Fate of Listeria monocytogenes, Salmonella spp., and Shiga Toxin-Producing Escherichia coli on Slices of an All-Beef Soppressata during Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Inoculation of Soppressata Slices
2.3. Chemical Analyses
2.4. Microbiological Analyses
2.5. Statistical Methods
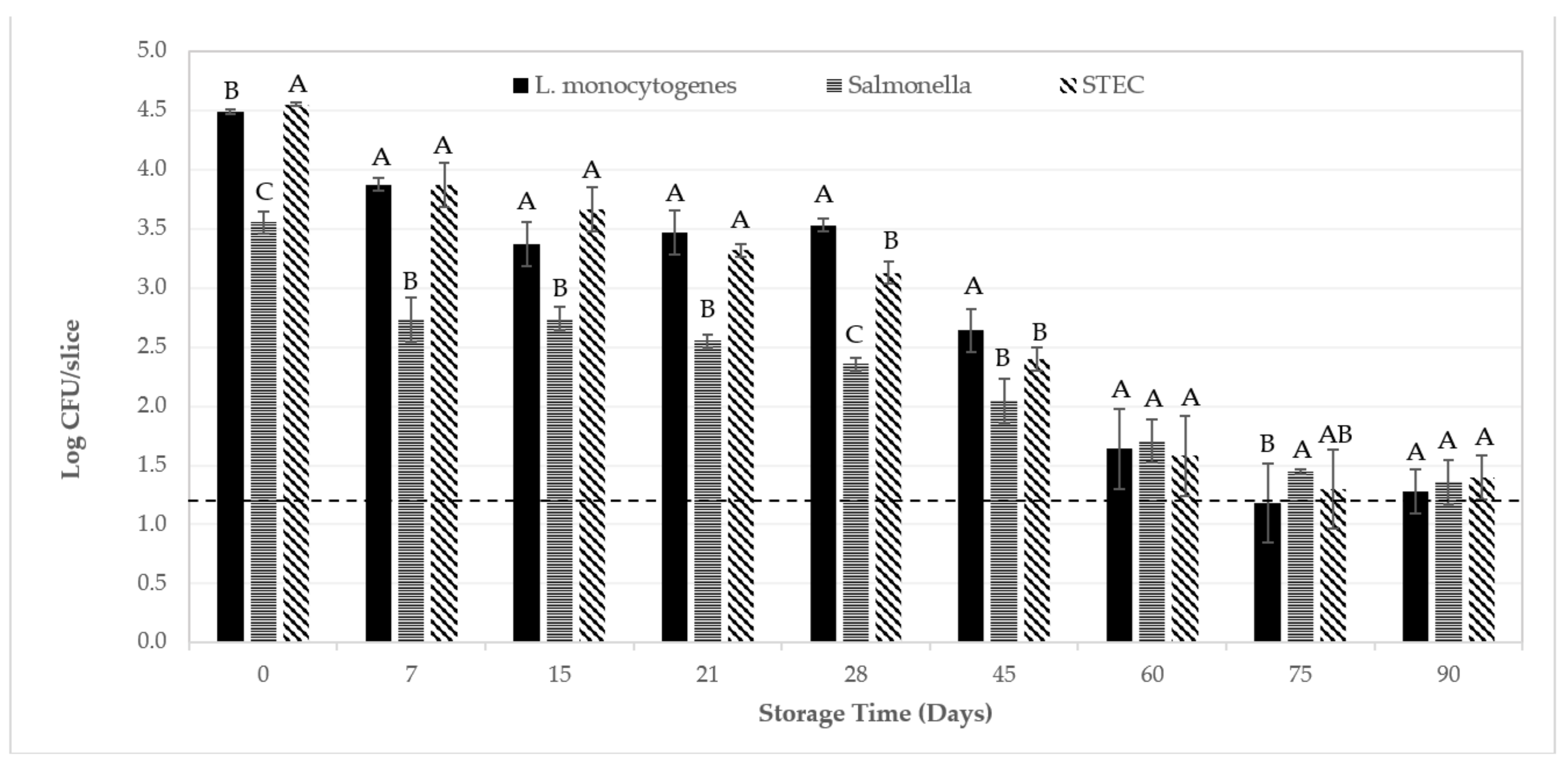
3. Results and Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hartman, P.A. The evolution of food microbiology. In Food Microbiology—Fundamentals and Frontiers, 4th ed.; Doyle, M.P., Buchanan, R.L., Eds.; ASM Press: Washington, DC, USA, 1997; pp. 3–12. [Google Scholar]
- Mitchell, E. History of Food Preservation Timeline–When did People Start to Preserve Food? 2022. Available online: https://dehydratorlab.com/history-of-food-preservation/ (accessed on 31 August 2022).
