Effect of High Hydrostatic Pressure Intensity on Structural Modifications in Mealworm (Tenebrio molitor) Proteins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Methods
2.2.1. Soluble Protein Recovery
2.2.2. Proximate Composition of Mealworm Protein Extracts
2.2.3. High Hydrostatic Pressure Treatments of Mealworm Proteins
2.3. Analysis
2.3.1. Turbidity Measurement
2.3.2. Particle-Size Measurement
2.3.3. Intrinsic Fluorescence Spectroscopy Measurements
2.3.4. Surface Hydrophobicity
2.3.5. Transmission Electron Microscopy
2.3.6. Determination of Protein Profiles of Control and Pressure-Treated Mealworm Protein Extracts
2.3.7. Protein Identification by Mass Spectrometry
2.4. Statistical Analysis
3. Results
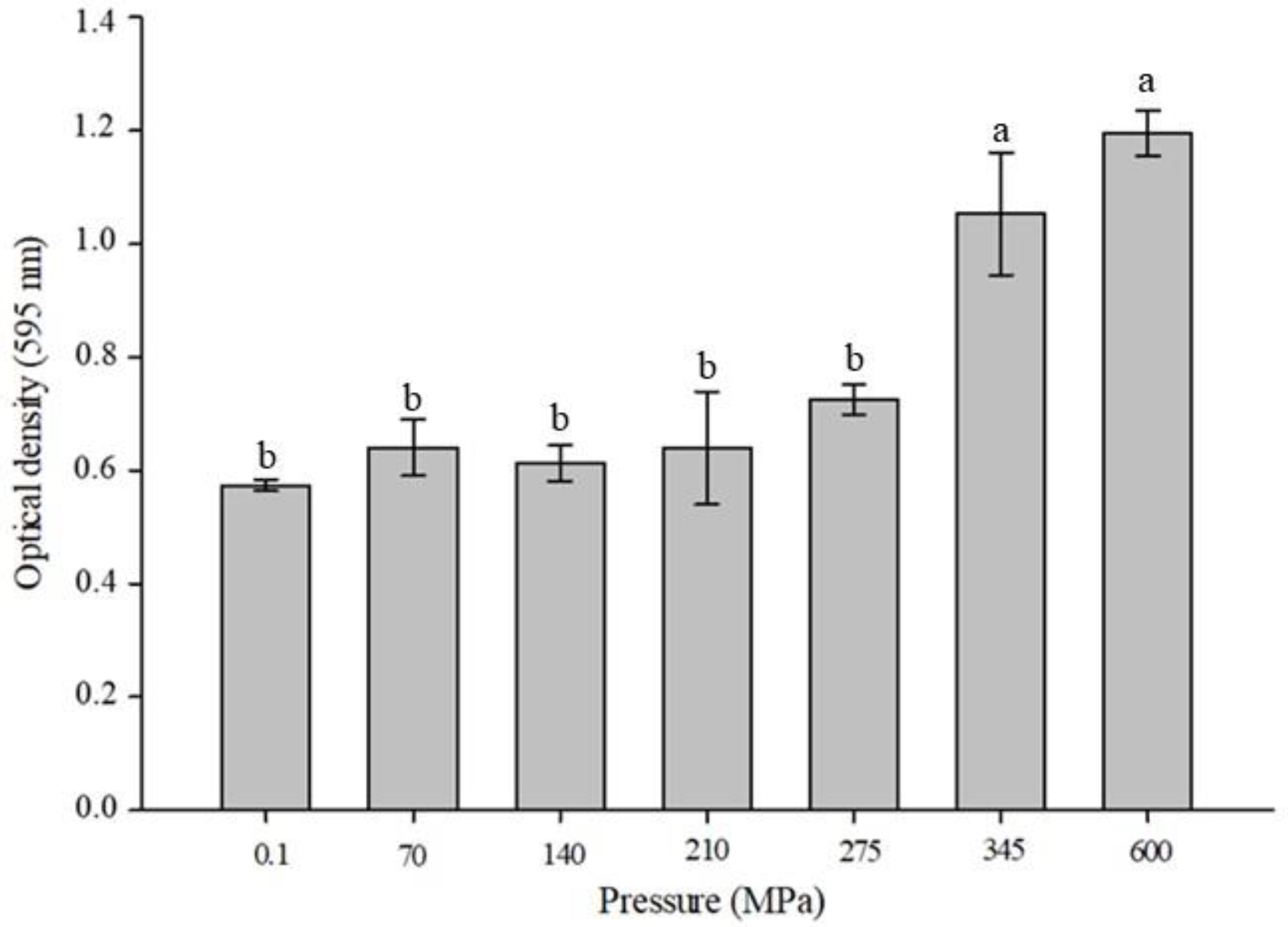
3.1. Change in Turbidity of Mealworm Proteins under HHP
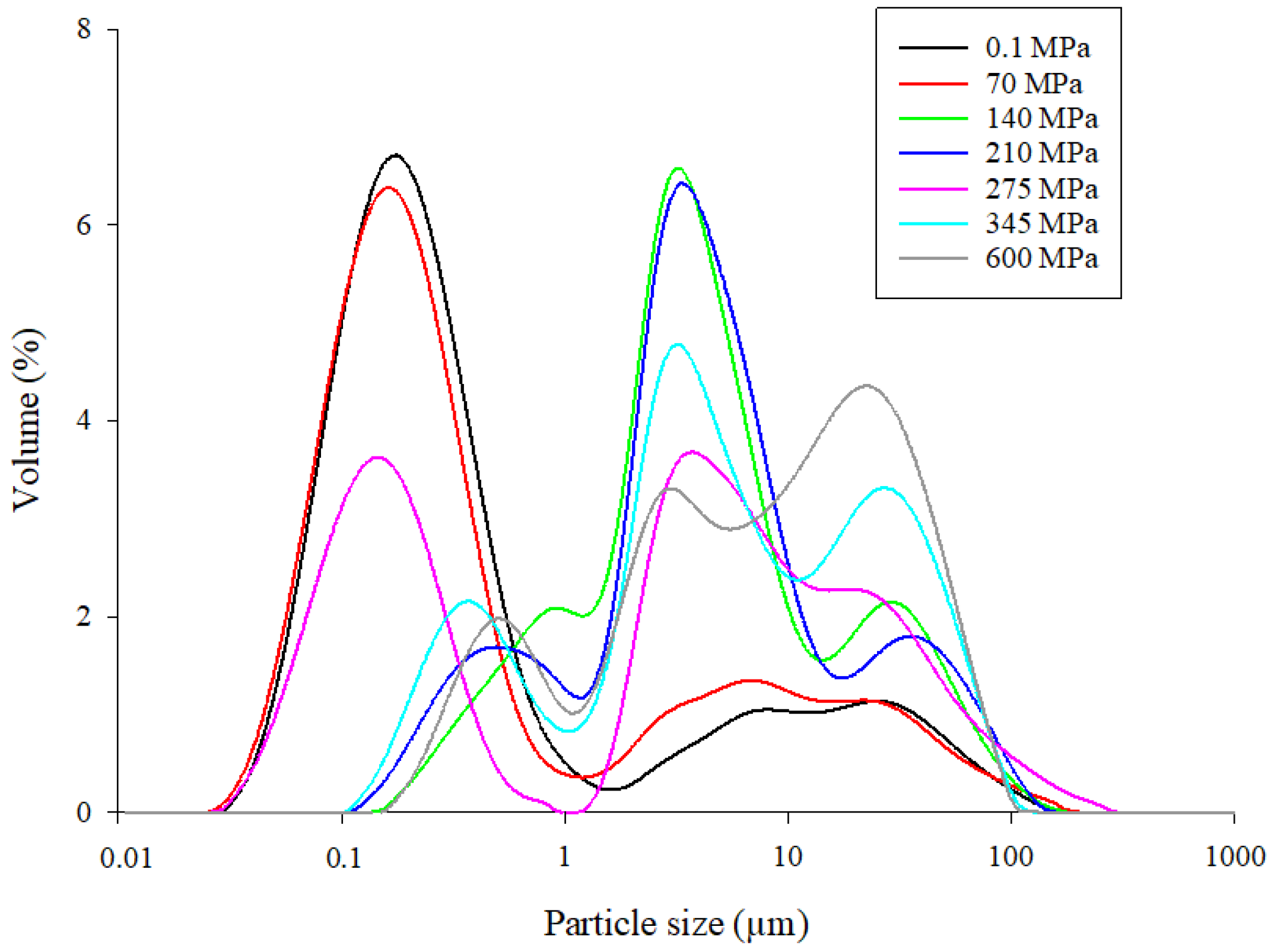
3.2. Particle-Size Distribution of Mealworm Proteins under HHP
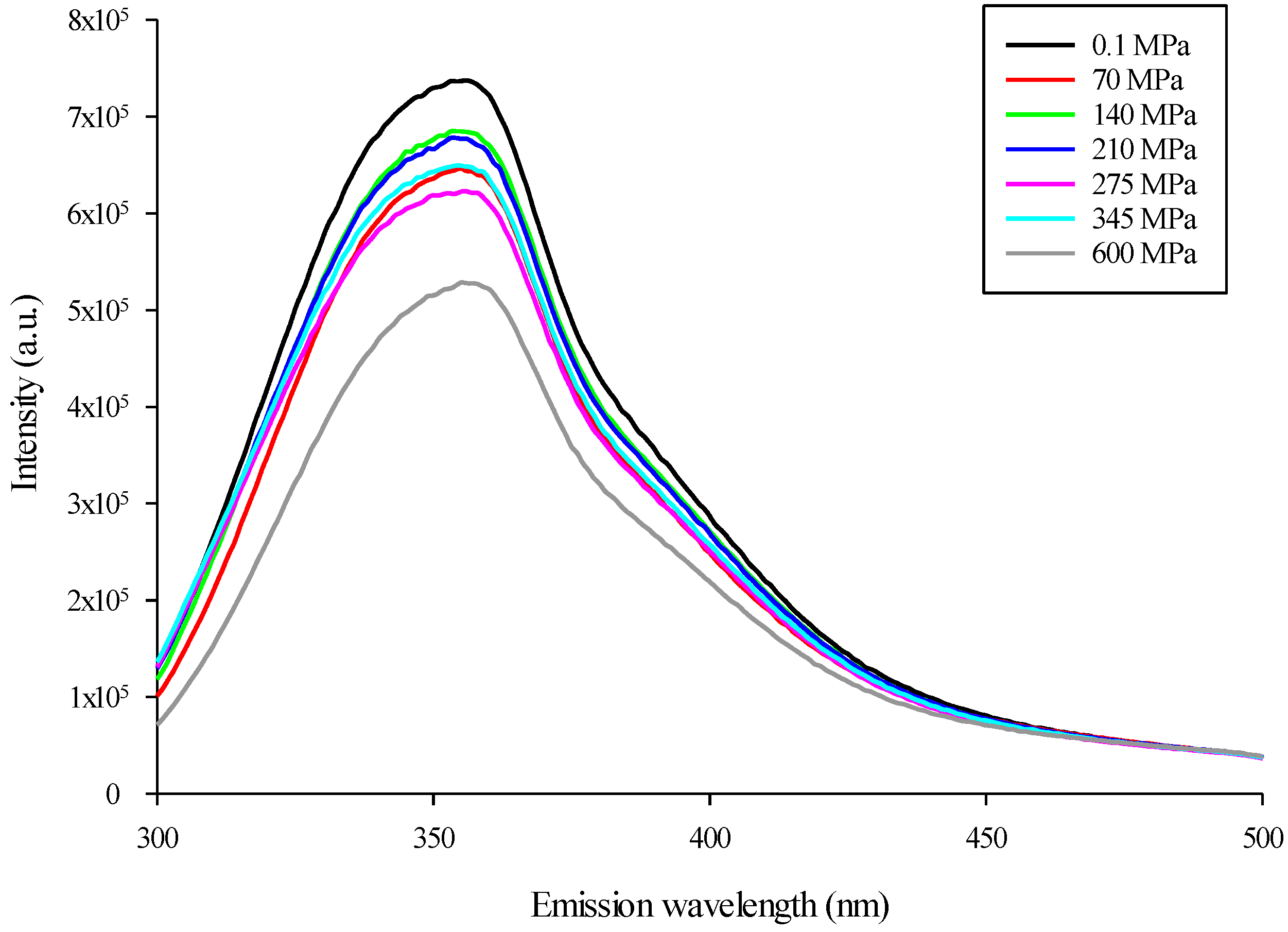
3.3. Modification of Mealworm Protein Intrinsic Fluorescence after HHP Treatment
3.4. Surface Hydrophobicity of HHP-Treated Mealworm Proteins
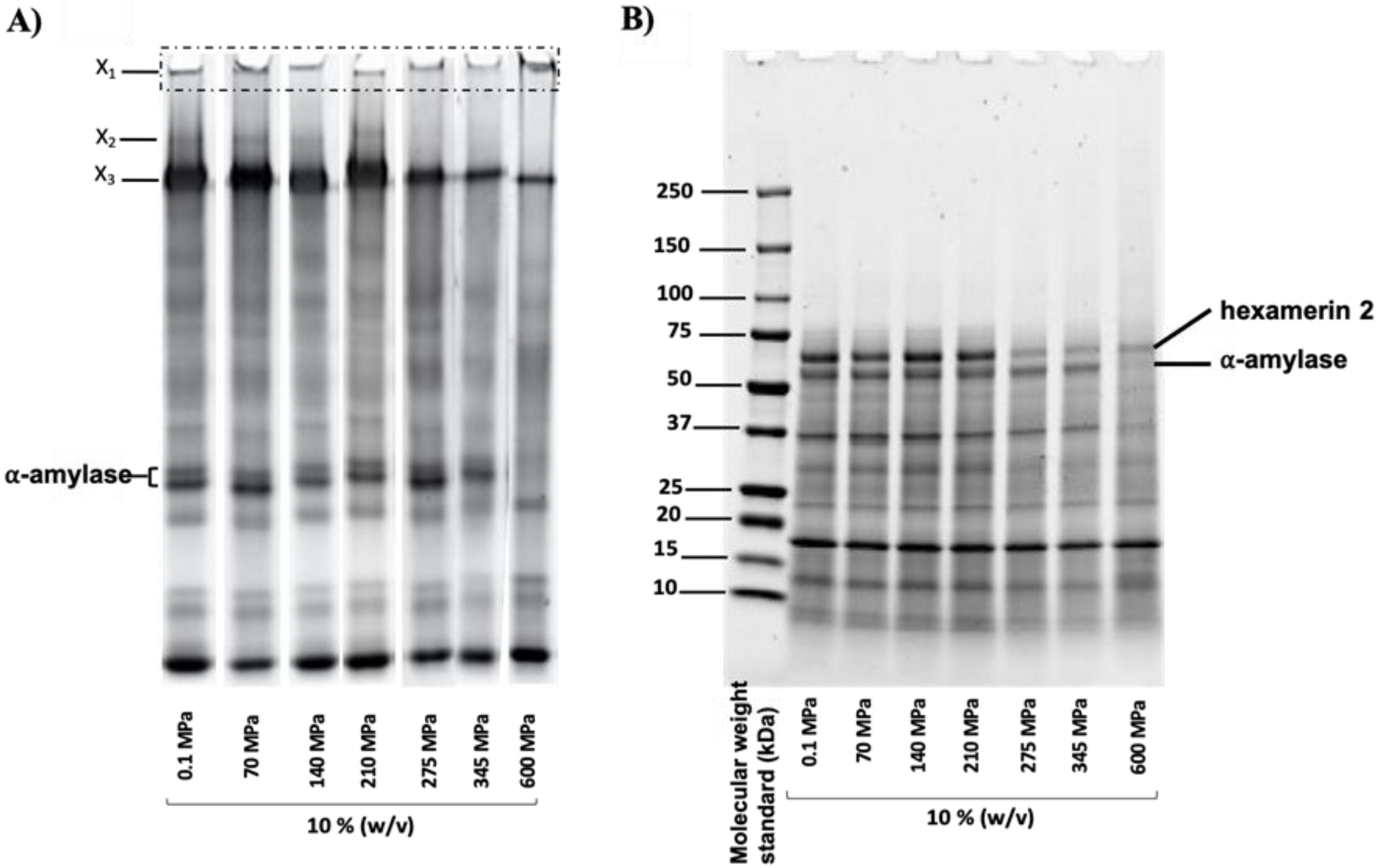
3.5. Protein Profile and Identification of Pressure-Induced Protein Aggregates
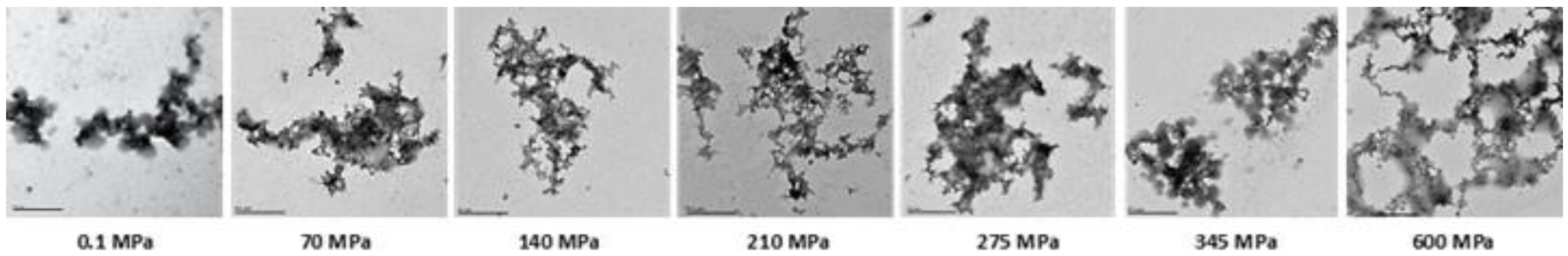
3.6. Microstructure of Pressure-Treated Mealworm Proteins
4. Discussion
4.1. Impact of High Hydrostatic Pressure on Mealworm Protein Structural Changes
4.2. Effect of Pressurization on Protein Profiles and Determination of Proteins Involved in Aggregate Formation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; Food and Agriculture Organization of the United Nations: Rome, Italia, 2013; ISBN 92-5-107596-4. [Google Scholar]
- Meyerrochow, V.B. Can Insects Help to Ease Problem of World Food Shortage. Search 1975, 6, 261–262. [Google Scholar]
- Hall, F.G.; Jones, O.G.; O’Haire, M.E.; Liceaga, A.M. Functional Properties of Tropical Banded Cricket (Gryllodes sigillatus) Protein Hydrolysates. Food Chem. 2017, 224, 414–422. [Google Scholar] [CrossRef]
- Hall, F.; Johnson, P.E.; Liceaga, A. Effect of Enzymatic Hydrolysis on Bioactive Properties and Allergenicity of Cricket (Gryllodes Sigillatus) Protein. Food Chem. 2018, 262, 39–47. [Google Scholar] [CrossRef]
