Effects of Synbiotic Lacticaseibacillus paracasei, Bifidobacterium breve, and Prebiotics on the Growth Stimulation of Beneficial Gut Microbiota
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Medium
2.2. Assessment of Probiotic Properties
2.2.1. In Vitro Survival Test for GI Tract Simulation
2.2.2. Adhesion Ability to Caco-2 Cell Lines
2.2.3. Antimicrobial Activity
2.2.4. Bile Salt Hydrolase (BSH) Activity
2.2.5. In Vitro Evaluation of Cholesterol Lowering
2.3. Safety Assessments of Probiotics
2.3.1. Antibiotics Susceptibility
2.3.2. Hemolytic Activity
2.4. Anti-Inflammatory Properties of Probiotics in Caco-2 Epithelial Cell Lines
2.5. Synbiotic Powder Preparation
2.6. In Vitro Test of Gut Microbiota Stimulation in Feces
2.7. Quantification of Gut Microbiota in Feces Using Real-Time Quantitative PCR
2.8. Determination of Organic Acids
2.9. Stability Assessments of Synbiotics
2.10. Statistical Analysis
3. Results and Discussion
3.1. Probiotic Properties
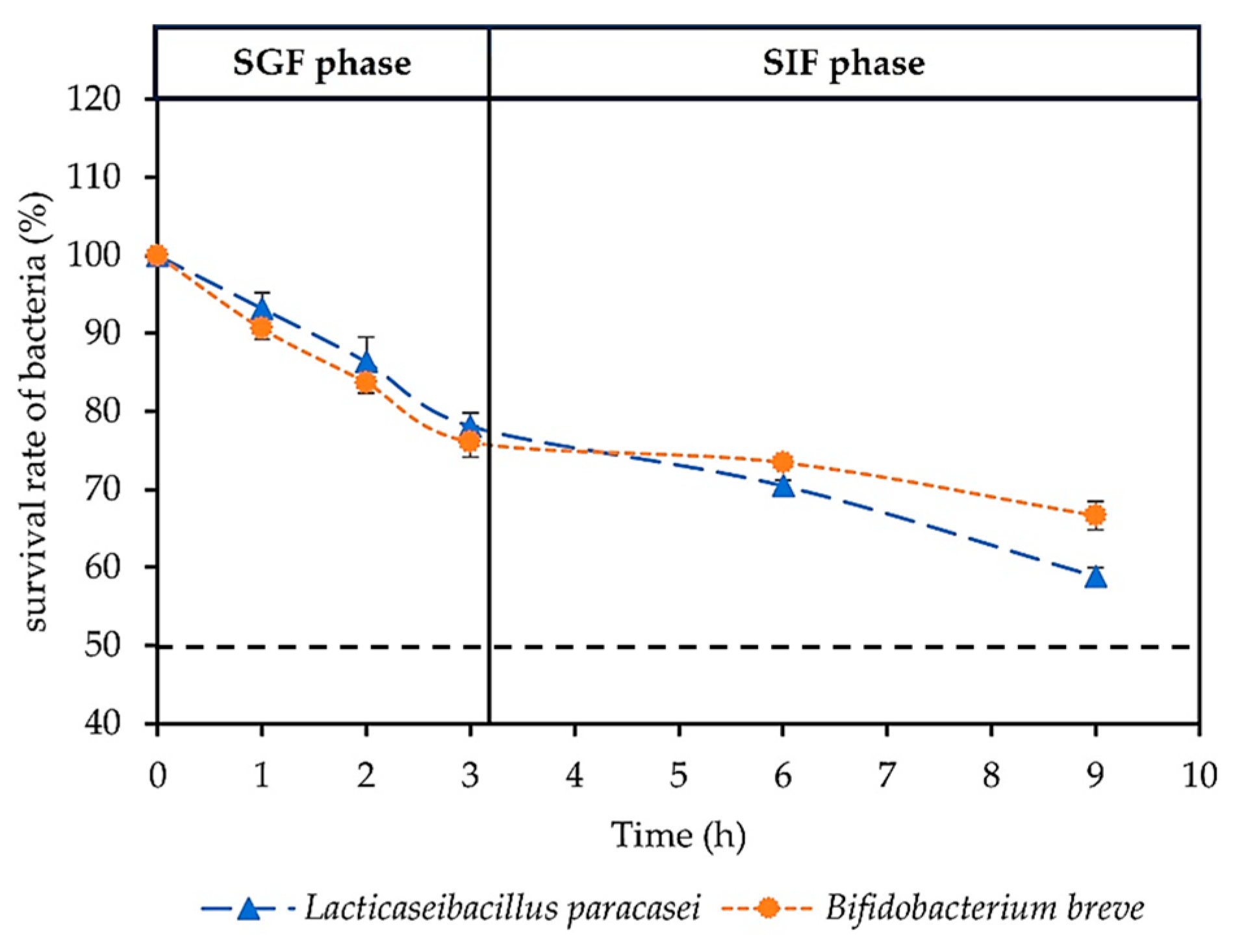
3.1.1. In Vitro Survival Rate of Probiotics in GI Tract Simulation
3.1.2. Adhesion Ability of Probiotics to Caco-2 Cell Lines
3.1.3. Antimicrobial Activity
3.1.4. Bile Salt Hydrolase (BSH) Activity
3.1.5. Cholesterol Lowering
3.2. Safety of Probiotics
3.2.1. Antibiotic-Resistant Probiotics
3.2.2. Hemolytic Activity
3.3. Anti-Inflammatory Properties of Probiotic Supernatants in Caco-2 Epithelial Cell Lines
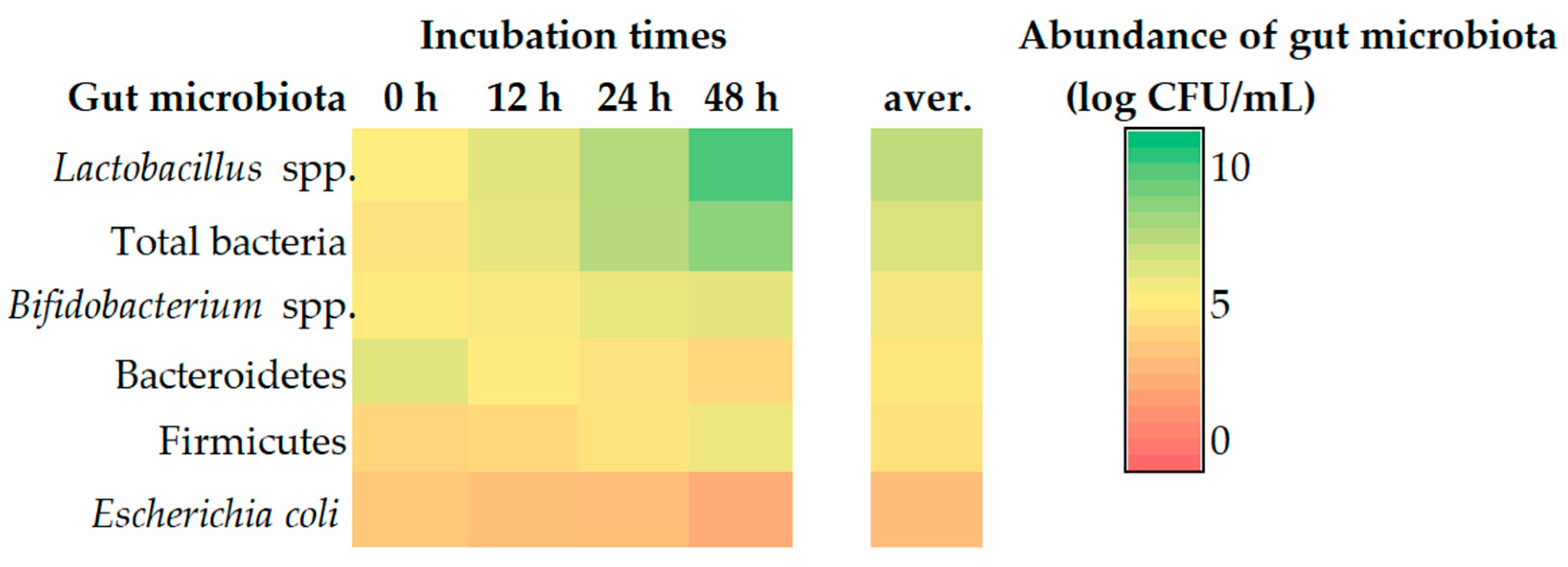
3.4. Gut Microbiota Using Feces as a Representation by Real-Time PCR
3.5. Organic Acid Contents
3.6. Stability Assessment of Synbiotics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petricevic, L.; Domig, K.J.; Nierscher, F.J.; Sandhofer, M.J.; Fidesser, M.; Krondorfer, I.; Husslein, P.; Kneifel, W.; Kiss, H. Characterisation of the vaginal Lactobacillus microbiota associated with preterm delivery. Sci. Rep. 2014, 4, 5136. [Google Scholar] [CrossRef]
- Hemarajata, P.; Versalovic, J. Effects of probiotics on gut microbiota: Mechanisms of intestinal immunomodulation and neuromodulation. Ther. Adv. Gastroenterol. 2013, 6, 39–51. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, P.; Zhang, X. Probiotics regulate gut microbiota: An effective method to improve immunity. Molecules 2021, 26, 6076. [Google Scholar] [CrossRef]
- García-Ruiz, A.; de Llano, D.G.; Esteban-Fernández, A.; Requena, T.; Bartolomé, B.; Moreno-Arribas, M.V. Assessment of probiotic properties in lactic acid bacteria isolated from wine. Food Microbiol. 2014, 44, 220–225. [Google Scholar] [CrossRef]
- Olveira, G.; González-Molero, I. An update on probiotics, prebiotics and symbiotics in clinical nutrition. Endocrinol. Y Nutr. 2016, 63, 482–494. [Google Scholar] [CrossRef]
- Guarner, F.; Khan, A.G.; Garisch, J.; Eliakim, R.; Gangl, A.; Thomson, A.; Krabshuis, J.; Lemair, T.; Kaufmann, P.; De Paula, J.A. World gastroenterology organisation global guidelines: Probiotics and prebiotics october 2011. J. Clin. Gastroenterol. 2012, 46, 468–481. [Google Scholar] [CrossRef]
- Gomez Quintero, D.F.; Kok, C.R.; Hutkins, R. The Future of Synbiotics: Rational Formulation and Design. Front. Microbiol. 2022, 13, 919725. [Google Scholar] [CrossRef]
