Use of Tandem Mass Spectrometry Quantitative Proteomics to Identify Potential Biomarkers to Follow the Effects of Cold and Frozen Storage of Muscle Tissue of Litopenaeus vannamei
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Sample Preparation
2.3. Quality Determination
2.4. Protein Extraction and Peptide Digestion
2.5. TMT Labeling of Peptide and Peptide Classification
2.6. UPLC-QE-MS/MS Analysis
2.7. Protein Database Searching and Analysis
2.8. Bioinformatics and Statistical Analysis
3. Results and Discussion
3.1. Quality Analysis of L. vannamei Muscles
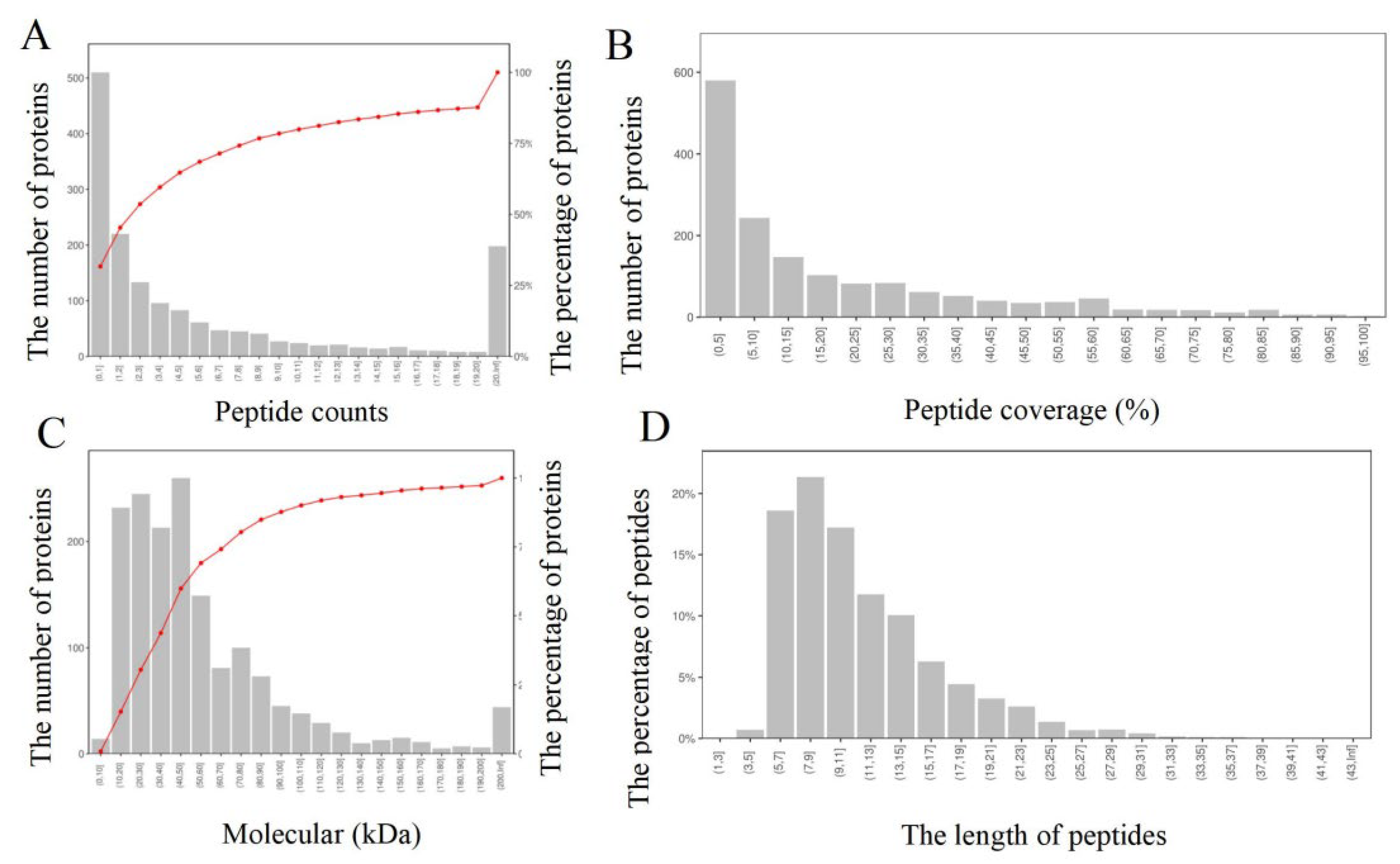
3.2. Protein Identification and Statistical Analysis
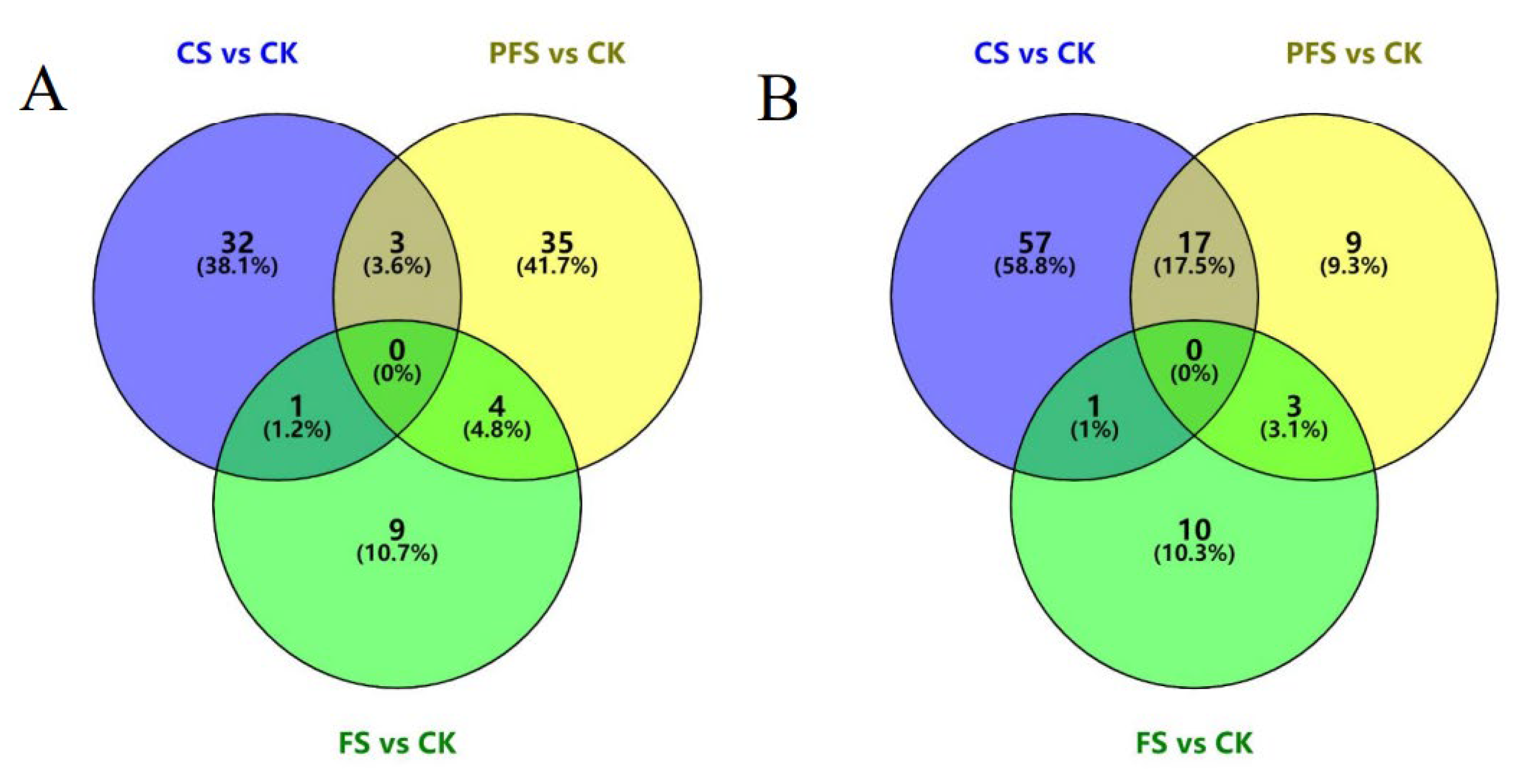
3.3. Statistics Analysis of Differential Proteins
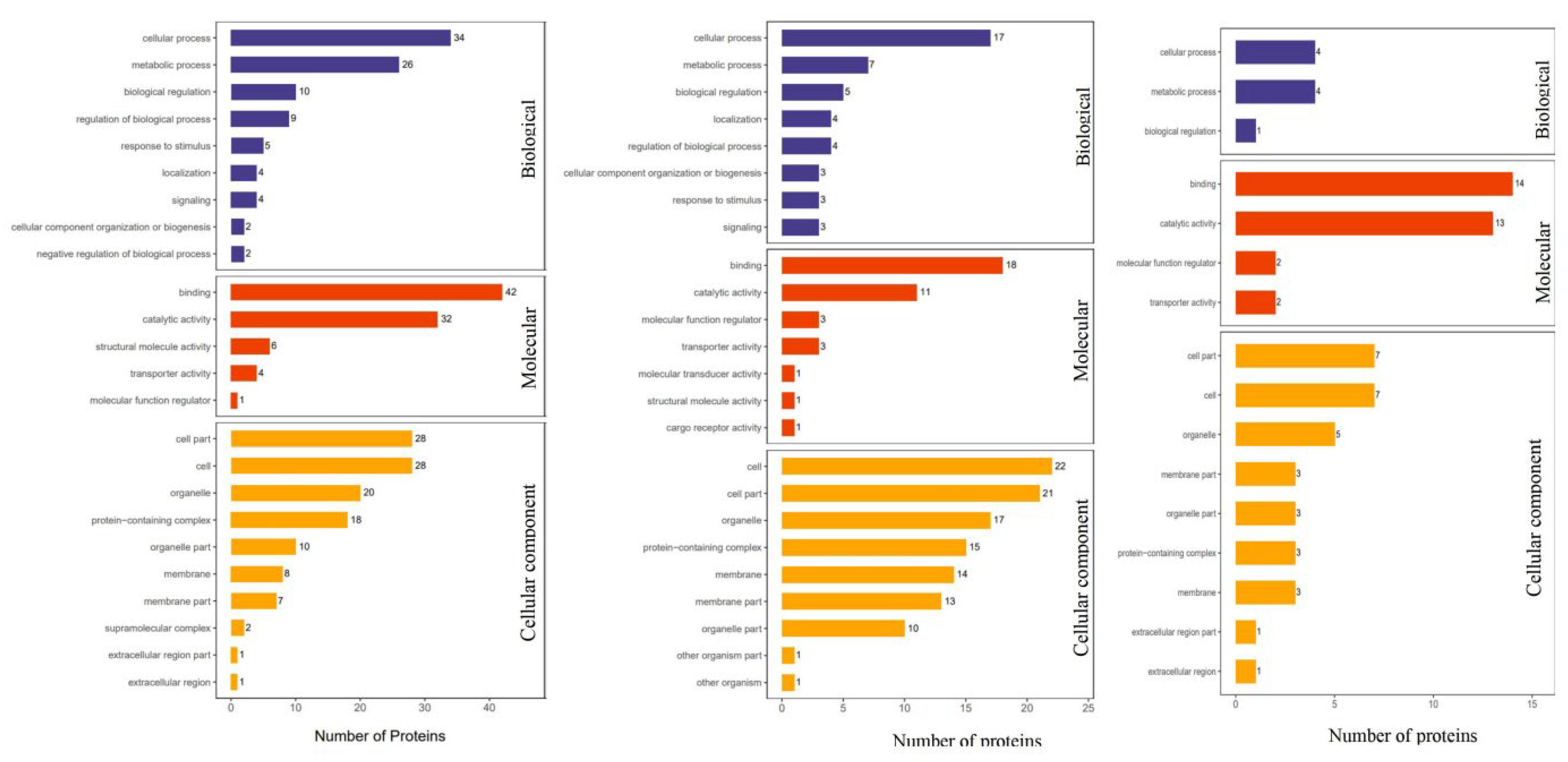
3.4. Functional Analysis of Differential Proteins
3.5. Correlation Analysis between Differential Protein and Quality Index
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schmitz, M.J.; Colombo, G.M.; Simiao, C.; Ortiz, C.R.; Fonseca, C.L.; Nascimento, D.S.T.; Ramos, P.B.; Yunes, J.S.; Wasielesky, W.J.; Tesser, M.B.; et al. Modulation of nodularin toxicity in shrimp Litopenaeus vannamei (BOONE, 1931) fed with dietary acai (Euterpe oleracea) inclusion. Fish Shellfish Immunol. 2020, 103, 464–471. [Google Scholar] [CrossRef]
- Kimbuathong, N.; Leelaphiwat, P.; Harnkarnsujarit, N. Inhibition of melanosis and microbial growth in Pacific white shrimp (Litopenaeus vannamei) using high CO2 modified atmosphere packaging. Food Chem. 2020, 312, 126114. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Tan, S.; Shiekh, K.A.; Benjakul, S.; Nirmal, N.P. Ethanolic guava leaf extracts with different chlorophyll removal processes: Anti-melanosis, antibacterial properties and the impact on qualities of Pacific white shrimp during refrigerated storage. Food Chem. 2021, 341, 128251. [Google Scholar] [CrossRef]