- Purschke, B.; Brüggen, H.; Scheibelberger, R.; Jäger, H. Effect of Pre-Treatment and Drying Method on Physico-Chemical Properties and Dry Fractionation Behaviour of Mealworm Larvae (Tenebrio molitor L.). Eur. Food Res. Technol. 2018, 244, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Janssen, R.H.; Vincken, J.-P.; van den Broek, L.A.; Fogliano, V.; Lakemond, C.M. Nitrogen-to-Protein Conversion Factors for Three Edible Insects: Tenebrio Molitor, Alphitobius Diaperinus, and Hermetia Illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef]
- Wu, R.A.; Ding, Q.; Yin, L.; Chi, X.; Sun, N.; He, R.; Luo, L.; Ma, H.; Li, Z. Comparison of the Nutritional Value of Mysore Thorn Borer (Anoplophora chinensis) and Mealworm Larva (Tenebrio molitor): Amino Acid, Fatty Acid, and Element Profiles. Food Chem. 2020, 323, 126818. [Google Scholar] [CrossRef]
- Tan, H.S.G.; Fischer, A.R.; Tinchan, P.; Stieger, M.; Steenbekkers, L.P.A.; van Trijp, H.C. Insects as Food: Exploring Cultural Exposure and Individual Experience as Determinants of Acceptance. Food Qual. Prefer. 2015, 42, 78–89. [Google Scholar] [CrossRef]
- Stone, A.K.; Tanaka, T.; Nickerson, M.T. Protein Quality and Physicochemical Properties of Commercial Cricket and Mealworm Powders. J. Food Sci. Technol. 2019, 56, 3355–3363. [Google Scholar] [CrossRef]
- Boukil, A.; Perreault, V.; Chamberland, J.; Mezdour, S.; Pouliot, Y.; Doyen, A. High Hydrostatic Pressure-Assisted Enzymatic Hydrolysis Affect Mealworm Allergenic Proteins. Molecules 2020, 25, 2685. [Google Scholar] [CrossRef]
- Dion-Poulin, A.; Laroche, M.; Doyen, A.; Turgeon, S.L. Functionality of Cricket and Mealworm Hydrolysates Generated after Pretreatment of Meals with High Hydrostatic Pressures. Molecules 2020, 25, 5366. [Google Scholar] [CrossRef]
- Mozhaev, V.V.; Heremans, K.; Frank, J.; Masson, P.; Balny, C. Exploiting the Effects of High Hydrostatic Pressure in Biotechnological Applications. Trends Biotechnol. 1994, 12, 493–501. [Google Scholar] [CrossRef]
- Considine, T.; Patel, H.A.; Anema, S.G.; Singh, H.; Creamer, L.K. Interactions of Milk Proteins during Heat and High Hydrostatic Pressure Treatments—A Review. Innov. Food Sci. Emerg. Technol. 2007, 8, 1–23. [Google Scholar] [CrossRef]
- Visschers, R.W.; de Jongh, H.H.J. Disulphide Bond Formation in Food Protein Aggregation and Gelation. Biotechnol. Adv. 2005, 23, 75–80. [Google Scholar] [CrossRef]
- Boukil, A.; Suwal, S.; Chamberland, J.; Pouliot, Y.; Doyen, A. Ultrafiltration Performance and Recovery of Bioactive Peptides after Fractionation of Tryptic Hydrolysate Generated from Pressure-Treated β-Lactoglobulin. J. Membr. Sci. 2018, 556, 42–53. [Google Scholar] [CrossRef]