- West, N.P.; Pyne, D.B.; Cripps, A.; Christophersen, C.T.; Conlon, M.A.; Fricker, P.A. Gut Balance, a synbiotic supplement, increases fecal Lactobacillus paracasei but has little effect on immunity in healthy physically active individuals. Gut Microbes 2012, 3, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Hashikura, N.; Murakami, R.; Sakurai, T.; Horigome, A.; Toda, K.; Xiao, J.-z.; Odamaki, T. Synbiotics of Bifidobacterium breve MCC1274 and lactulose enhances production of tryptophan metabolites in fermented human fecal communities. Food Res. Int. 2023, 163, 112308. [Google Scholar] [CrossRef] [PubMed]
- Nishida, C.; Uauy, R.; Kumanyika, S.; Shetty, P. The joint WHO/FAO expert consultation on diet, nutrition and the prevention of chronic diseases: Process, product and policy implications. Public Health Nutr. 2004, 7, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Ayama, H.; Sumpavapol, P.; Chanthachum, S. Effect of encapsulation of selected probiotic cell on survival in simulated gastrointestinal tract condition. Songklanakarin J. Sci. Technol. 2014, 36, 291–299. [Google Scholar]
- Panicker, A.S.; Ali, S.A.; Anand, S.; Panjagari, N.R.; Kumar, S.; Mohanty, A.K.; Behare, P.V. Evaluation of some in vitro probiotic properties of Lactobacillus fermentum Strains. J. Food Sci. Technol. 2018, 55, 2801–2807. [Google Scholar] [CrossRef]
- Shehata, M.; El Sohaimy, S.; El-Sahn, M.A.; Youssef, M. Screening of isolated potential probiotic lactic acid bacteria for cholesterol lowering property and bile salt hydrolase activity. Ann. Agric. Sci. 2016, 61, 65–75. [Google Scholar] [CrossRef]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Jordan, B.F. Gut microbiota-mediated inflammation in obesity: A link with gastrointestinal cancer. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 671–682. [Google Scholar] [CrossRef]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and butyrate-producing colon bacteria: Importance and strategies for their stimulation in the human gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [PubMed]
- Getahun, A.; Tesfaye, A.; Muleta, D. Investigation of the potential benefits and risks of probiotics and prebiotics and their synergy in fermented foods. Singap. J. Chem. Biol. 2016, 6, 1–16. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.; Harris, H.M.; Mattarelli, P.; O’toole, P.W.; Pot, B.; Vandamme, P.; Walter, J. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Sadeghi, A. In vitro Assessment of Some Probiotic Properties of Lactobacillus fermentum Isolated from Pickled Garlic. J. Food Qual. Hazards Control. 2016, 3, 67–72. [Google Scholar]
- Campana, R.; van Hemert, S.; Baffone, W. Strain-specific probiotic properties of lactic acid bacteria and their interference with human intestinal pathogens invasion. Gut Pathog. 2017, 9, 1–12. [Google Scholar] [CrossRef]
