván, J.M.; Amigo-Benavent, M.; del Castillo, M.D. Antioxidant Properties of Soy-Based Drinks and Effects of Processing. In Processing and Impact on Antioxidants in Beverages; Elsevier: Amsterdam, The Netherlands, 2014; pp. 225–232. [Google Scholar]
- Rizzo, G.; Baroni, L. Soy foods and their role in vegetarian diets. Nutrients 2018, 10, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanderhout, S.M.; Aglipay, M.; Torabi, N.; Jüni, P.; da Costa, B.R.; Birken, C.S.; O’Connor, D.L.; Thorpe, K.E.; Maguire, J.L. Whole milk compared with reduced-fat milk and childhood overweight: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2020, 111, 266–279. [Google Scholar] [CrossRef]
- Khan, I.T.; Nadeem, M.; Imran, M.; Ayaz, M.; Ajmal, M.; Ellahi, M.Y.; Khalique, A. Antioxidant capacity and fatty acids characterization of heat treated cow and buffalo milk. Lipids Health Dis. 2017, 16, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.; Murphy, P.A.; Sala, I. Isoflavone profiles of soymilk as affected by high-pressure treatments of soymilk and soybeans. Food Chem. 2008, 111, 592–598. [Google Scholar] [CrossRef]
- Ren, C.; Xiong, W.; Peng, D.; He, Y.; Zhou, P.; Li, J.; Li, B. Effects of thermal sterilization on soy protein isolate/polyphenol complexes: Aspects of structure, in vitro digestibility and antioxidant activity. Food Res. Int. 2018, 112, 284–290. [Google Scholar] [CrossRef]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein–phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- El-Sayed, S.M.; Youssef, A.M. Potential application of herbs and spices and their effects in functional dairy products. Heliyon 2019, 5, e01989. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, S.; Chakraborty, C.; Moitra, S.; Bandyopadhyay, K. Potential application of milk and milk products as carrier for herbs and spices: A Review. Int. J. Eng. Res. Sci. Technol. 2017, 6, 113–124. [Google Scholar]
- Yamamoto, N.; Shoji, M.; Hoshigami, H.; Watanabe, K.; Takatsuzu, T.; Yasuda, S.; Igoshi, K.; Kinoshita, H. Antioxidant capacity of soymilk yogurt and exopolysaccharides produced by lactic acid bacteria. Biosci. Microbiota Food Health 2019, 18-017. [Google Scholar] [CrossRef] [Green Version]
- Pratap, D.; Singh, R.; Ravichandran, C.; Ojha, A.; Upadhyay, A.; Kaur, B.P.; Senthilkumar, T. Evaluation of physicochemical, antioxidant, and sensory characteristics of khoa prepared from blends of soy and standardized milk. J. Food Process. Preserv. 2019, 43, e14215. [Google Scholar] [CrossRef]
- Fardet, A.; Rock, E. In vitro and in vivo antioxidant potential of milks, yoghurts, fermented milks and cheeses: A narrative review of evidence. Nutr. Res. Rev. 2018, 31, 52–70. [Google Scholar] [CrossRef] [Green Version]
- Park, H.; Lee, M.; Kim, K.-T.; Park, E.; Paik, H.-D. Antioxidant and antigenotoxic effect of dairy products supplemented with red ginseng extract. J. Dairy Sci. 2018, 101, 8702–8710. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Fan, F.; Xu, X.; Liang, Y. Interactions of black and green tea polyphenols with whole milk. Food Res. Int. 2013, 53, 449–455. [Google Scholar] [CrossRef]