- Pérez-Álvarez, J.A.; Viuda-Martos, M.; Fernández-López, J. Salchichón (Spanish Dry-Cured Sausage). In Pork, 1st ed.; Munekata, P.E.S., Pateiro, M., Franco, D., Lorenzo, J.M., Eds.; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar] [CrossRef]
- Roca, M.; Incze, K. Fermented sausages. Food Rev. Int. 1990, 6, 91–118. [Google Scholar] [CrossRef]
- Toldrá, F. Improving the sensory quality of cured and fermented meat products. In Processed Meats—Improving Safety, Nutrition and Quality; Kerry, J.P., Kerry, J., Eds.; Woodhead Publishing: Cambridge, UK, 2011; pp. 508–526. [Google Scholar] [CrossRef]
- Zanardi, E.; Novelli, E. Italian salami, a comprehensive analysis. In Pork, 1st ed.; Munekata, P.E.S., Pateiro, M., Franco, D., Lorenzo, J.M., Eds.; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar] [CrossRef]
- Fatima, M. Beef Salami Guide and History: Is it a Healthy Meat? 2022. Available online: https://meatnmarrow.com/beef/beef-salami-guide/ (accessed on 1 September 2022).
- Miner, B. What is Soppressata? It’s Definitely Cured Meat but What Makes It Unique? 2020. Available online: www.ilporcellinodenver.com/blogs/bills-blog/what-is-soppressata (accessed on 17 December 2022).
- Centers for Disease Control and Prevention. Escherichia coli O157:H7 outbreak linked to commercially distributed dry-cured salami-Washington and California, 1994. Morb. Mortal. Wkly. Rep. 1995, 44, 157–160. [Google Scholar]
- Centers for Disease Control and Prevention. Community outbreak of hemolytic uremic syndrome attributable to Escherichia coli O111:NM—South Australia, 1995. Morb. Mortal. Wkly. Rep. 1995, 44, 550–557. [Google Scholar]
- Centers for Disease Control and Prevention. Multistate Outbreak of E. coli O157:H7 Infections Associated with Lebanon Bologna (FINAL UPDATE). 2011. Available online: https://www.cdc.gov/ecoli/2011/lebanon-bologna-3-23-11.html (accessed on 11 January 2023).
- Gieraltowski, L.; Julian, E.; Pringle, J.; Macdonald, K.; Quilliam, D.; Marsden-Haug, N.; Saathoff-Huber, L.; Von Stein, D.; Kissler, B.; Parish, M.; et al. Nationwide outbreak of Salmonella Montevideo infections associated with contaminated imported black and red pepper: Warehouse membership cards provide critical clues to identify the source. Epidemiol. Infect. 2013, 141, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Mataragas, M.; Bellio, A.; Rovetto, F.; Astegiano, S.; Greci, C.; Hertel, C.; Decatelli, L.; Cocolin, L. Quantification of persistence of the food-borne pathogens Listeria monocytogenes and Salmonella enterica during manufacture of Italian fermented sausages. Food Control 2015, 47, 552–559. [Google Scholar] [CrossRef]
- Meloni, D. Presence of Listeria monocytogenes in Mediterranean-style dry fermented sausages. Foods 2015, 4, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.E. Gastrointestinal outbreak associated with fermented meats. Meat Sci. 2004, 67, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Omer, M.K.; Ordonez, A.A.; Prieto, M.; Skjerve, E.; Asehun, T.; Alvseike, O.A. A systematic review of bacterial foodborne outbreaks related to red meat and meat products. Foodborne Pathog. Dis. 2018, 15, 598–610. [Google Scholar] [CrossRef]
- Thévenot, D.; Delignette-Muller, M.L.; Christieans, S.; Vernozy-Rozand, C. Prevalence of Listeria monocytogenes in 13 dried sausage processing plants and their products. Meat Sci. 2005, 102, 85–94. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture, Food Safety and Inspection Service. Pennsylvania Firm Recalls Lebanon Bologna Products Due to Possible E.coli O157:H7 Contamination. 2011. Available online: https://www.fsis.usda.gov/recalls-alerts/pennsylvania-firm-recalls-lebanon-bologna-products-due-possible-e.-coli-o157h7 (accessed on 30 January 2023).