- Li, D.Y.; Li, N.; Dong, X.H.; Tan, Z.F.; Na, X.K.; Liu, X.Y.; Zhou, D.Y. Effect of phytic acid combined with lactic acid on color and texture deterioration of ready-to-eat shrimps during storage. Food Chem. 2022, 396, 133702. [Google Scholar] [CrossRef]
- Licciardello, F.; Kharchoufi, S.; Muratore, G.; Restuccia, C. Effect of edible coating combined with pomegranate peel extract on the quality maintenance of white shrimps (Parapenaeus longirostris) during refrigerated storage. Food Packag. Shelf Life 2018, 17, 114–119. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Yan, J.K.; Ding, Y.; Ma, H. Effects of ultrasound on the thawing of quick-frozen small yellow croaker (Larimichthys polyactis) based on TMT-labeled quantitative proteomic. Food Chem. 2022, 366, 130600. [Google Scholar] [CrossRef]
- Yuan, G.; Lv, H.; Tang, W.; Zhang, X.; Sun, H. Effect of chitosan coating combined with pomegranate peel extract on the quality of Pacific white shrimp during iced storage. Food Control 2016, 59, 818–823. [Google Scholar] [CrossRef]
- Melwani, A.R.; Thompson, E.L.; Raftos, D.A. Differential proteomic response of Sydney rock oysters (Saccostrea glomerata) to prolonged environmental stress. Aquat. Toxicol. 2016, 173, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, J.; Hu, W.; Chen, J.; Li, H. Protein changes in post mortem large yellow croaker (Pseudosciaena crocea) monitored by SDS-PAGE and proteome analysis. Food Control. 2014, 41, 49–55. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, L.; Lei, Y.; Shen, H.; Yu, X.; Luo, Y. Differential proteomic analysis to identify proteins associated with quality traits of frozen mud shrimp (Solenocera melantho) using an iTRAQ-based strategy. Food Chem. 2018, 251, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Kruk, Z.A.; Yun, H.; Rutley, D.L.; Lee, E.J.; Kim, Y.J.; Jo, C. The effect of high pressure on microbial population, meat quality and sensory characteristics of chicken breast fillet. Food Control 2011, 22, 6–12. [Google Scholar] [CrossRef]