- Varunjikar, M.S.; Belghit, I.; Gjerde, J.; Palmblad, M.; Oveland, E.; Rasinger, J.D. Shotgun proteomics approaches for authentication, biological analyses, and allergen detection in feed and food-grade insect species. Food Control 2022, 137, 108888. [Google Scholar] [CrossRef]
- Giulia, L.; Tullia, T.; Pratesi, F.; Claudia, F.; Puxeddu, I.; Migliorini, P.; Natasja, G.; Johan, J.; Stefaan, D.; Caligiani, A. Shotgun Proteomics, in-Silico Evaluation and Immunoblotting Assays for Allergenicity Assessment of Lesser Mealworm, Black Soldier Fly and Their Protein Hydrolysates. Sci. Rep. 2020, 10, 1228. [Google Scholar]
- Yi, L.; Van Boekel, M.A.; Boeren, S.; Lakemond, C.M. Protein Identification and in Vitro Digestion of Fractions from Tenebrio Molitor. Eur. Food Res. Technol. 2016, 242, 1285–1297. [Google Scholar] [CrossRef] [Green Version]
- de Gier, S.; Verhoeckx, K. Insect (Food) Allergy and Allergens. Mol. Immunol. 2018, 100, 82–106. [Google Scholar] [CrossRef]
- Gravel, A.; Doyen, A. The Use of Edible Insect Proteins in Food: Challenges and Issues Related to Their Functional Properties. Innov. Food Sci. Emerg. Technol. 2020, 59, 102272. [Google Scholar] [CrossRef]
- Gkinali, A.-A.; Matsakidou, A.; Vasileiou, E.; Paraskevopoulou, A. Potentiality of Tenebrio Molitor Larva-Based Ingredients for the Food Industry: A Review. Trends Food Sci. Technol. 2022, 119, 495–507. [Google Scholar] [CrossRef]
- Kim, T.-K.; Yong, H.I.; Kang, M.-C.; Jung, S.; Jang, H.W.; Choi, Y.-S. Effects of High Hydrostatic Pressure on Technical Functional Properties of Edible Insect Protein. Food Sci. Anim. Resour. 2021, 41, 185–195. [Google Scholar] [CrossRef]
- Ugur, A.E.; Bolat, B.; Oztop, M.H.; Alpas, H. Effects of High Hydrostatic Pressure (HHP) Processing and Temperature on Physicochemical Characterization of Insect Oils Extracted from Acheta Domesticus (House cricket) and Tenebrio Molitor (Yellow Mealworm). Waste Biomass Valor 2021, 12, 4277–4286. [Google Scholar] [CrossRef]
- Bolat, B.; Ugur, A.E.; Oztop, M.H.; Alpas, H. Effects of High Hydrostatic Pressure Assisted Degreasing on the Technological Properties of Insect Powders Obtained from Acheta Domesticus & Tenebrio Molitor. J. Food Eng. 2021, 292, 110359. [Google Scholar] [CrossRef]
- Yi, L.; Lakemond, C.M.; Sagis, L.M.; Eisner-Schadler, V.; van Huis, A.; van Boekel, M.A. Extraction and Characterisation of Protein Fractions from Five Insect Species. Food Chem. 2013, 141, 3341–3348. [Google Scholar] [CrossRef]