- U.S. Department of Agriculture, Food Safety and Inspection Service. Olli Salumeria Americana Firm Recalls Ready-to-Eat Meat Products Due to Possible Listeria Contamination. 2018. Available online: https://www.fsis.usda.gov/wps/portal/fsis/topics/recalls-and-public-health-alerts/recall-case-archive/archive/2018/recall-018-2018-release (accessed on 11 January 2023).
- U.S. Department of Agriculture, Food Safety and Inspection Service. Ezzo Sausage Company Recalls Meat Products Due to Possible Listeria Contamination. 2019. Available online: https://www.fsis.usda.gov/wps/portal/fsis/topics/recalls-and-public-health-alerts/recall-case-archive/archive/2019/recall-111-2019-release (accessed on 11 January 2023).
- U.S. Department of Agriculture, Food Safety and Inspection Service. Daniele International LLC Recalls Ready-to-Eat Sausage Products due to Possible Listeria contamination. 2023. Available online: https://www.fsis.usda.gov/recalls-alerts/daniele-international-llc-recalls-ready-eat-sausage-products-due-possible-listeria (accessed on 11 March 2023).
- Centers for Disease Control and Prevention. Outbreak of Listeria Infections Linked to Deli Meats. 2021. Available online: https://www.cdc.gov/listeria/outbreaks/delimeat-10-20/index.html (accessed on 11 January 2023).
- Centers for Disease Control and Prevention. Salmonella Outbreak Linked to Italian-Style Meats. 2021. Available online: https://www.cdc.gov/salmonella/italian-style-meat-08-21/index.html (accessed on 11 January 2023).
- Centers for Disease Control and Prevention. Salmonella Outbreak Linked to Salami Sticks. 2021. Available online: https://www.cdc.gov/salmonella/i45-10-21/index.html (accessed on 11 January 2023).
- Centers for Disease Control and Prevention. List of Multistate Foodborne Outbreak Notices. 2022. Available online: www.cdc.gov/foodsafety/outbreaks/lists/outbreaks-list.html (accessed on 17 January 2023).
- Tilden, J.; Young, W.; McNamara, A.M.; Custer, C.; Boesel, B.; Lambert-Fair, M.A.; Majkowski, J.; Vugia, D.; Werner, S.B.; Hollingsworth, J.; et al. A new route of transmission for Escherichia coli O157:H7: Infection from dry fermented salami. Public Health Briefs 1996, 86, 1142–1145. [Google Scholar] [CrossRef]
- Alexander, E.R.; Boase, J.; Davis, M.; Kirchner, L.; Osaki, C.; Tanino, T.; Samadpour, P.; Goldoft, M.; Bradley, P.; Hinton, B.; et al. Escherichia coli O157:H7 outbreak linked to commercially distributed dry-cured salami. J. Am. Med. Assoc. 1995, 273, 985–986. [Google Scholar]
- Ethelberg, S.; Smith, B.; Torpdahl, M.; Lisby, M.; Boel, J.; Jensen, T.; Nielsen, E.M.; Malbak, K. Outbreak of non-O157 Shiga toxin-producing Escherichia coli infection from consumption of beef sausage. Clin. Infect. Dis. 2009, 48, e78–e81. [Google Scholar] [CrossRef] [PubMed]
- Barbuti, S.; Parolari, G. Validation of manufacturing process to control pathogenic bacteria in typical dry fermented products. Meat Sci. 2002, 62, 323–329. [Google Scholar] [CrossRef]