- Pennacchia, C.; Vaughan, E.E.; Villani, F. Potential probiotic Lactobacillus strains from fermented sausages: Further investigations on their probiotic properties. Meat Sci. 2006, 73, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Lin, P.R.; Ng, C.C.; Shyu, Y.T. Probiotic properties of Lactobacillus strains isolated from the feces of breast-fed infants and Taiwanese pickled cabbage. Anaerobe 2010, 16, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Grover, S.; Batish, V.K. Bile salt hydrolase (Bsh) activity screening of Lactobacilli: In vitro selection of indigenous Lactobacillus strains with potential bile salt hydrolysing and cholesterol-lowering ability. Probiotics Antimicrob. Proteins 2012, 4, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.I.; McCartney, A.L.; Gibson, G.R. An in vitro study of the probiotic potential of a bile-salt-hydrolyzing Lactobacillus fermentum strain, and determination of its cholesterol-lowering properties. Appl. Environ. Microbiol. 2003, 69, 4743–4752. [Google Scholar] [CrossRef]
- Bauer, L.C.; Santana, D.d.A.; Macedo, M.d.S.; Torres, A.G.; Souza, N.E.d.; Simionato, J.I. Method validation for simultaneous determination of cholesterol and cholesterol oxides in milk by RP-HPLC-DAD. J. Braz. Chem. Soc. 2014, 25, 161–168. [Google Scholar] [CrossRef]
- Bordoni, A.; Amaretti, A.; Leonardi, A.; Boschetti, E.; Danesi, F.; Matteuzzi, D.; Roncaglia, L.; Raimondi, S.; Rossi, M. Cholesterol-lowering probiotics: In vitro selection and in vivo testing of bifidobacteria. Appl. Microbiol. Biotechnol. 2013, 97, 8273–8281. [Google Scholar] [CrossRef]
- Tomaro-Duchesneau, C.; Jones, M.L.; Shah, D.; Jain, P.; Saha, S.; Prakash, S. Cholesterol assimilation by Lactobacillus probiotic bacteria: An in vitro investigation. BioMed Res. Int. 2014, 2014, 380316. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J. 2012, 10, 2740. [Google Scholar]
- Yasmin, I.; Saeed, M.; Khan, W.A.; Khaliq, A.; Chughtai, M.F.J.; Iqbal, R.; Tehseen, S.; Naz, S.; Liaqat, A.; Mehmood, T. In vitro probiotic potential and safety evaluation (hemolytic, cytotoxic activity) of Bifidobacterium strains isolated from raw camel milk. Microorganisms 2020, 8, 354. [Google Scholar] [CrossRef]
- De Marco, S.; Sichetti, M.; Muradyan, D.; Piccioni, M.; Traina, G.; Pagiotti, R.; Pietrella, D. Probiotic cell-free supernatants exhibited anti-inflammatory and antioxidant activity on human gut epithelial cells and macrophages stimulated with LPS. Evid.-Based Complement. Altern. Med. 2018, 2018, 1756308. [Google Scholar] [CrossRef]
- Aghamohammad, S.; Sepehr, A.; Miri, S.T.; Najafi, S.; Pourshafie, M.R.; Rohani, M. Anti-inflammatory and immunomodulatory effects of Lactobacillus spp. as a preservative and therapeutic agent for IBD control. Immun. Inflamm. Dis. 2022, 10, e635. [Google Scholar] [CrossRef] [PubMed]
- Kaewarsar, E.; Chaiyasut, C.; Lailerd, N.; Makhamrueang, N.; Peerajan, S.; Sirilun, S. Optimization of mixed inulin, fructooligosaccharides, and galactooligosaccharides as prebiotics for stimulation of probiotics growth and function. Foods 2023, 12, 1591. [Google Scholar] [CrossRef] [PubMed]
- Takagi, R.; Sasaki, K.; Sasaki, D.; Fukuda, I.; Tanaka, K.; Yoshida, K.-i.; Kondo, A.; Osawa, R. A single-batch fermentation system to simulate human colonic microbiota for high-throughput evaluation of prebiotics. PLoS ONE 2016, 11, e0160533. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gibson, G.R.; Walton, G.E. An in vitro approach to study effects of prebiotics and probiotics on the faecal microbiota and selected immune parameters relevant to the elderly. PLoS ONE 2016, 11, e0162604. [Google Scholar] [CrossRef]