- Li, D.; Zhao, H.; Muhammad, A.I.; Song, L.; Guo, M.; Liu, D. The comparison of ultrasound-assisted thawing, air thawing and water immersion thawing on the quality of slow/fast freezing bighead carp (Aristichthys nobilis) fillets. Food Chem. 2020, 320, 126614. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Dixit, P.K. Cellular lactate and pyruvate are key intermediates for intra-cellular energy metabolism regulated by Lactate dehydrogenase (LDH) in agerelated phenomenon. Int. J. Clin. Biochem. Res. 2020, 2, 285–290. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, J. Analysis of Proteins Associated with Quality Deterioration of Grouper Fillets Based on TMT Quantitative Proteomics during Refrigerated Storage. Molecules 2019, 24, 2641. [Google Scholar] [CrossRef]
- Duan, Y.; Xiong, D.; Wang, Y.; Zhang, Z.; Li, H.; Dong, H.; Zhang, J. Toxicological effects of microplastics in Litopenaeus vannamei as indicated by an integrated microbiome, proteomic and metabolomic approach. Sci. Total Environ. 2021, 761, 143311. [Google Scholar] [CrossRef]
- Lu, X.; Luan, S.; Dai, P.; Meng, X.; Cao, B.; Luo, K.; Kong, J. iTRAQ-based comparative proteome analysis for molecular mechanism of defense against acute ammonia toxicity in Pacific White shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2018, 74, 52–61. [Google Scholar] [CrossRef]
- Ebinezer, L.B.; Franchin, C.; Trentin, A.R.; Carletti, P.; Trevisan, S.; Agrawal, G.K.; Rakwal, R.; Quaggiotti, S.; Arrigoni, G.; Masi, A. Quantitative Proteomics of Maize Roots Treated with a Protein Hydrolysate: A Comparative Study with Transcriptomics Highlights the Molecular Mechanisms Responsive to Biostimulants. J. Agric Food Chem. 2020, 68, 7541–7553. [Google Scholar] [CrossRef]
- Zhang, B.; Yao, H.; Qi, H.; Zhang, X. Trehalose and alginate oligosaccharides increase the stability of muscle proteins in frozen shrimp (Penaeus vannamei). Food Funct. 2020, 2, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zheng, M.; Sun, S.; Wang, H.; Yue, Z.; Zhu, Y.; Han, X.; Yang, J.; Zhou, Y.; Cai, Y.; et al. Chaperonin containing TCP1 subunit 5 is a tumor associated antigen of non-small cell lung cancer. Oncotarget 2017, 8, 64170–64179. [Google Scholar] [CrossRef] [PubMed]