- Luchansky, J.B.; Shoyer, B.A.; Jung, Y.; Shane, L.E.; Osoria, M.; Porto-Fett, A.C.S. Viability of Shiga toxin-producing Escherichia coli, Salmonella, and Listeria monocytogenes within plant versus beef burgers during cold storage and following pan frying. J. Food Prot. 2020, 83, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International, 19th ed.; Association of Official Analytical Chemists (AOAC): Gaithersburg, MD, USA, 2012. [Google Scholar]
- Luchansky, J.B.; Porto, A.C.S.; Wallace, F.M.; Call, J.E. Recovery of Listeria monocytogenes from vacuum-sealed packages of frankfurters: Comparison of the U.S. Department of Agriculture (USDA) Food Safety and Inspection Service product composite enrichment method, the USDA Agricultural Research Service (ARS) product composite rinse method, and the USDA-ARS package rinse method. J. Food Prot. 2002, 65, 567–570. [Google Scholar]
- Downes, F.P.; Ito, K. Compendium of Methods for the Microbiological Examination of Foods, 4th ed.; American Public Health Association (APHA): Washington, DC, USA, 2001. [Google Scholar]
- SAS Institute. SAS v9.4, TS1M7, SAS/STAT v15.2; SAS Institute Inc.: Cary, NC, USA, 2021. [Google Scholar]
- Saxton, A.M. A macro for converting mean separation output to letter groupings in Proc Mixed. In Proceedings of the 23rd SAS Users Group International; Nashville, TN, USA, 22–25 March 1998, SAS Institute: Cary, NC, USA, 1998; pp. 1243–1246. [Google Scholar]
- Howard, H. Charcuterie: What’s Old Is New Again. Specialty Meats and Cheeses Still Reign Supreme in Delis. 2020. Available online: https://www.delibusiness.com/charcuterie-whats-old-is-new-again/ (accessed on 17 December 2022).
- Leroy, F.; De Vuyst, L. Fermented foods: Fermented meat products. In Encyclopedia of Food and Health, 1st ed.; Caballero, B., Finglas, P., Toldrá, F., Eds.; Academic Press: Waltham, MA, USA, 2016; pp. 656–660. [Google Scholar]
- Allen, L. The Perfect Charcuterie Board. 2021. Available online: https://tastesbetterfromscratch.com/charcuterie-board/ (accessed on 18 December 2022).
- Bisceglia, B. Get on Board with Charcuterie. 2022. Available online: https://asicentral.com/news/web-exclusive/july-2022/infographic-get-on-board-with-charcuterie/ (accessed on 18 December 2022).
- Schilling, W. Charcuterie: A Marriage of Art and Science. 2022. Available online: https://www.ift.org/news-and-publications/food-technology-magazine/issues/2022/february/columns/processing-applied-science-artisan-meat (accessed on 10 February 2023).
- Calicioglu, M.; Faith, N.G.; Buege, D.; Luchansky, J.B. Viability of Escherichia coli O157:H7 in fermented semidry low temperature-cooked, beef summer sausage. J. Food Prot. 1997, 60, 1158–1162. [Google Scholar] [CrossRef]
- Gill, A.O.; Ramaswamy, H.S. Application of high pressure processing to kill Escherichia coli O157 in ready-to-eat meats. J. Food Prot. 2008, 71, 2182–2189. [Google Scholar] [CrossRef]
- Holck, A.L.; Axelsson, L.; Rode, T.M.; Høy, M.; Måge, I.; Alvseike, O.; L’Abée-Lund, T.M.; Omer, M.K.; Granum, P.E.; Heir, E. Reduction of verotoxigenic Escherichia coli in production of fermented sausages. Meat Sci. 2011, 89, 286–295. [Google Scholar] [CrossRef]
- Jacob, R.; Porto-Fett, A.C.S.; Call, J.E.; Luchansky, J.B. Fate of surface inoculated Escherichia coli O157:H7, Listeria monocytogenes, and Salmonella typhimurium on kippered beef during storage at refrigeration and abusive temperatures. J. Food Prot. 2009, 72, 403–407. [Google Scholar] [CrossRef]
