Arsenic Bioaccessibility in Rice and Its Application to Derive Health-Based Limits in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rice Sample Collection and Pretreatment
2.2. Arsenic Concentrations in Rice
2.3. Arsenic Bioaccessibility in Rice via PBET Method
2.4. Health Risk Assessment and Derivation of Health-Based Limits for iAs in Rice
2.5. Statistical Analysis
3. Results
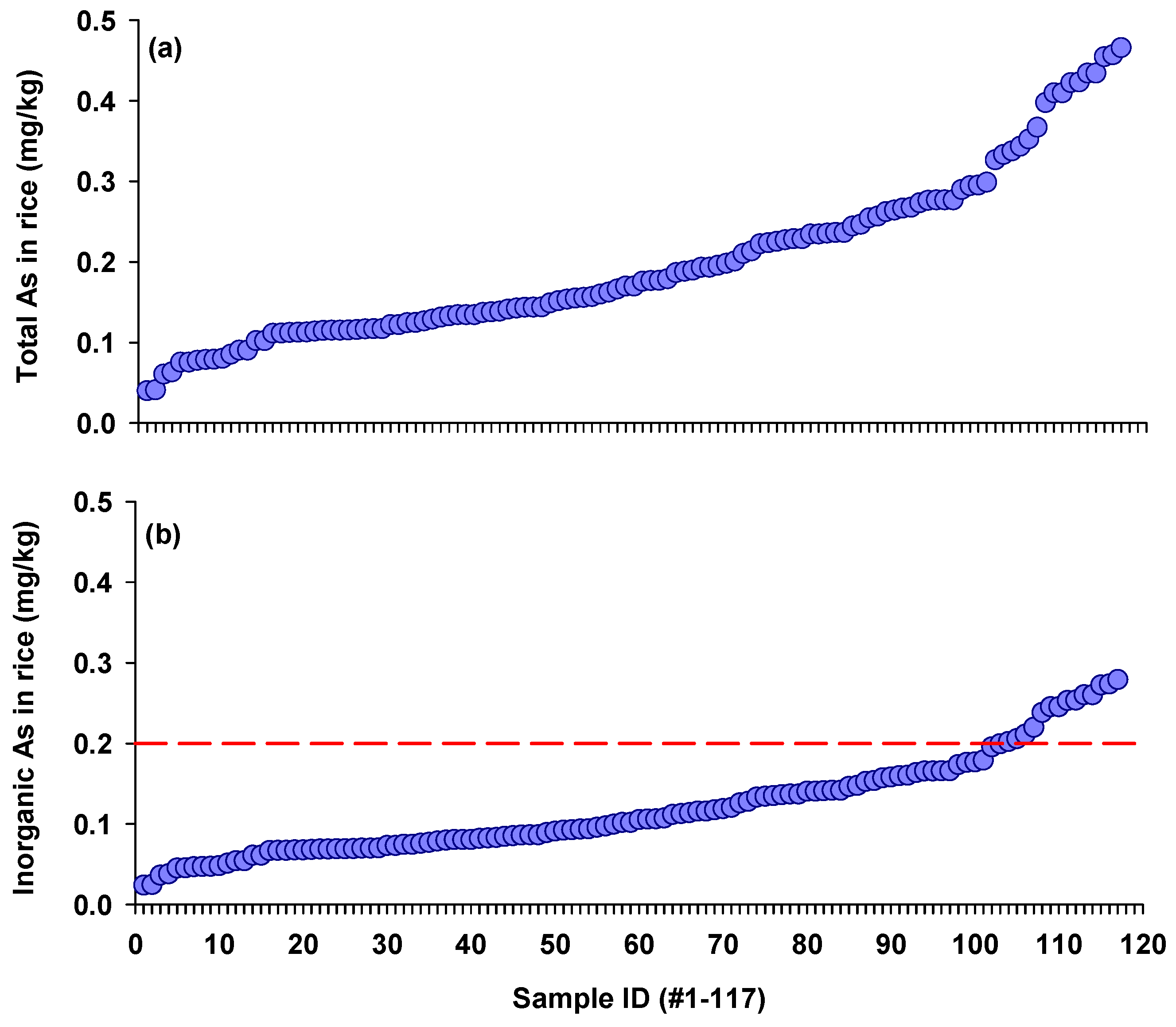
3.1. Total and Inorganic Arsenic Concentrations in Rice
3.2. Arsenic Bioaccessibility in Rice Samples
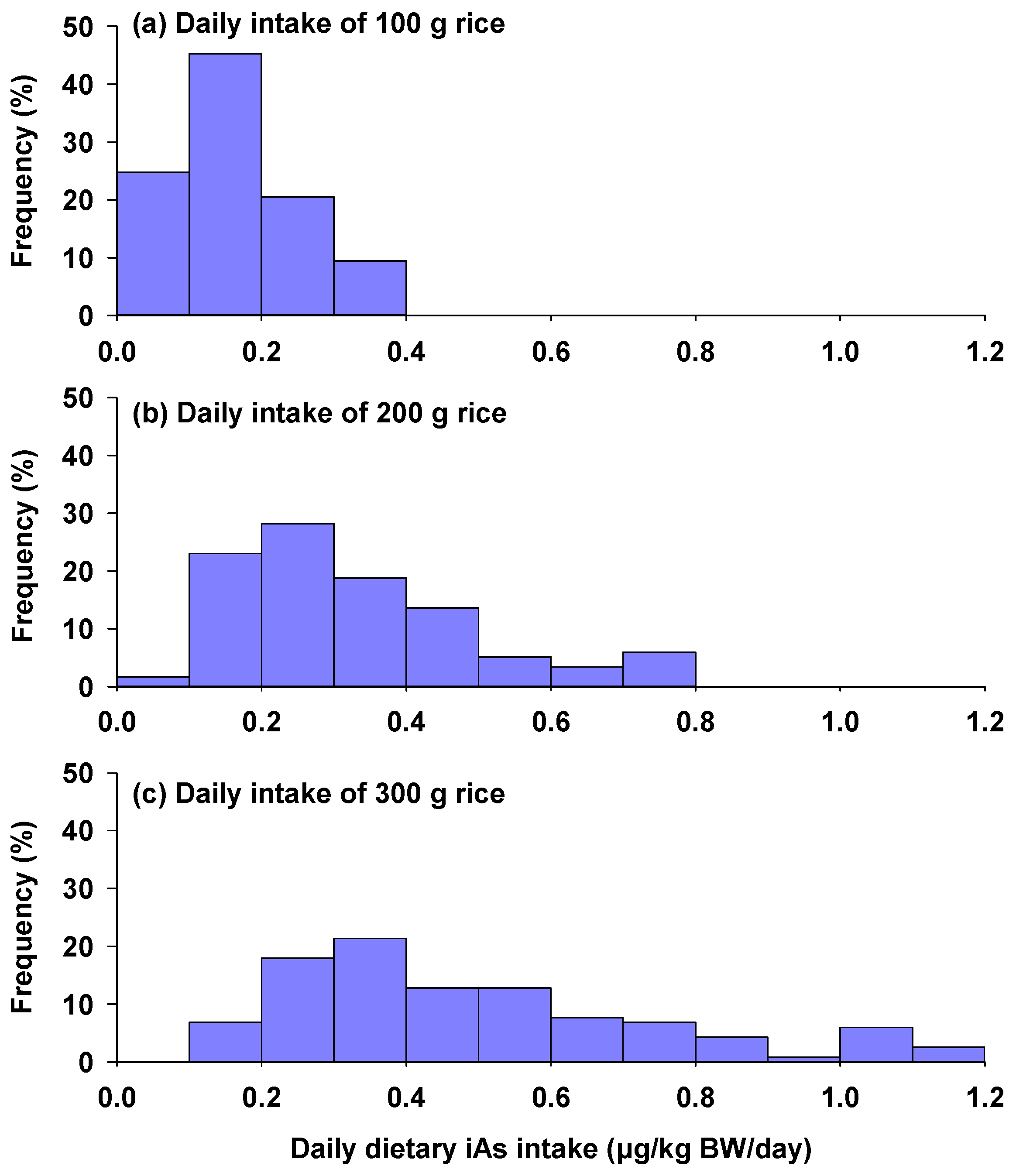
3.3. Estimated Daily Intake of Inorganic Arsenic
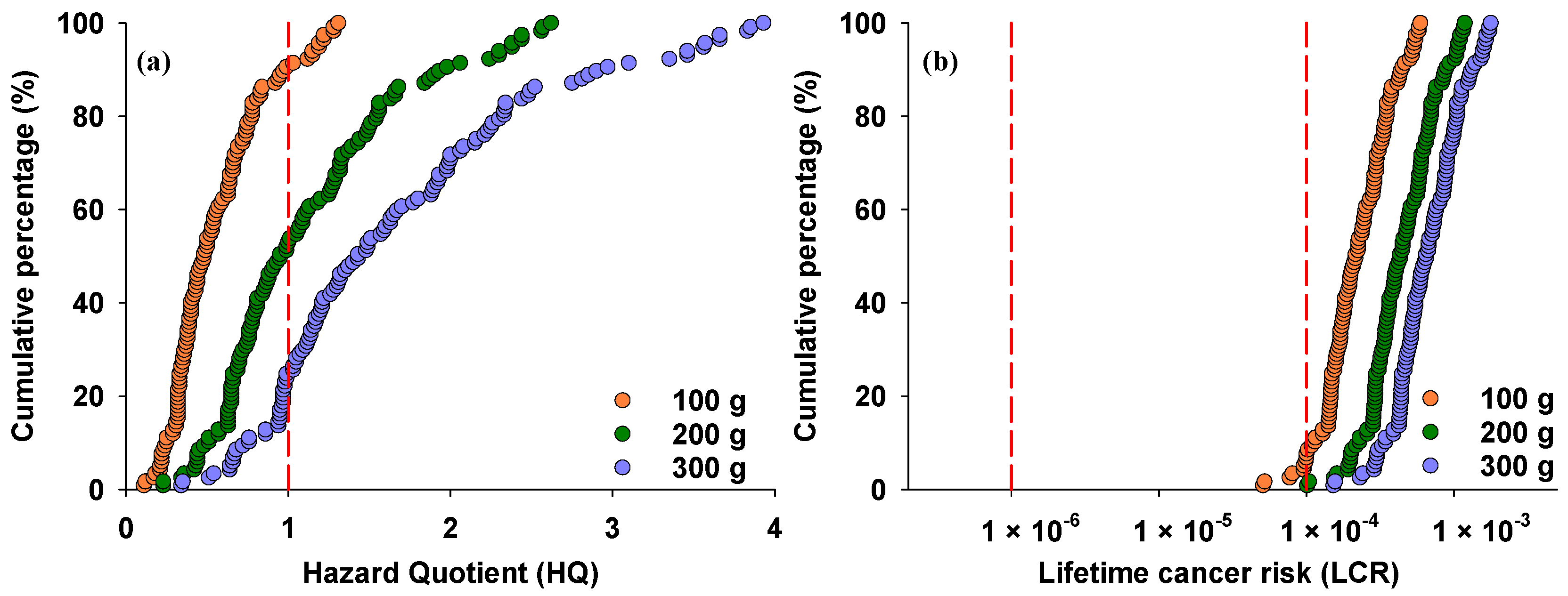
3.4. Health Risk Assessment of iAs in Rice
3.5. Deriving Health-Based Limits for iAs in Rice
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, R.; Singh, S.; Parihar, P.; Singh, V.P.; Prasad, S.M. Arsenic contamination, consequences and remediation techniques: A review. Ecotoxicol. Environ. Saf. 2015, 112, 247–270. [Google Scholar] [CrossRef] [PubMed]
- Podgorski, J.; Berg, M. Global threat of arsenic in groundwater. Science 2020, 368, 845–850. [Google Scholar] [CrossRef]
- Davis, M.A.; Mackenzie, T.A.; Cottingham, K.L.; Gilbert-Diamond, D.; Punshon, T.; Karagas, M.R. Rice consumption and urinary arsenic concentrations in U.S. children. Environ. Health Perspect. 2012, 120, 1418–1424. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, J.Y.; Yin, D.X.; Li, M.Y.; Chen, X.Q.; Juhasz, A.L.; Luo, J.; Navas-Acien, A.; Li, H.B.; Ma, L.Q. Arsanilic acid contributes more to total arsenic than roxarsone in chicken meat from Chinese markets. J. Hazard Mater. 2020, 383, 121178. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.J.; McGrath, S.P.; Meharg, A.A. Arsenic as a food chain contaminant: Mechanisms of plant uptake and metabolism and mitigation strategies. Annu. Rev. Plant Biol. 2010, 61, 535–559. [Google Scholar] [CrossRef]
- Sui, F.Q.; Chang, J.D.; Tang, Z.; Liu, W.J.; Huang, X.Y.; Zhao, F.J. Nramp5 expression and functionality likely explain higher cadmium uptake in rice than in wheat and maize. Plant Soil 2018, 433, 377–389. [Google Scholar] [CrossRef]
- Liu, Q.; Lu, W.; Bai, C.; Xu, C.; Ye, M.; Zhu, Y.; Yao, L. Cadmium, arsenic, and mineral nutrients in rice and potential risks for human health in South China. Environ. Sci. Pollut. Res. Int. 2023, 30, 76842–76852. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Banik, P. Geographical variation of arsenic distribution in paddy soil, rice and rice-based products: A meta-analytic approach and implications to human health. J. Environ. Manag. 2019, 233, 184–199. [Google Scholar] [CrossRef]