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The protein kinase complement of the human genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef]
- Priyadarshini, M.B.; Xavier, K.M.; Dhanabalan, V.; Nayak, B.B.; Balange, A.K. Development of ready-to-cook shrimp analogue from surimi: Effect of natural plant extracts on the chemical quality during refrigerated storage. LWT 2021, 135, 110239. [Google Scholar] [CrossRef]
- Xu, D.; Sun, L.; Li, C.; Wang, Y.; Ye, R. Inhibitory effect of glucose oxidase from Bacillus sp. CAMT22370 on the quality deterioration of Pacific white shrimp during cold storage. LWT 2018, 92, 339–346. [Google Scholar] [CrossRef]
- GB 5009.228-2016; National Standard for Food Safety: Determination of Volatile Base Nitrogen in Food. Ministry of Health, People’s Republic of China: Beijing, China, 2016.
- Bereman, M.S.; Egertson, J.D.; MacCoss, M.J. Comparison between procedures using SDS for shotgun proteomic analyses of complex samples. Proteomics 2011, 11, 2931–2935. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Wang, L.; Wang, Z. Proteomic characterization of the hepatopancreas in the Pacific white shrimp Litopenaeus vannamei under cold stress: Revealing the organism homeostasis mechanism. Fish Shellfish Immunol. 2019, 92, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Balti, R.; Mansour, M.B.; Zayoud, N.; Le Balc’h, R.; Brodu, N.; Arhaliass, A.; Masse, A. Active exopolysaccharides based edible coatings enriched with red seaweed (Gracilaria gracilis) extract to improve shrimp preservation during refrigerated storage. Food Biosci. 2020, 34, 100522. [Google Scholar] [CrossRef]
- Srinivasan, P.; Robinson, J.; Geevaretnam, J.; Rayappan, J.B.B. Development of electronic nose (Shrimp-Nose) for the determination of perishable quality and shelf-life of cultured Pacific white shrimp (Litopenaeus vannamei). Sens. Actuators B Chem. 2020, 317, 128192. [Google Scholar] [CrossRef]