- Karolenko, C.E.; Bhusal, A.; Nelson, J.L.; Muriana, P.M. Processing of biltong (dried beef) to achieve USDA-FSIS 5-log reduction of Salmonella without a heat lethality step. Microorganisms 2020, 8, 791. [Google Scholar] [CrossRef]
- Luchansky, J.B.; Mayhew, M.; Jung, Y.; Klinedinst, A.; Harkins, L.; Shane, L.E.; Osoria, M.; Mcgeary, L.; Traugher, Z.; Shoyer, B.A.; et al. Meat bars: A survey to assess consumer familiarity and preparation parameters and a challenge study to quantify viability of Shiga toxin-producing Escherichia coli cells during processing and storage. J. Food Prot. 2019, 82, 1249–1264. [Google Scholar] [CrossRef]
- Luchansky, J.B.; Shoyer, B.A.; Shane, L.E.; Osoria, M.; Campano, S.G.; Porto-Fett, A.C.S. Inactivation of Listeria monocytogenes and Shiga toxin-producing Escherichia coli in “soupie”, a homemade soppressata. Food Prot. Trends 2022, 42, 48–57. [Google Scholar] [CrossRef]
- Miraglia, V.; Finazzi, G.; Daminelli, P.; Bonometti, E.; Gregorelli, M.; Boni, P. Behaviour of Listeria monocytogenes in chunked or sliced seasoned Bresaola della Vatellina IGP. Ind. Alimentari. 2009, 48, 58–64. [Google Scholar]
- Porto-Fett, A.C.S.; Hwang, C.-A.; Call, J.E.; Juneja, V.K.; Ingham, S.C.; Ingham, B.H.; Luchansky, J.B. Viability of multi-strain mixtures of Listeria monocytogenes, Salmonella typhimurium, or Escherichia coli O157:H7 inoculated into the batter or onto the surface of a soudjouk-style fermented semi-dry sausage. Food Microbiol. 2008, 25, 793–801. [Google Scholar] [CrossRef]
- Porto-Fett, A.C.S.; Call, J.E.; Luchansky, J.B. Validation of a commercial process for inactivation of Escherichia coli O157:H7, Salmonella, and Listeria monocytogenes on the surface of whole muscle beef jerky strips. J. Food Prot. 2008, 71, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Porto-Fett, A.C.S.; Pierre, J.; Shoyer, B.A.; Luchansky, J.B. Effect of storage temperature and cooking times on viability of Listeria monocytogenes and Escherichia coli O157:H7 in/on goetta. J. Food Saf. 2013, 33, 128–136. [Google Scholar] [CrossRef]
- Porto-Fett, A.C.S.; McCoy, A.; Shane, L.E.; Henry, E.; Osoria, M.; Shoyer, B.A.; Campano, S.G.; Burson, D.; Luchansky, J.B. Fate of Listeria monocytogenes and Shiga toxin-producing Escherichia coli on bresaola slices during storage. Meat Muscle Biol. 2022, 6, 13918. [Google Scholar] [CrossRef]
- Stoltenberg, S.K.; Getty, K.J.K.; Thippareddi, H.; Phebus, R.K.; Loughin, T.M. Fate of Escherichia coli O157:H7 during production of snack sticks made from beef or a venison/beef fat blend and directly acidified with citric or lactic acid. J. Food Sci. 2006, 71, M228–M235. [Google Scholar] [CrossRef]
- Watson, S.; Gaydos, N.J.; Egolf, S.R.; Campbell, J.A. Fate of Escherichia coli O157:H7, Salmonella spp., and Listeria monocytogenes during curing and drying of beef bresaola. Meat Muscle Biol. 2021, 5, 1–8. [Google Scholar] [CrossRef]
- Berry, D. Keeping Charcuterie Safe. 2022. Available online: https://www.meatpoultry.com/articles/27733-keeping-charcuterie-safe (accessed on 10 January 2023).