- Stiverson, J.; Williams, T.; Chen, J.; Adams, S.; Hustead, D.; Price, P.; Guerrieri, J.; Deacon, J.; Yu, Z. Prebiotic oligosaccharides: Comparative evaluation using in vitro cultures of infants’ fecal microbiomes. Appl. Environ. Microbiol. 2014, 80, 7388–7397. [Google Scholar] [CrossRef]
- Mitsou, E.K.; Kakali, A.; Antonopoulou, S.; Mountzouris, K.C.; Yannakoulia, M.; Panagiotakos, D.B.; Kyriacou, A. Adherence to the Mediterranean diet is associated with the gut microbiota pattern and gastrointestinal characteristics in an adult population. Br. J. Nutr. 2017, 117, 1645–1655. [Google Scholar] [CrossRef]
- Jian, C.; Luukkonen, P.; Yki-Järvinen, H.; Salonen, A.; Korpela, K. Quantitative PCR provides a simple and accessible method for quantitative microbiota profiling. PLoS ONE 2020, 15, e0227285. [Google Scholar] [CrossRef]
- Rinttilä, T.; Kassinen, A.; Malinen, E.; Krogius, L.; Palva, A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J. Appl. Microbiol. 2004, 97, 1166–1177. [Google Scholar] [CrossRef]
- Ignacio, A.; Fernandes, M.; Rodrigues, V.; Groppo, F.; Cardoso, A.; Avila-Campos, M.; Nakano, V. Correlation between body mass index and faecal microbiota from children. Clin. Microbiol. Infect. 2016, 22, 258.e1–258.e8. [Google Scholar] [CrossRef]
- Guo, X.; Xia, X.; Tang, R.; Zhou, J.; Zhao, H.; Wang, K. Development of a real-time PCR method for Firmicutes and Bacteroidetes in faeces and its application to quantify intestinal population of obese and lean pigs. Lett. Appl. Microbiol. 2008, 47, 367–373. [Google Scholar] [CrossRef]
- Wang, I.-K.; Lai, H.-C.; Yu, C.-J.; Liang, C.-C.; Chang, C.-T.; Kuo, H.-L.; Yang, Y.-F.; Lin, C.-C.; Lin, H.-H.; Liu, Y.-L. Real-time PCR analysis of the intestinal microbiotas in peritoneal dialysis patients. Appl. Environ. Microbiol. 2012, 78, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, N.A.; Walker, A.W.; Berry, S.H.; Duncan, S.H.; Farquarson, F.M.; Louis, P.; Thomson, J.M.; Satsangi, J.; Flint, H.J. The impact of different DNA extraction kits and laboratories upon the assessment of human gut microbiota composition by 16S rRNA gene sequencing. PLoS ONE 2014, 9, e88982. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Stability testing of active pharmaceutical ingredients and finished pharmaceutical products, Annex 10. WHO Expert Comm. Specif. Pharm. Prep. 2018, 52, 309–351. [Google Scholar]
- Anderson, S.R. Circular dichroism spectra of the complexes of 1-anilino-8-naphthalenesulfonate with bovine serum albumin. Evidence for heterogeneity of binding. Biochemistry 1969, 8, 4838–4842. [Google Scholar] [CrossRef]
- Somashekaraiah, R.; Shruthi, B.; Deepthi, B.; Sreenivasa, M. Probiotic properties of lactic acid bacteria isolated from neera: A naturally fermenting coconut palm nectar. Front. Microbiol. 2019, 10, 1382. [Google Scholar] [CrossRef]