- Ekanem, J.O. Microbial, sensory and nutritional properties of laboratory prepared sorrel (zobo) drinks fortified with spices and sugar. J. Glob. Biosci. 2018, 7, 5573–5584. [Google Scholar]
- Manirakiza, P.; Covaci, A.; Schepens, P. Comparative study on total lipid determination using Soxhlet, Roese-Gottlieb, Bligh & Dyer, and modified Bligh & Dyer extraction methods. J. Food Compos. Anal. 2001, 14, 93–100. [Google Scholar]
- Sáyago-Ayerdi, S.G.; Arranz, S.; Serrano, J.; Goñi, I. Dietary fiber content and associated antioxidant compounds in roselle flower (Hibiscus sabdariffa L.) beverage. J. Agric. Food Chem. 2007, 55, 7886–7890. [Google Scholar] [CrossRef]
- Meyer, S.; Markova, M.; Pohl, G.; Marschall, T.A.; Pivovarova, O.; Pfeiffer, A.F.H.; Schwerdtle, T. Development, validation and application of an ICP-MS/MS method to quantify minerals and (ultra-)trace elements in human serum. J. Trace Elem. Med. Biol. 2018, 49, 157–163. [Google Scholar] [CrossRef]
- Temminghoff, E.; Houba, V. Plant Analyses Procedures; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004. [Google Scholar]
- Vázquez, C.V.; Rojas, M.G.V.; Ramírez, C.A.; Chávez-Servín, J.L.; García-Gasca, T.; Martínez, R.A.F.; García, O.P.; Rosado, J.L.; López-Sabater, C.M.; Castellote, A.I. Total phenolic compounds in milk from different species. Design of an extraction technique for quantification using the Folin–Ciocalteu method. Food Chem. 2015, 176, 480–486. [Google Scholar] [CrossRef]
- Serpen, A.; Gökmen, V.; Fogliano, V. Solvent effects on total antioxidant capacity of foods measured by direct Quencher procedure. J. Food Compos. Anal. 2012, 26, 52–57. [Google Scholar] [CrossRef]
- Benabderrahim, M.A.; Yahia, Y.; Bettaieb, I.; Elfalleh, W.; Nagaz, K. Antioxidant activity and phenolic profile of a collection of medicinal plants from Tunisian arid and Saharan regions. Ind. Crops Prod. 2019, 138, 111427. [Google Scholar] [CrossRef]
- Hajirostamloo, B.; Mahastie, P. Comparison of nutritional and chemical parameters of soymilk and cow milk. World Acad. Sci. Eng. Technol. 2009, 57, 436–438. [Google Scholar]
- Woodside, J.V.; Brennan, S.; Cantwell, M. Are Soy-Milk Products Viable Alternatives to Cow’s Milk? In Beverage Impacts on Health and Nutrition; Springer: Berlin/Heidelberg, Germany, 2016; pp. 151–162. [Google Scholar]
- Han, J.; Chang, Y.; Britten, M.; St-Gelais, D.; Champagne, C.P.; Fustier, P.; Lacroix, M. Interactions of phenolic compounds with milk proteins. Eur. Food Res. Technol. 2019, 245, 1881–1888. [Google Scholar] [CrossRef]
- Bandyopadhyay, M.; Chakraborty, R.; Raychaudhuri, U. Incorporation of herbs into sandesh, an Indian sweet dairy product, as a source of natural antioxidants. Int. J. Dairy Technol. 2007, 60, 228–233. [Google Scholar] [CrossRef]
- Kihara, J.; Bolo, P.; Kinyua, M.; Rurinda, J.; Piikki, K. Micronutrient deficiencies in African soils and the human nutritional nexus: Opportunities with staple crops. Environ. Geochem. Health 2020, 42, 3015–3033. [Google Scholar] [CrossRef] [Green Version]