- Liguori, A.; Belsito, E.L.; Di Gioia, M.L.; Leggio, A.; Malagrinò, F.; Romio, E.; Siciliano, C.; Tagarelli, A. GC/MS analysis of fatty acids in Italian dry fermented sausages. Open Food Sci. J. 2015, 9, 5–13. [Google Scholar] [CrossRef]
- Jung, J.-H.; Shim, K.-S.; Shin, D. Effects of ripening duration and rosemary powder addition on salchichon modified sausage quality. Asian-Australas. J. Anim. Sci. 2015, 28, 671–676. [Google Scholar] [CrossRef]
- Romeo, F.V.; Runcio, A.; Piscopo, A.; Iaccarino, T.; Mincione, A.; Poiana, M. Characterization of four typical Calabrian cured meat products: Spicy sausage, soppressata, ’nduja and capocollo. Acta Aliment. 2014, 43, 564–573. [Google Scholar] [CrossRef]
- Coppola, R.; Iorizzo, M.; Saotta, R.; Sorrentino, E.; Grazia, L. Characterization of micrococci and staphylococci isolated from soppressata molisana, a Southern Italy fermented sausage. Food Microbiol. 1997, 14, 47–53. [Google Scholar] [CrossRef]
- Matthews, K.R.; Kniel, K.E.; Montville, T.J. Listeria monocytogenes. In Food Microbiology: An Introduction, 4th ed.; ASM Press: Washington, DC, USA, 2017; pp. 223–242. [Google Scholar]
- Jay, J.M. Foodborne gastroenteritis caused by Salmonella and Shigella. In Modern Food Microbiology, 6th ed.; Aspen Publishers: Gaithersburg, MD, USA, 2000; pp. 511–530. [Google Scholar]
- Bailey, S.; Richardson, L.J.; Cox, N.A.; Cosby, D.E. Salmonella. In Pathogens and Toxins in Foods: Challenges and Interventions; Juneja, V.K., Sofos, J.N., Eds.; ASM Press: Washington, DC, USA, 2010; pp. 108–118. [Google Scholar]
- Glass, K.A.; Loeffelholz, J.M.; Ford, J.P.; Doyle, M.P. Fate of Escherichia coli O157:H7 as affected by pH or sodium chloride and in fermented, dry sausage. Appl. Environ. Microbiol. 1992, 58, 2513–2516. [Google Scholar] [CrossRef]
- Getty, K.J.K.; Phebus, R.K.; Marsden, J.L.; Fung, D.Y.C.; Kastner, C.L. Escherichia coli O157:H7 and fermented sausages: A review. J. Rapid Methods Autom. Microbiol. 2000, 8, 141–225. [Google Scholar] [CrossRef]
- Sperber, W.H. Influence of water activity on foodborne bacteria—A review. J. Food Prot. 1983, 46, 142–150. [Google Scholar] [CrossRef] [PubMed]
- De Cesare, A.; Mioni, R.; Manfreda, G. Prevalence of Listeria monocytogenes in fresh and fermented Italian sausages and ribotyping of contaminating strains. Int. J. Food Microbiol. 2007, 120, 124–130. [Google Scholar] [CrossRef]
- Quaglia, N.C.; Storelli, M.M.; Ioanna, F.; Celano, G.; Celano, G.V.; Conversano, C.; De Rosa, M.; Dambrosio, A. Listeria monocytogenes and enterotoxigenic Staphylococcus aureus in dry fermented sausages belonging to “Traditional Agri-Food Product” produced in Southern Italy. J. Food Saf. 2019, 39, e12685. [Google Scholar] [CrossRef]
- Hussein, H.S.; Bollinger, L.M. Prevalence of Shiga toxin-producing Escherichia coli in beef. Meat Sci. 2005, 71, 676–689. [Google Scholar] [CrossRef]
- Levine, P.; Rose, B.; Green, S.; Ransom, G.; Hill, W. Pathogen testing of ready-to-eat meat and poultry products collected at federally inspected establishments in the United States, 1990 to 1999. J. Food Prot. 2001, 64, 1188–1193. [Google Scholar] [CrossRef]
- Cabedo, L.; Picart i Barrot, L.; Teixidó i Canelles, A. Prevalence of Listeria monocytogenes and Salmonella in ready-to-eat food in Catalonia, Spain. J. Food Prot. 2008, 71, 855–859. [Google Scholar] [CrossRef]
- Glass, K.A.; Kaspar, C.W.; Sindelar, J.J.; Milkowski, A.L.; Lotz, B.M.; Kang, J.; Faith, N.G.; Enache, E.; Katoaka, A.; Henry, C. Validation of pepperoni process for control of Shiga toxin-producing Escherichia coli. J. Food Prot. 2012, 75, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Heir, E.; Holck, A.L.; Omer, M.K.; Alvseike, O.; Mage, I.; Hoy, M.; Rode, T.M.; Sidhu, M.S.; Axelsson, L. Effects of post-processing treatments on sensory quality and Shiga toxigenic Escherichia coli reductions in dry-fermented sausages. Meat Sci. 2013, 94, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Hinkens, J.C.; Faith, N.G.; Lorang, T.D.; Bailey, P.; Buege, D.; Kaspar, C.W.; Luchansky, J.B. Validation of pepperoni processes for control of Escherichia coli O157:H7. J. Food Prot. 1996, 59, 1260–1266. [Google Scholar] [CrossRef]
- Ihnot, A.M.; Roering, A.M.; Wierzba, R.K.; Faith, N.G.; Luchansky, J.B. Behavior of Salmonella typhimurium DT104 during the manufacture and storage of pepperoni. Int. J. Food Microbiol. 1998, 40, 117–121. [Google Scholar] [CrossRef]
- Nelson, A. Consumers Continue to Crave Craft Meats. 2018. Available online: https://www.foodbusinessnews.net/articles/12821-consumers-continue-to-crave-craft-meats (accessed on 3 January 2023).