- Wessels, M.L.J.; Azzollini, D.; Fogliano, V. Frozen Storage of Lesser Mealworm Larvae (Alphitobius diaperinus) Changes Chemical Properties and Functionalities of the Derived Ingredients. Food Chem. 2020, 320, 126649. [Google Scholar] [CrossRef]
- Möller, M.; Denicola, A. Protein tryptophan accessibility studied by fluorescence quenching. Biochem. Mol. Biol. Educ. 2002, 30, 175–178. [Google Scholar] [CrossRef]
- Mark, B.L.; McKenna, S.A.; Khajehpour, M. 1.11—Protein Structural Analysis. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Academic Press: Burlington, VT, USA, 2011; pp. 139–153. ISBN 978-0-08-088504-9. [Google Scholar]
- Nakai, S. Measurement of Protein Hydrophobicity. Curr. Protoc. Food Anal. Chem. 2003, 9, B5.2.1–B5.2.13. [Google Scholar] [CrossRef]
- Azagoh, C.; Ducept, F.; Garcia, R.; Rakotozafy, L.; Cuvelier, M.-E.; Keller, S.; Lewandowski, R.; Mezdour, S. Extraction and Physicochemical Characterization of Tenebrio Molitor Proteins. Food Res. Int. 2016, 88, 24–31. [Google Scholar] [CrossRef]
- Marciniak, A.; Suwal, S.; Brisson, G.; Britten, M.; Pouliot, Y.; Doyen, A. Studying a Chaperone-like Effect of Beta-Casein on Pressure-Induced Aggregation of Beta-Lactoglobulin in the Presence of Alpha-Lactalbumin. Food Hydrocoll. 2018, 84, 9–15. [Google Scholar] [CrossRef]
- Chao, D.; He, R.; Jung, S.; Aluko, R.E. Effect of Pressure or Temperature Pretreatment of Isolated Pea Protein on Properties of the Enzymatic Hydrolysates. Food Res. Int. 2013, 54, 1528–1534. [Google Scholar] [CrossRef]
- Hayakawa, S.; Nakai, S. Relationships of Hydrophobicity and Net Charge to the Solubility of Milk and Soy Proteins. J. Food Sci. 2006, 50, 486–491. [Google Scholar] [CrossRef]
- Chen, J.; Mu, T.; Zhang, M.; Goffin, D.; Sun, H.; Ma, M.; Liu, X.; Zhang, D. Structure, Physicochemical, and Functional Properties of Protein Isolates and Major Fractions from Cumin (Cuminum cyminum) Seeds. Int. J. Food Prop. 2018, 21, 685–701. [Google Scholar] [CrossRef] [Green Version]
- Lundgren, D.H.; Hwang, S.-I.; Wu, L.; Han, D.K. Role of Spectral Counting in Quantitative Proteomics. Expert Rev. Proteom. 2010, 7, 39–53. [Google Scholar] [CrossRef]
- Yong, Y.H.; Foegeding, E.A. Caseins: Utilizing Molecular Chaperone Properties to Control Protein Aggregation in Foods. J. Agric. Food Chem. 2010, 58, 685–693. [Google Scholar] [CrossRef]
- de Souza, H.K.S.; Bai, G.; Gonçalves, M.D.P.; Bastos, M. Whey Protein Isolate–Chitosan Interactions: A Calorimetric and Spectroscopy Study. Thermochim. Acta 2009, 495, 108–114. [Google Scholar] [CrossRef]