- Wu, S. Effect of chitosan-based edible coating on preservation of white shrimp during partially frozen storage. Int. J. Biol. Macromol. 2014, 65, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Pan, C.; Chen, S.; Liu, S.; Hao, S.; Huang, H.; Wang, D.; Xiang, H. Quality changes and indicator proteins of Litopenaeus vannamei based on label-free proteomics analysis during partial freezing storage. Curr. Res. Food Sci. 2023, 6, 100415. [Google Scholar] [CrossRef] [PubMed]
- Consortium, G.O. The gene ontology project in 2008. Nucleic Acids Res. 2008, 36 (Suppl. S1), D440–D444. [Google Scholar] [CrossRef]
- Roncaglia, P.; Martone, M.E.; Hill, D.P.; Berardini, T.Z.; Foulger, R.E.; Imam, F.T.; Drabkin, H.; Mungall, C.J.; Lomax, J. The Gene Ontology (GO) Cellular Component Ontology: Integration with SAO (Subcellular Anatomy Ontology) and other recent developments. J. Biomed. Semant. 2013, 4, 20. [Google Scholar] [CrossRef]

| Quality Characteristics | q | CS | PFS | FS |
|---|---|---|---|---|
| Water holding capacity | 90.83 ± 0.06 a | 81.43 ± 1.38 c | 85.31 ± 0.81 b | 88.38 ± 1.2 a |
| L* | 29.70 ± 0.41 a | 41.12 ± 0.32 a | 39.66 ± 0.15 b | 31.48 ± 0.18 c |
| Springiness (N) | 0.93 ± 0.00 a | 0.68 ± 0.01 c | 0.95 ± 0.00 | 0.98 ± 0.00 a |
| Cohesiveness | 0.44 ± 0.01 a | 0.33 ± 0.01 c | 0.41 ± 0.01 b | 0.43 ± 0.02 a |
| Chewiness (mJ) | 752.92 ± 5.01 a | 703.21 ± 28.86 a | 591.42 ± 27.61 a | 609.95 ± 40.78 b |
| Hardness (g) | 1733.54 ± 35.17 a | 2120.72 ± 77.57 a | 1435.13 ± 16.87 b | 1204.87 ± 36.08 b |
| TVB-N | 6.57 ± 0.54 a | 22.11 ± 0.19 a | 18.57 ± 0.41 b | 19.11 ± 0.29 b |
| Comparisons | CS/CK | PFS/CK | FS/CK | |
|---|---|---|---|---|
| BP | cellular processes | 34 | 26 | 10 |
| metabolic processes | 26 | 7 | 4 | |
| biological regulation processes | 10 | 5 | 1 | |
| MF | binding | 42 | 18 | 14 |
| catalytic activity | 32 | 11 | 13 | |
| CC | cells | 28 | 22 | 7 |
| cell parts | 28 | 21 | 7 | |
| organelles | 20 | 17 | 5 | |
| Accession | Protein Name | Springiness | Cohesiveness | Chewiness | Hardness | L* | a* | b* | Water Holding Capacity | TVB-N |