- Porto-Fett, A.C.S.; Call, J.E.; Shoyer, B.A.; Hill, D.E.; Pshebniski, C.; Cocoma, G.J.; Luchansky, J.B. Evaluation of fermentation, drying, and/or high pressure processing on viability of Listeria monocytogenes, Escherichia coli O157:H7, Salmonella spp., and Trichinella spiralis in raw pork and genoa salami. Int. J. Food Microbiol. 2010, 140, 61–75. [Google Scholar] [CrossRef]
- Bonilauri, P.; Grisenti, M.S.; Daminelli, P.; Merialdi, G.; Ramini, M.; Bardasi, L.; Taddei, R.; Coscian-Cunico, E.; Dalzini, E.; Frustoli, M.A.; et al. Reduction of Salmonella spp. populations in Italian salami during production process and high pressure processing treatment: Validation of processes to export to the U.S. Meat Sci. 2019, 157, 107869. [Google Scholar] [CrossRef] [PubMed]

| Bacterial Strains | Strain Designation | Source | Type 1 |
|---|---|---|---|
| L. monocytogenes | MFS2 | Environmental isolate from a pork processing plant | 1/2a |
| H7776 | Frankfurter isolate, 1998 outbreak | 4b | |
| ScottA | Clinical isolate, 1983 Massachusetts pasteurized milk outbreak | 4b | |
| LM-101M | Beef and pork sausage isolate | 4b | |
| F6854 | Turkey frankfurter isolate | 1/2a | |
| S. Typhimurium | MFS 248 | Hog carcass isolate | |
| S. Typhimurium | MFS 330 | Hog carcass isolate | |
| S. Typhimurium | FSIS OB060362 | Clinical isolate associated with pork sausage | |
| S. Typhimurium | H3380 | Clinical isolate | DT104 |
| S. Copenhagen | MFS 3446 | Pork isolate | |
| STEC | H30 | Infant with diarrhea | O26:H11 |
| CDC 96-3285 | Human stool | O45:H2 | |
| CDC 90-3128 | Human stool | O103:H2 | |
| ATCC BAA-2326 | Human stool | O104:H4 | |
| JB1-95 | Clinical isolate | O111:H- | |
| CDC 97-3068 | Human stool | O121:H19 | |
| 83-75 | Human stool | O145:NM | |
| USDA FSIS 011-82 | Meat isolate | O157:H7 |
| Analyte | Brand A 2 | Brand B 2 | Brand C 2 | Average All Brands |
|---|---|---|---|---|
| Ash (%) | 6.90 ± 0.18 3,A | 6.48 ± 0.05 B | 5.20 ± 0.03 C | 6.19 ± 0.88 |
| Carbohydrates (%) | 2.85 ± 0.70 A | 3.38 ± 1.78 A | 1.13 ± 0.74 A | 2.45 ± 1.18 |
| Fat (%) | 26.34 ± 5.03 A | 32.07 ± 4.04 A | 23.77 ± 0.42 A | 27.39 ± 4.25 |
| Moisture (%) | 35.51 ± 2.85 A | 30.66 ± 5.55 A | 42.06 ± 0.85 A | 36.07 ± 5.72 |
| Protein (%) | 28.42 ± 1.29 A | 27.42 ± 0.33 A | 27.85 ± 1.20 A | 27.90 ± 0.50 |
| Salt (%) | 5.43 ± 0.17 A | 5.27 ± 0.08 A | 4.02 ± 0.02 B | 4.90 ± 0.77 |
| Nitrite (ppm) | <5.0 ppm ± 0.0 A | <5.0 ppm ± 0.0 A | <5.0 ppm ± 0.0 A | <5.0 ppm ± 0.0 |
| Acidity (%; as lactic acid) | 2.95 ± 0.91 A | 2.39 ± 0.63 A | 2.54 ± 0.07 A | 2.62 ± 0.29 |
| Water activity (aw) | 0.850 ± 0.007 AB | 0.825 ± 0.034 B | 0.911 ± 0.006 A | 0.86 ± 0.04 |