- Scholz-Ahrens, K.E.; Ahrens, F.; Barth, C.A. Nutritional and health attributes of milk and milk imitations. Eur. J. Nutr. 2020, 59, 19–34. [Google Scholar] [CrossRef]
- Nieves, J.W. Osteoporosis: The role of micronutrients. Am. J. Clin. Nutr. 2005, 81, 1232S–1239S. [Google Scholar] [CrossRef] [Green Version]
- Koffi, E.; Shewfelt, R.; Wicker, L. Storage stability and sensory analysis of uht-processed whey-banana beverages. J. Food Qual. 2005, 28, 386–401. [Google Scholar] [CrossRef]
- Nti, C.A.; Plahar, W.A.; Annan, N.T. Development and quality characteristics of shelf-stable soy-agushie: A residual by-product of soymilk production. Food Sci. Nutr. 2016, 4, 315–321. [Google Scholar] [CrossRef]
- Alozie Yetunde, E.; Udofia, U.S. Nutritional and sensory properties of almond (Prunus amygdalu Var. Dulcis) seed milk. World J. Dairy Food Sci. 2015, 10, 117–121. [Google Scholar]
- Marshall, M.R. Ash analysis. In Food analysis; Springer: Berlin/Heidelberg, Germany, 2010; pp. 105–115. [Google Scholar]
- Ertan, K.; Bayana, D.; Gokce, O.; Alatossava, J.T.; Yilmaz, Y.; Gursoy, O. Total antioxidant capacity and phenolic content of pasteurized and UHT-treated cow milk samples marketed in Turkey. Acad. Food J. 2017, 15, 103–108. [Google Scholar]
- Chávez-Servín, J.L.; Andrade-Montemayor, H.M.; Vázquez, C.V.; Barreyro, A.A.; García-Gasca, T.; Martínez, R.A.F.; Ramírez, A.M.O.; de la Torre-Carbot, K. Effects of feeding system, heat treatment and season on phenolic compounds and antioxidant capacity in goat milk, whey and cheese. Small Rumin. Res. 2018, 160, 54–58. [Google Scholar] [CrossRef]
- Apostolidis, E.; Kwon, Y.-I.; Shinde, R.; Ghaedian, R.; Shetty, K. Inhibition of Helicobacter pylori by fermented milk and soymilk using select lactic acid bacteria and link to enrichment of lactic acid and phenolic content. Food Biotechnol. 2011, 25, 58–76. [Google Scholar] [CrossRef]
- McClements, D.J.; Newman, E.; McClements, I.F. Plant-based milks: A review of the science underpinning their design, fabrication, and performance. Compr. Rev. Food Sci. Food Saf. 2019, 18, 2047–2067. [Google Scholar] [CrossRef] [Green Version]
- Ogori, A.F.; Amove, J.; Aduloju, P.; Sardo, G.; Okpala, C.O.R.; Bono, G.; Korzeniowska, M. Functional and Quality Characteristics of Ginger, Pineapple, and Turmeric Juice Mix as Influenced by Blend Variations. Foods 2021, 10, 525. [Google Scholar] [CrossRef]
- Amenu, K.; Agga, G.E.; Kumbe, A.; Shibiru, A.; Desta, H.; Tiki, W.; Dego, O.K.; Wieland, B.; Grace, D.; Alonso, S. Milk Symposium review: Community-tailored training to improve the knowledge, attitudes, and practices of women regarding hygienic milk production and handling in Borana pastoral area of southern Ethiopia. J. Dairy Sci. 2020, 103, 9748–9757. [Google Scholar] [CrossRef]
- Niero, G.; Penasa, M.; Currò, S.; Masi, A.; Trentin, A.; Cassandro, M.; De Marchi, M. Development and validation of a near infrared spectrophotometric method to determine total antioxidant activity of milk. Food Chem. 2017, 220, 371–376. [Google Scholar] [CrossRef]