- Mendonça, A.A.; Pinto-Neto, W.d.P.; da Paixão, G.A.; Santos, D.d.S.; De Morais, M.A., Jr.; De Souza, R.B. Journey of the probiotic bacteria: Survival of the fittest. Microorganisms 2022, 11, 95. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.; Etienne-Mesmin, L.; Livrelli, V.; Denis, S.; Blanquet-Diot, S.; Alric, M. Relevance and challenges in modeling human gastric and small intestinal digestion. Trends Biotechnol. 2012, 30, 591–600. [Google Scholar] [CrossRef]
- Wang, R.-M.; Li, N.; Zheng, K.; Hao, J.-F. Enhancing acid tolerance of the probiotic bacterium Lactobacillus acidophilus NCFM with trehalose. FEMS Microbiol. Lett. 2018, 365, fny217. [Google Scholar] [CrossRef]
- Mau, M.M.; Sarker, S.; Terry, B.S. Ingestible devices for long-term gastrointestinal residency: A review. Prog. Biomed. Eng. 2021, 3, 042001. [Google Scholar] [CrossRef]
- Arnold, J.W.; Simpson, J.B.; Roach, J.; Kwintkiewicz, J.; Azcarate-Peril, M.A. Intra-species genomic and physiological variability impact stress resistance in strains of probiotic potential. Front. Microbiol. 2018, 9, 242. [Google Scholar] [CrossRef]
- Nyanga-Koumou, A.P.; Ouoba, L.I.I.; Kobawila, S.C.; Louembe, D. Response mechanisms of lactic acid bacteria to alkaline environments: A review. Crit. Rev. Microbiol. 2012, 38, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Tham, C.S.-C.; Peh, K.-K.; Bhat, R.; Liong, M.-T. Probiotic properties of bifidobacteria and lactobacilli isolated from local dairy products. Ann. Microbiol. 2012, 62, 1079–1087. [Google Scholar] [CrossRef]
- Haddaji, N.; Mahdhi, A.K.; Krifi, B.; Ismail, M.B.; Bakhrouf, A. Change in cell surface properties of Lactobacillus casei under heat shock treatment. FEMS Microbiol. Lett. 2015, 362, fnv047. [Google Scholar] [CrossRef] [PubMed]
- Gheziel, C.; Russo, P.; Arena, M.P.; Spano, G.; Ouzari, H.-I.; Kheroua, O.; Saidi, D.; Fiocco, D.; Kaddouri, H.; Capozzi, V. Evaluating the probiotic potential of Lactobacillus plantarum strains from Algerian infant feces: Towards the design of probiotic starter cultures tailored for developing countries. Probiotics Antimicrob. Proteins. 2019, 11, 113–123. [Google Scholar] [CrossRef]
- Yadav, R.; Puniya, A.K.; Shukla, P. Probiotic properties of Lactobacillus plantarum RYPR1 from an indigenous fermented beverage Raabadi. Front. Microbiol. 2016, 7, 1683. [Google Scholar] [CrossRef]
- Kotsou, M.G.; Mitsou, E.K.; Oikonomou, I.G.; Kyriacou, A.A. In vitro assessment of probiotic properties of Lactobacillus strains from infant gut microflora. Food Biotechnol. 2008, 22, 1–17. [Google Scholar] [CrossRef]
- Ma, T.; Shen, X.; Shi, X.; Sakandar, H.A.; Quan, K.; Li, Y.; Jin, H.; Kwok, L.-Y.; Zhang, H.; Sun, Z. Targeting gut microbiota and metabolism as the major probiotic mechanism—An evidence-based review. Trends Food Sci. Technol. 2023, 138, 178–198. [Google Scholar] [CrossRef]
- Damodharan, K.; Lee, Y.S.; Palaniyandi, S.A.; Yang, S.H.; Suh, J.-W. Preliminary probiotic and technological characterization of Pediococcus pentosaceus strain KID7 and in vivo assessment of its cholesterol-lowering activity. Front. Microbiol. 2015, 6, 768. [Google Scholar] [CrossRef]