| pH | 5.05 ± 0.08 A | 5.02 ± 0.16 A | 4.92 ± 0.02 A | 5.00 ± 0.07 |
| Microorganism | Brand A | Brand B | Brand C |
|---|---|---|---|
| Aerobic plate count | 5.40 ± 1.41 A | 1.22 ± 0.37 B | 2.77 ± 0.22 AB |
| Lactic acid bacteria | 5.01 ± 0.55 A | <1 log/g 2,B | 5.22 ± 0.02 A |
| Shiga toxin-producing Escherichia coli O157:H7 | Negative | Negative | Negative 3 |
| Shiga toxin-producing Escherichia coli “Top Six” 4 | Negative | Negative | Negative |
| Listeria monocytogenes | Negative | Negative | Negative |
| Salmonella spp. | Negative | Negative | Negative |
| Storage Temperature | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 °C | 20 °C | |||||||||||
| Storage Days | L. monocytogenes | Salmonella | STEC | L. monocytogenes | Salmonella | STEC | ||||||
| DP 1,3 | E 2,3 | DP | E | DP | E | DP | E | DP | E | DP | E | |
| 0 | 9/9 4 | 0/0 5 | 9/9 | 0/0 | 9/9 | 0/0 | 9/9 | 0/0 | 9/9 | 0/0 | 9/9 | 0/0 |
| 7 | 9/9 | 0/0 | 9/9 | 0/0 | 9/9 | 0/0 | 9/9 | 0/0 | 9/9 | 0/0 | 9/9 | 0/0 |
| 15 | 9/9 | 0/0 | 9/9 | 0/0 | 9/9 | 0/0 | 9/9 | 0/0 | 9/9 | 0/0 | 9/9 | 0/0 |
| 21 | 9/9 | 0/0 | 9/9 | 0/0 | 9/9 | 0/0 | 8/9 | 0/1 | 9/9 | 0/0 | 6/9 | 3/3 |
| 28 | 9/9 | 0/0 | 9/9 | 0/0 | 9/9 | 0/0 | 1/9 | 0/8 | 7/9 | 2/2 | 8/9 | 1/2 |
| 45 | 9/9 | 0/0 | 9/9 | 0/0 | 9/9 | 0/0 | 0/9 | 0/9 | 0/9 | 9/9 | 3/9 | 1/6 |
| 60 | 9/9 | 0/0 | 8/9 | 0/1 | 8/9 | 0/1 | 0/9 | 0/9 | 0/9 | 4/9 | 0/9 | 3/9 |
| 75 | 3/9 | 0/6 | 7/9 | 2/2 | 3/9 | 2/6 | 6/9 | 2/3 | 0/9 | 1/9 | 0/9 | 0/9 |
| 90 | 8/9 | 0/1 | 5/9 | 2/4 | 5/9 | 2/4 | 0/9 | 0/9 | 0/9 | 1/9 | 0/9 | 0/9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luchansky, J.B.; Shane, L.E.; Osoria, M.; Vinyard, B.T.; Shoyer, B.A.; Campano, S.G.; Porto-Fett, A.C.S. Fate of Listeria monocytogenes, Salmonella spp., and Shiga Toxin-Producing Escherichia coli on Slices of an All-Beef Soppressata during Storage. Foods 2023, 12, 1954. https://doi.org/10.3390/foods12101954
Luchansky JB, Shane LE, Osoria M, Vinyard BT, Shoyer BA, Campano SG, Porto-Fett ACS. Fate of Listeria monocytogenes, Salmonella spp., and Shiga Toxin-Producing Escherichia coli on Slices of an All-Beef Soppressata during Storage. Foods. 2023; 12(10):1954. https://doi.org/10.3390/foods12101954
Chicago/Turabian StyleLuchansky, John B., Laura E. Shane, Manuela Osoria, Bryan T. Vinyard, Bradley A. Shoyer, Stephen G. Campano, and Anna C. S. Porto-Fett. 2023. "Fate of Listeria monocytogenes, Salmonella spp., and Shiga Toxin-Producing Escherichia coli on Slices of an All-Beef Soppressata during Storage" Foods 12, no. 10: 1954. https://doi.org/10.3390/foods12101954