- Hsu, K.-C.; Ko, W.-C. Effect of Hydrostatic Pressure on Aggregation and Viscoelastic Properties of Tilapia (Orechromis niloticus) Myosin. J. Food Sci. 2001, 66, 1158–1162. [Google Scholar] [CrossRef]
- Kanno, C.; Mu, T.-H.; Hagiwara, T.; Ametani, M.; Azuma, N. Gel Formation from Industrial Milk Whey Proteins under Hydrostatic Pressure: Effect of Hydrostatic Pressure and Protein Concentration. J. Agric. Food Chem. 1998, 46, 417–424. [Google Scholar] [CrossRef]
- Van der Plancken, I.; Van Loey, A.; Hendrickx, M.E.G. Changes in Sulfhydryl Content of Egg White Proteins Due to Heat and Pressure Treatment. J. Agric. Food Chem. 2005, 53, 5726–5733. [Google Scholar] [CrossRef]
- Wang, J.-M.; Yang, X.-Q.; Yin, S.-W.; Zhang, Y.; Tang, C.-H.; Li, B.-S.; Yuan, D.-B.; Guo, J. Structural Rearrangement of Ethanol-Denatured Soy Proteins by High Hydrostatic Pressure Treatment. J Agric. Food Chem. 2011, 59, 7324–7332. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Lee, M.C.; Ravanfar, R.; Padilla-Zakour, O.I.; Abbaspourrad, A. The Impact of High-Pressure Processing on the Structure and Sensory Properties of Egg White-Whey Protein Mixture at Acidic Conditions. Food Bioprocess Technol. 2020, 13, 379–389. [Google Scholar] [CrossRef]
- David-Birman, T.; Raften, G.; Lesmes, U. Effects of Thermal Treatments on the Colloidal Properties, Antioxidant Capacity and in-Vitro Proteolytic Degradation of Cricket Flour. Food Hydrocoll. 2018, 79, 48–54. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Zhang, Z.; Chen, Z.; Jing, X.; Wang, X. Effect of High Hydrostatic Pressure Treatment on the Structure and Physicochemical Properties of Millet Gliadin. LWT 2022, 154, 112755. [Google Scholar] [CrossRef]
- Perreault, V.; Hénaux, L.; Bazinet, L.; Doyen, A. Pretreatment of Flaxseed Protein Isolate by High Hydrostatic Pressure: Impacts on Protein Structure, Enzymatic Hydrolysis and Final Hydrolysate Antioxidant Capacities. Food Chem. 2017, 221, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.A.; Singh, H.; Anema, S.G.; Creamer, L.K. Effects of Heat and High Hydrostatic Pressure Treatments on Disulfide Bonding Interchanges among the Proteins in Skim Milk. J. Agric. Food Chem. 2006, 54, 3409–3420. [Google Scholar] [CrossRef]