|---|---|---|---|---|---|---|---|---|---|---|
| Protein biosynthesis | ||||||||||
| A0A3R7Q994 | 40S ribosomal protein S8 | 0.585 | 0.815 | 0.989 * | −0.465 | −0.993 ** | 0.416 | −0.472 | 0.946 | −0.804 |
| A0A3R7QE43 | 40S ribosomal protein S27 | 0.732 | 0.916 | 0.997 ** | −0.624 | −0.975 * | 0.559 | −0.275 | 0.994 ** | −0.827 |
| A0A423TWT2 | S10e ribosomal protein | 0.682 | 0.878 | 0.997 ** | −0.575 | −0.997 ** | 0.534 | −0.400 | 0.969 * | −0.766 |
| A0A076NBT3 | 60S ribosomal protein L40 | 0.906 | 0.993 ** | 0.921 | −0.831 | −0.868 | 0.761 | 0.028 | 0.974 * | −0.747 |
| A0A423U985 | Putative eukaryotic translation initiation factor 4-like | 0.947 | 1 ** | 0.886 | −0.887 | −0.833 | 0.828 | 0.077 | 0.947 | −0.67 |
| A0A3R7P4W6 | CCT-epsilon | 0.994 ** | 0.967 * | 0.747 | −0.967 * | −0.678 | 0.915 | 0.284 | 0.842 | −0.529 |
| A0A3R7N5V9 | Mitogen-activated protein kinase 7-interacting protein | 0.569 | 0.797 | 0.981 * | −0.454 | −0.996 ** | 0.421 | −0.526 | 0.927 | −0.752 |
| Protein hydrolysis | ||||||||||
| A0A423T969 | Proteasome subunit alpha type | −0.471 | −0.709 | −0.855 | 0.333 | 0.801 | −0.198 | 0.162 | −0.868 | 0.995 ** |
| A0A423SW39 | Acyl-peptide hydrolase | −0.966 * | −0.996 ** | −0.859 | 0.918 | 0.813 | −0.876 | −0.080 | −0.92 | 0.593 |
| RNA binding | ||||||||||
| A0A3R7PPQ9 | Putative RNA-binding protein-like isoform X4 | 0.603 | 0.819 | 0.984 * | −0.493 | −0.999 ** | 0.465 | −0.507 | 0.933 | −0.732 |
| A0A423TSY3 | Sex-lethal | 0.938 | 0.994 ** | 0.877 | −0.873 | −0.814 | 0.800 | 0.124 | 0.947 | −0.712 |
| Accession | Protein name | Springiness | Cohesiveness | Chewiness | Hardness | L* | a* | b* | Water holding capacity | TVB−N |
| GTP binding | ||||||||||
| A0A3R7MKG0 | J domain-containing protein | 0.757 | 0.922 | 0.993 ** | −0.662 | −0.985 * | 0.623 | −0.322 | 0.980 * | −0.736 |
| A0A3R7QNM6 | RhoA | −0.937 | −0.996 ** | −0.883 | 0.872 | 0.822 | −0.801 | −0.110 | −0.950 * | 0.711 |
| Other proteins | ||||||||||