- Meharg, A.A.; Williams, P.N.; Adomako, E.; Lawgali, Y.Y.; Deacon, C.; Villada, A.; Cambell, R.C.J.; Sun, G.; Zhu, Y.G.; Feldmann, J.; et al. Geographical variation in total and inorganic arsenic content of polished (white) rice. Environ. Sci. Technol. 2009, 43, 1612–1617. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; McGrath, S.P.; Meharg, A.A.; Zhao, F.J. Growing rice aerobically markedly decreases arsenic accumulation. Environ. Sci. Technol. 2008, 42, 5574–5579. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, M.; Mao, X.; Qian, Y.; Chen, T.; Zhang, Y. Concentrations of inorganic arsenic in milled rice from China and associated dietary exposure assessment. J. Agric. Food Chem. 2015, 63, 10838–10845. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, L.; Jia, Y.; Yang, Z. Arsenic speciation in locally grown rice grains from Hunan Province, China: Spatial distribution and potential health risk. Sci. Total Environ. 2016, 557–558, 438–444. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Sun, G.X.; Lei, M.; Teng, M.; Liu, Y.X.; Chen, N.C.; Wang, L.H.; Carey, A.M.; Deacon, C.; Raab, A.; et al. High percentage inorganic arsenic content of mining impacted and nonimpacted Chinese rice. Environ. Sci. Technol. 2008, 42, 5008–5013. [Google Scholar] [CrossRef]
- Kumarathilaka, P.; Seneweera, S.; Ok, Y.S.; Meharg, A.A.; Bundschuh, J. Mitigation of arsenic accumulation in rice: An agronomical, physico-chemical, and biological approach–A critical review. Crit. Rev. Environ. Sci. Technol. 2019, 50, 31–71. [Google Scholar] [CrossRef]