- Öztürk, M.; Hacıbeyoğlu, K.; Önal, C.; Kılıçsaymaz, Z. Construction of R16F and D19L mutations in the loop I of bile salt hydrolase (BSH) enzyme from Lactobacillus plantarum B14 and structural and functional analysis of the mutant BSHs. Food Biotechnol. 2019, 33, 125–141. [Google Scholar] [CrossRef]
- Jarocki, P.; Podleśny, M.; Glibowski, P.; Targoński, Z. A new insight into the physiological role of bile salt hydrolase among intestinal bacteria from the genus Bifidobacterium. PLoS ONE 2014, 9, e114379. [Google Scholar] [CrossRef]
- Ooi, L.G.; Liong, M.T. Cholesterol-lowering effects of probiotics and prebiotics: A review of in vivo and in vitro findings. Int. J. Mol. Sci. 2010, 11, 2499–2522. [Google Scholar] [CrossRef]
- Momin, E.S.; Khan, A.A.; Kashyap, T.; Pervaiz, M.A.; Akram, A.; Mannan, V.; Sanusi, M.; Elshaikh, A.O.; Pervaiz Sr, M.A. The effects of probiotics on cholesterol levels in patients with metabolic syndrome: A systematic review. Cureus 2023, 15, e37567. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zhang, S.; Lu, J.; Zhang, C.; Pang, X.; Lv, J. Screening for cholesterol-lowering probiotics from lactic acid bacteria isolated from corn silage based on three hypothesized pathways. Int. J. Mol. Sci. 2019, 20, 2073. [Google Scholar] [CrossRef]
- Wong, A.; Ngu, D.Y.S.; Dan, L.A.; Ooi, A.; Lim, R.L.H. Detection of antibiotic resistance in probiotics of dietary supplements. Nutr. J. 2015, 14, 95. [Google Scholar] [CrossRef]
- Narayanan, R.; Narayanan, R. Antibiotic susceptibility profile of lactic acid bacteria with probiotic potential isolated from humans. Biomed. J. Sci. Tech. Res. 2019, 17, 12964–12966. [Google Scholar]
- Duche, R.T.; Singh, A.; Wandhare, A.G.; Sangwan, V.; Sihag, M.K.; Nwagu, T.N.; Panwar, H.; Ezeogu, L.I. Antibiotic resistance in potential probiotic lactic acid bacteria of fermented foods and human origin from Nigeria. BMC Microbiol. 2023, 23, 142. [Google Scholar] [CrossRef] [PubMed]
- Gueimonde, M.; Sánchez, B.; de los Reyes-Gavilán, C.G.; Margolles, A. Antibiotic resistance in probiotic bacteria. Front. Microbiol. 2013, 4, 202. [Google Scholar] [CrossRef]
- Halder, D.; Mandal, M.; Chatterjee, S.S.; Pal, N.K.; Mandal, S. Indigenous probiotic Lactobacillus isolates presenting antibiotic like activity against human pathogenic bacteria. Biomedicines 2017, 5, 31. [Google Scholar] [CrossRef]
- Plessas, S.; Nouska, C.; Karapetsas, A.; Kazakos, S.; Alexopoulos, A.; Mantzourani, I.; Chondrou, P.; Fournomiti, M.; Galanis, A.; Bezirtzoglou, E. Isolation, characterization and evaluation of the probiotic potential of a novel Lactobacillus strain isolated from Feta-type cheese. Food Chem. 2017, 226, 102–108. [Google Scholar] [CrossRef]
- Moradi, J.; Fathollahi, M.; Halimi, S.; Alvandi, A.; Abiri, R.; Vaziri, S.; Rezaei, A. Characterization of the resistome in Lactobacillus genomic sequences from the human gut. J. Glob. Antimicrob. Resist. 2022, 30, 451–458. [Google Scholar] [CrossRef]
- Pieniz, S.; Andreazza, R.; Anghinoni, T.; Camargo, F.; Brandelli, A. Probiotic potential, antimicrobial and antioxidant activities of Enterococcus durans strain LAB18s. Food Control 2014, 37, 251–256. [Google Scholar] [CrossRef]