- Patel, H.A.; Singh, H.; Havea, P.; Considine, T.; Creamer, L.K. Pressure-Induced Unfolding and Aggregation of the Proteins in Whey Protein Concentrate Solutions. J. Agric. Food Chem. 2005, 53, 9590–9601. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, R.; Palmer, J.; Hemar, Y.; Yang, Z. Impact of High Hydrostatic Pressure on the Gelation Behavior and Microstructure of Quinoa Protein Isolate Dispersions. ACS Food Sci. Technol. 2021, 1, 2144–2151. [Google Scholar] [CrossRef]
- Bhattacharjee, C.; Das, K.P. Thermal Unfolding and Refolding of β-Lactoglobulin. Eur. J. Biochem. 2000, 267, 3957–3964. [Google Scholar] [CrossRef]
- Li, S.; Li, L.; Zhu, X.; Ning, C.; Cai, K.; Zhou, C. Conformational and Charge Changes Induced by L-Arginine and l-Lysine Increase the Solubility of Chicken Myosin. Food Hydrocoll. 2019, 89, 330–336. [Google Scholar] [CrossRef]
- Acero-Lopez, A.; Ullah, A.; Offengenden, M.; Jung, S.; Wu, J. Effect of High Pressure Treatment on Ovotransferrin. Food Chem. 2012, 135, 2245–2252. [Google Scholar] [CrossRef]
- Qu, W.; Zhang, X.; Han, X.; Wang, Z.; He, R.; Ma, H. Structure and Functional Characteristics of Rapeseed Protein Isolate-Dextran Conjugates. Food Hydrocoll. 2018, 82, 329–337. [Google Scholar] [CrossRef]
- Queirós, R.P.; Saraiva, J.A.; da Silva, J.A.L. Tailoring Structure and Technological Properties of Plant Proteins Using High Hydrostatic Pressure. Crit. Rev. Food Sci. Nutr. 2018, 58, 1538–1556. [Google Scholar] [CrossRef] [PubMed]
- Puppo, C.; Chapleau, N.; Speroni, F.; de Lamballerie-Anton, M.; Michel, F.; Añón, C.; Anton, M. Physicochemical Modifications of High-Pressure-Treated Soybean Protein Isolates. J. Agric. Food Chem. 2004, 52, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Guo, X.; Lin, Y.; Chen, J.; Liao, X.; Hu, X.; Wu, J. Effects of High Hydrostatic Pressure on Physicochemical and Functional Properties of Walnut (Juglans regia L.) Protein Isolate. J. Sci. Food Agric. 2013, 93, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, J.; Ji, D.; Lee, C. Effects of Heating Time and Temperature on Functional Properties of Proteins of Yellow Mealworm Larvae (Tenebrio molitor L.). Food Sci. Anim. Resour. 2019, 39, 296–308. [Google Scholar] [CrossRef]
- Rivero-Pino, F.; Espejo-Carpio, F.J.; Pérez-Gálvez, R.; Guadix, A.; Guadix, E.M. Effect of Ultrasound Pretreatment and Sequential Hydrolysis on the Production of Tenebrio Molitor Antidiabetic Peptides. Food Bioprod. Processing 2020, 123, 217–224. [Google Scholar] [CrossRef]
- Huppertz, T.; Fox, P.F.; Kelly, A.L. High Pressure Treatment of Bovine Milk: Effects on Casein Micelles and Whey Proteins. J. Dairy Res. 2004, 71, 97–106. [Google Scholar] [CrossRef]