| A0A423SU24 | Putative dysferlin-like | −0.754 | −0.929 | −0.991 ** | 0.648 | 0.963 * | −0.577 | 0.226 | −0.998 ** | 0.836 |
| K7WFT8 | Troponin I | 0.832 | 0.967 * | 0.978 * | −0.743 | −0.950 * | 0.690 | −0.176 | 0.993 ** | −0.755 |
| A0A423TC50 | Activation factor subunit spp27 | 0.83 | 0.966 * | 0.963 * | −0.736 | −0.918 | 0.657 | −0.085 | 0.997 ** | −0.822 |
| A0A423U5T5 | Putative ensconsin-like isoform X8 | 0.729 | 0.914 | 0.994 ** | −0.62 | −0.968 * | 0.549 | −0.254 | 0.995 ** | −0.845 |
| Uncharacterized protein | ||||||||||
| A0A423SPE6 | Uncharacterized protein | 0.707 | 0.881 | 0.982 * | −0.614 | −0.992 ** | 0.595 | −0.421 | 0.948 | −0.677 |
| A0A423SR08 | Uncharacterized protein | 0.674 | 0.872 | 0.996 ** | −0.568 | −0.998 ** | 0.529 | −0.414 | 0.965 * | −0.758 |
| A0A3R7PAK4 | Uncharacterized protein | 0.604 | 0.811 | 0.973 * | −0.501 | −0.996 ** | 0.485 | −0.532 | 0.917 | −0.683 |
| A0A3R7PV59 | Uncharacterized protein | 0.644 | 0.858 | 0.997 ** | −0.529 | −0.992 ** | 0.473 | −0.398 | 0.970 * | −0.818 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Ning, Q.; Wu, Z.; Zhou, H.; Liao, J.; Sun, X.; Lin, J.; Pang, J. Use of Tandem Mass Spectrometry Quantitative Proteomics to Identify Potential Biomarkers to Follow the Effects of Cold and Frozen Storage of Muscle Tissue of Litopenaeus vannamei. Foods 2023, 12, 2920. https://doi.org/10.3390/foods12152920
Chen Y, Ning Q, Wu Z, Zhou H, Liao J, Sun X, Lin J, Pang J. Use of Tandem Mass Spectrometry Quantitative Proteomics to Identify Potential Biomarkers to Follow the Effects of Cold and Frozen Storage of Muscle Tissue of Litopenaeus vannamei. Foods. 2023; 12(15):2920. https://doi.org/10.3390/foods12152920
Chicago/Turabian StyleChen, Yu, Qian Ning, Zhenzhen Wu, Hanlin Zhou, Jun Liao, Xiangyun Sun, Jing Lin, and Jie Pang. 2023. "Use of Tandem Mass Spectrometry Quantitative Proteomics to Identify Potential Biomarkers to Follow the Effects of Cold and Frozen Storage of Muscle Tissue of Litopenaeus vannamei" Foods 12, no. 15: 2920. https://doi.org/10.3390/foods12152920