- Casarotti, S.N.; Carneiro, B.M.; Todorov, S.D.; Nero, L.A.; Rahal, P.; Penna, A.L.B. In vitro assessment of safety and probiotic potential characteristics of Lactobacillus strains isolated from water buffalo mozzarella cheese. Ann. Microbiol. 2017, 67, 289–301. [Google Scholar] [CrossRef]
- Choeisoongnern, T.; Sirilun, S.; Waditee-Sirisattha, R.; Pintha, K.; Peerajan, S.; Chaiyasut, C. Potential probiotic Enterococcus faecium OV3-6 and its bioactive peptide as alternative bio-preservation. Foods 2021, 10, 2264. [Google Scholar] [CrossRef] [PubMed]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: A door to the body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Levitan, O.; Ma, L.; Giovannelli, D.; Burleson, D.B.; McCaffrey, P.; Vala, A.; Johnson, D.A. The gut microbiome—Does stool represent right? Heliyon 2023, 9, e13602. [Google Scholar] [CrossRef] [PubMed]
- Saulnier, D.M.; Gibson, G.R.; Kolida, S. In vitro effects of selected synbiotics on the human faecal microbiota composition. FEMS Microbiol. Ecol. 2008, 66, 516–527. [Google Scholar] [CrossRef]
- Song, H.; Jeon, D.; Unno, T. Evaluation of prebiotics through an in vitro gastrointestinal digestion and fecal fermentation experiment: Further idea on the implementation of machine learning technique. Foods 2022, 11, 2490. [Google Scholar] [CrossRef]
- Parvez, S.; Malik, K.A.; Ah Kang, S.; Kim, H.Y. Probiotics and their fermented food products are beneficial for health. J. Appl. Microbiol. 2006, 100, 1171–1185. [Google Scholar] [CrossRef]
- Chaiongkarn, A.; Dathong, J.; Phatvej, W.; Saman, P.; Kuancha, C.; Teetavet, S. Stability of spray-dried synbiotics containing Lactobacillus plantarum DSM 2648 and exopolysaccharide from Pediococcus acidilactici TISTR 2612 and its vivo effectiveness. J. Food Sci. Agric. Technol. (JFAT) 2019, 5, 193–199. [Google Scholar]


| Target Bacterial Group | Sequence (5′ → 3′) | Amplicon Length (bp) | Reference |
|---|---|---|---|
| Bifidobacterium spp. | F: TCGCGTCCGGTGTGAAAG | 243 | [38] |
| R: CCACATCCAGCATCCAC | |||
| Lactobacillus spp. | F: AGCAGTAGGGAATCTTCCA | 345 | [39] |
| R: ATT(C/T)CACCGCTACACATG | |||
| Bacteroidetes | F: GGA(A/G)CATGTGGTTTAATTCGATGAT | 124 | [40] |
| R: AGCTGACGACAACCATGCAG | |||
| Firmicutes | F: GGAG(C/T)ATGTGGTTTAATTCGAAGCA | 127 | [40] |
| R: AGCTGACGACAACCATGCAC | |||
| Escherichia coli | F: CATGCCGCGTGTATGAAGAA | 400 | [41] |
| R: CGGGTAACGTCAATGAGCAAA | |||
| Total bacteria | F: GTGCTGCATGGCTGTCGTCA | 148 | [42] |
| R: ACGTCATCCCCACCTTCCTC |
| Pathogenic Bacteria | Antimicrobial Activity | |||
|---|---|---|---|---|
| Agar Spot Test | Agar Well Diffusion | |||
| L. paracasei | B. breve | L. paracasei | B. breve | |
| Bacillus cereus ATCC 11778 | ++ | ++ | + | ++ |
| Escherichia coli ATCC 25922 | +++ | ++ | ++ | +++ |
| Pseudomonas aeruginosa ATCC 27853 | +++ | + | ++ | ++ |
| Salmonella enterica subsp. enterica ser. Typhi DMST 22842 | +++ | ++ | +++ | ++ |
| Salmonella enterica subsp. enterica ser. Typhimurium TISTR 1469 | ++ | ++ | ++ | + |
| Shigella sonnei ATCC 25931 | ++ | ++ | +++ | ++ |
| Staphylococcus aureus ATCC 5923 | +++ | +++ | ++ | +++ |
| Conditions | BSH Activity | |||||
|---|---|---|---|---|---|---|