- Jegouic, M.; Grinberg, V.Y.; Guingant, A.; Haertlé, T. Baric Oligomerization in α-Lactalbumin/β-Lactoglobulin Mixtures. J. Agric. Food Chem. 1997, 45, 19–22. [Google Scholar] [CrossRef]
- Hagner-Holler, S.; Schoen, A.; Erker, W.; Marden, J.H.; Rupprecht, R.; Decker, H.; Burmester, T. A Respiratory Hemocyanin from an Insect. Proc. Natl. Acad. Sci. USA 2004, 101, 871–874. [Google Scholar] [CrossRef] [Green Version]
- Burmester, T.; Schellen, K. Common Origin of Arthropod Tyrosinase, Arthropod Hemocyanin, Insect Hexamerin, and Dipteran Arylphorin Receptor. J. Mol. Evol. 1996, 42, 713–728. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, Y.; Zhao, Y. Structure-Based Modelling of Hemocyanin Allergenicity in Squid and Its Response to High Hydrostatic Pressure. Sci. Rep. 2017, 7, 40021. [Google Scholar] [CrossRef]
- Reinhart, G.; Gratton, E.; Mantulin, W.W. Dissociation of Large Oligomeric Proteins by High Hydrostatic Pressure: Dynamic Light Scattering Studies. In High Pressure Chemistry, Biochemistry and Materials Science; Winter, R., Jonas, J., Eds.; NATO ASI Series; Springer: Dordrecht, The Netherlands, 1993; pp. 619–626. ISBN 978-94-011-1699-2. [Google Scholar]
- Grauwet, T.; der Plancken, I.V.; Vervoort, L.; Hendrickx, M.E.; Loey, A.V. Investigating the Potential of Bacillus Subtilis α-Amylase as a Pressure-Temperature-Time Indicator for High Hydrostatic Pressure Pasteurization Processes. Biotechnol. Prog. 2009, 25, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.R.A.; Clark, R.; Ledward, D.A. Effects of High Pressure on Amylases and Starch in Wheat and Barley Flours. Food Chem. 1998, 63, 363–372. [Google Scholar] [CrossRef]
- Krüger, M.; Linke, W.A. The Giant Protein Titin: A Regulatory Node That Integrates Myocyte Signaling Pathways. J. Biol. Chem. 2011, 286, 9905–9912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivalain, N.; Roquain, J.; Demazeau, G. Development of High Hydrostatic Pressure in Biosciences: Pressure Effect on Biological Structures and Potential Applications in Biotechnologies. Biotechnol. Adv. 2010, 28, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Murchie, L.W.; Cruz-Romero, M.; Kerry, J.P.; Linton, M.; Patterson, M.F.; Smiddy, M.; Kelly, A.L. High Pressure Processing of Shellfish: A Review of Microbiological and Other Quality Aspects. Innov. Food Sci. Emerg. Technol. 2005, 6, 257–270. [Google Scholar] [CrossRef]
- Ko, W.-C.; Hwang, J.-S.; Jao, C.-L.; Hsu, K.-C. Denaturation of Tilapia Myosin Fragments by High Hydrostatic Pressure. J. Food Sci. 2004, 69, C604–C607. [Google Scholar] [CrossRef]