- Niero, G.; Currò, S.; Costa, A.; Penasa, M.; Cassandro, M.; Boselli, C.; Giangolini, G.; De Marchi, M. Phenotypic characterization of total antioxidant activity of buffalo, goat, and sheep milk. J. Dairy Sci. 2018, 101, 4864–4868. [Google Scholar] [CrossRef]
- Michlova, T.; Dragounova, H.; Horníčková, Š.; Hejtmankova, A. Factors influencing the content of vitamins A and E in sheep and goat milk. Czech J. Food Sci. 2015, 33, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz-Ersan, L.; Ozcan, T.; Akpinar-Bayizit, A.; Sahin, S. Comparison of antioxidant capacity of cow and ewe milk kefirs. J. Dairy Sci. 2018, 101, 3788–3798. [Google Scholar] [CrossRef] [PubMed]
- Baghbadorani, S.T.; Ehsani, M.R.; Mirlohi, M.; Ezzatpanah, H.; Azadbakht, L.; Babashahi, M. Antioxidant capability of ultra-high temperature milk and ultra-high temperature soy milk and their fermented products determined by four distinct spectrophotometric methods. Adv. Biomed. Res. 2017, 6, 62. [Google Scholar] [PubMed]
- Naik, L.; Ankitha, R.; Sharma, A. Salubrious Curcumin Fortified Whey Beverage Formulation and Study its Antioxidant Property. Int. J. Sci. Eng. Manag. 2018, 3, 1–7. [Google Scholar]


| GAE (g/kg Sample) | |||
|---|---|---|---|
| Sample | Unboiled | Boiled | |
| Whole milk | 0% Turmeric | 0.01 ± 0.5 a,1 | 0.03 ± 0.2 a,1 |
| 2% Turmeric | 0.03 ± 0.2 a,2 | 0.04 ± 0.2 a,2 | |
| 6% Turmeric | 0.05 ± 0.4 a,3 | 0.05 ± 1.6 a,2 | |
| Skimmed milk | 0% Turmeric | 0.01 ± 0.1 a,1 | 0.02 ± 0.6 a,1 |
| 2% Turmeric | 0.04 ± 0.9 a,2 | 0.05 ± 2.2 a,1 | |
| 6% Turmeric | 0.08± 1.5 b,3 | 0.08 ± 1.2 a,1 | |
| Soya milk | 0% Turmeric | 0.09 ± 0.2 b,1 | 0.10 ± 1.7 b,1 |
| 2% Turmeric | 0.10 ± 1.1 b,1 | 0.11 ± 0.7 b,2 | |
| 6% Turmeric | 0.14 ± 1.6 c,2 | 0.13 ± 2.2 b,3 | |
| TEAC (mmol Trolox/kg Sample) | |||
|---|---|---|---|
| Sample | Unboiled | Boiled | |
| Whole milk | 0% Turmeric | 0.5± 0.1 a,1 | 0.1 ± 0.1 a,1 |
| 2% Turmeric | 3.8 ± 0.4 ab,2 | 1.4 ± 0.1 a,2 | |
| 6% Turmeric | 5.1 ± 1.1 a,2 | 5.3 ± 0.1 a,3 | |
| Skimmed milk | 0% Turmeric | 0.5 ± 0.4 a,1 | 0.4 ± 0.2 b,1 |
| 2% Turmeric | 2.9 ± 0.3 a,2 | 2.6 ± 0.4 a,2 | |
| 6% Turmeric | 5.6 ± 0.5 a,3 | 5.6 ± 0.1 a,3 | |
| Soymilk | 0% Turmeric | 3.4 ± 0.1 b,1 | 7.5 ± 0.3 a,1 |
| 2% Turmeric | 4.6 ± 0.2 b,1 | 11.6 ± 0.7 b,2 | |
| 6% Turmeric | 4.5 ± 0.8 a,1 | 17.7 ± 0.9 b,3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idowu-Adebayo, F.; Fogliano, V.; Linnemann, A. Turmeric-Fortified Cow and Soya Milk: Golden Milk as a Street Food to Support Consumer Health. Foods 2022, 11, 558. https://doi.org/10.3390/foods11040558
Idowu-Adebayo F, Fogliano V, Linnemann A. Turmeric-Fortified Cow and Soya Milk: Golden Milk as a Street Food to Support Consumer Health. Foods. 2022; 11(4):558. https://doi.org/10.3390/foods11040558
Chicago/Turabian StyleIdowu-Adebayo, Folake, Vincenzo Fogliano, and Anita Linnemann. 2022. "Turmeric-Fortified Cow and Soya Milk: Golden Milk as a Street Food to Support Consumer Health" Foods 11, no. 4: 558. https://doi.org/10.3390/foods11040558