| L. paracasei | B. breve | |||||
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| MRS + GCA | − | + | + | − | + | + |
| MRS + TCA | − | − | − | − | − | − |
| MRS + TDCA | − | + | + | − | + | + |
| Pathogenic Bacteria | Concentrations (µg/disc) | Antibiotic Susceptibility | |
|---|---|---|---|
| L. paracasei | B. breve | ||
| Ampicillin | 10 | S | S |
| Erythromycin | 15 | S | S |
| Gentamycin | 10 | S | S |
| Tetracycline | 30 | S | S |
| Vancomycin | 30 | R | R |
| Chloramphenicol | 30 | S | I |
| Kanamycin | 30 | R | I |
| Clindamycin | 2 | R | I |
| Streptomycin | 10 | S | S |
| Treatments | Secretion of Cytokines (pg/mL) | ||
|---|---|---|---|
| IL-6 | IL-10 | IL-12 | |
| Untreated | 5.85 ± 0.38 f | 7.81 ± 0.45 i | 0.53 ± 0.04 h |
| LPS | 55.15 ± 1.98 a | 83.93 ± 0.89 h | 7.56 ± 0.27 a |
| E. coli | 48.69 ± 1.19 b | 94.08 ± 2.00 g | 6.57 ± 0.28 b |
| S. Typhi | 43.60 ± 1.69 bc | 85.09 ± 1.45 h | 5.51 ± 0.15 c |
| L. paracasei | 17.85 ± 1.85 e | 264.65 ± 0.82 b | 2.71 ± 0.20 g |
| B. breve | 20.37 ± 0.94 e | 283.53 ± 4.10 a | 2.39 ± 0.21 g |
| LPS + L. paracasei | 43.66 ± 0.95 bc | 216.32 ± 5.57 c | 3.55 ± 0.07 f |
| LPS + B. breve | 45.69 ± 3.56 bc | 221.56 ± 3.09 c | 4.30 ± 0.04 e |
| E. coli + L. paracasei | 38.21 ± 1.77 d | 196.01 ± 2.39 e | 4.14 ± 0.07 e |
| E. coli + B. breve | 37.29 ± 1.99 d | 195.02 ± 1.19 ef | 4.40 ± 0.14 de |
| S. Typhi + L. paracasei | 41.15 ± 1.33 cd | 188.10 ± 2.00 f | 4.43 ± 0.18 de |
| S. Typhi + B. breve | 44.57 ± 1.61 bc | 208.41 ± 1.92 d | 4.87 ± 0.05 d |
| Month | Number of Survival Probiotics (log CFU/mL) | Moisture Content (%) | Water Solubility Index (%) | |||
|---|---|---|---|---|---|---|
| 5 ± 3 °C | 30 ± 2 °C | 5 ± 3 °C | 30 ± 2 °C | 5 ± 3 °C | 30 ± 2 °C | |
| 0 | 9.93 ± 0.01 | 9.91 ± 0.01 | 5.85 ± 0.03 | 5.86 ± 0.02 | 85.11 ± 0.07 | 85.17 ± 0.06 |
| 1 | 7.71 ± 0.03 *,** | 6.16 ± 0.02 *,** | 5.86 ± 0.02 | 5.83 ± 0.02 | 83.60 ± 0.26 * | 84.41 ± 0.17 * |
| 3 | 7.57 ± 0.02 *,** | 4.06 ± 0.02 *,** | 5.93 ± 0.02 * | 5.14 ± 0.02 * | 81.36 ± 0.07 *,** | 83.41 ± 0.16 *,** |
| 6 | 5.76 ± 0.02 *,** | 3.49 ± 0.04 *,** | 5.97 ± 0.02 * | 4.99 ± 0.03 * | 79.30 ± 0.31 *,** | 80.26 ± 0.05 *,** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaewarsar, E.; Chaiyasut, C.; Lailerd, N.; Makhamrueang, N.; Peerajan, S.; Sirilun, S. Effects of Synbiotic Lacticaseibacillus paracasei, Bifidobacterium breve, and Prebiotics on the Growth Stimulation of Beneficial Gut Microbiota. Foods 2023, 12, 3847. https://doi.org/10.3390/foods12203847
Kaewarsar E, Chaiyasut C, Lailerd N, Makhamrueang N, Peerajan S, Sirilun S. Effects of Synbiotic Lacticaseibacillus paracasei, Bifidobacterium breve, and Prebiotics on the Growth Stimulation of Beneficial Gut Microbiota. Foods. 2023; 12(20):3847. https://doi.org/10.3390/foods12203847
Chicago/Turabian StyleKaewarsar, Ekkachai, Chaiyavat Chaiyasut, Narissara Lailerd, Netnapa Makhamrueang, Sartjin Peerajan, and Sasithorn Sirilun. 2023. "Effects of Synbiotic Lacticaseibacillus paracasei, Bifidobacterium breve, and Prebiotics on the Growth Stimulation of Beneficial Gut Microbiota" Foods 12, no. 20: 3847. https://doi.org/10.3390/foods12203847