- Ko, W.C.; Jao, C.L.; Hsu, K.C. Effect of Hydrostatic Pressure on Molecular Conformation of Tilapia (Orechromis niloticus) Myosin. J. Food Sci. 2003, 68, 1192–1195. [Google Scholar] [CrossRef]
- Hsu, K.-C.; Hwang, J.-S.; Yu, C.-C.; Jao, C.-L. Changes in Conformation and in Sulfhydryl Groups of Actomyosin of Tilapia (Orechromis Niloticus) on Hydrostatic Pressure Treatment. Food Chem. 2007, 103, 560–564. [Google Scholar] [CrossRef]

| Pressure Level (MPa) | H0 (Slope × 106) * |
|---|---|
| 0.1 | 1.50 ± 0.03 b |
| 70 | 1.84 ± 0.14 b |
| 140 | 1.58 ± 0.20 b |
| 210 | 1.80 ± 0.11 b |
| 275 | 2.41 ± 0.01 a |
| 345 | 2.33 ± 0.02 a |
| 600 | 2.33 ± 0.02 a |
| Identified Proteins | UniProt ID | MW (kDa) | Total Spectrum Count (TSC) 1 | |
|---|---|---|---|---|
| 0.1 MPa | 600 MPa | |||
| Twitchin OS = Asbolus verrucosus OX = 1,661,398 GN = BDFB_000398 PE = 4 SV = 1 | A0A482W446_9CUCU | 995 | 230 | 79 |
| Hexamerin 2 OS = Tenebrio molitor OX = 7067 PE = 2 SV = 1 | Q95PI7_TENMO | 85 | 28 | 252 |
| Alpha-amylase OS = Tenebrio molitor OX = 7067 PE = 1 SV = 1 | AMY_TENMO | 51 | 4 | 122 |
| Myosin heavy chain, muscle isoform X13 OS = Asbolus verrucosus OX = 1,661,398 GN = BDFB_000378 PE = 3 SV = 1 | A0A482VBZ5_9CUCU | 256 | 17 | 5 |
| Actin-87E-like Protein OS = Tribolium castaneum OX = 7070 GN = TcasGA2_TC003326 PE = 3 SV = 1 | D6WF19_TRICA | 42 | 18 | 43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boukil, A.; Marciniak, A.; Mezdour, S.; Pouliot, Y.; Doyen, A. Effect of High Hydrostatic Pressure Intensity on Structural Modifications in Mealworm (Tenebrio molitor) Proteins. Foods 2022, 11, 956. https://doi.org/10.3390/foods11070956
Boukil A, Marciniak A, Mezdour S, Pouliot Y, Doyen A. Effect of High Hydrostatic Pressure Intensity on Structural Modifications in Mealworm (Tenebrio molitor) Proteins. Foods. 2022; 11(7):956. https://doi.org/10.3390/foods11070956
Chicago/Turabian StyleBoukil, Abir, Alice Marciniak, Samir Mezdour, Yves Pouliot, and Alain Doyen. 2022. "Effect of High Hydrostatic Pressure Intensity on Structural Modifications in Mealworm (Tenebrio molitor) Proteins" Foods 11, no. 7: 956. https://doi.org/10.3390/foods11070956
APA StyleBoukil, A., Marciniak, A., Mezdour, S., Pouliot, Y., & Doyen, A. (2022). Effect of High Hydrostatic Pressure Intensity on Structural Modifications in Mealworm (Tenebrio molitor) Proteins. Foods, 11(7), 956. https://doi.org/10.3390/foods11070956