- Sun, H.J.; Rathinasabapathi, B.; Wu, B.; Luo, J.; Pu, L.P.; Ma, L.Q. Arsenic and selenium toxicity and their interactive effects in humans. Environ. Int. 2014, 69, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Li, H.B.; Xu, J.Y.; Luo, J.; Ma, L.Q. Arsenic extraction and speciation in plants: Method comparison and development. Sci. Total Environ. 2015, 523, 138–145. [Google Scholar] [CrossRef]
- Nunes, L.M.; Li, G.; Chen, W.Q.; Meharg, A.A.; O’Connor, P.; Zhu, Y.G. Embedded health risk from arsenic in globally traded rice. Environ. Sci. Technol. 2022, 56, 6415–6425. [Google Scholar] [CrossRef]
- Li, G.; Sun, G.X.; Williams, P.N.; Nunes, L.; Zhu, Y.G. Inorganic arsenic in Chinese food and its cancer risk. Environ. Int. 2011, 37, 1219–1225. [Google Scholar] [CrossRef]
- Wei, R.; Chen, C.; Kou, M.; Liu, Z.; Wang, Z.; Cai, J.; Tan, W. Heavy metal concentrations in rice that meet safety standards can still pose a risk to human health. Commun. Earth Environ. 2023, 4, 84. [Google Scholar] [CrossRef]
- Diamond, G.L.; Bradham, K.D.; Brattin, W.J.; Burgess, M.; Griffin, S.; Hawkins, C.A.; Juhasz, A.L.; Klotzbach, J.M.; Nelson, C.; Lowney, Y.W.; et al. Predicting oral relative bioavailability of arsenic in soil from in vitro bioaccessibility. J. Toxicol. Environ. Health A 2016, 79, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B.; Li, J.; Zhao, D.; Li, C.; Wang, X.J.; Sun, H.J.; Juhasz, A.L.; Ma, L.Q. Arsenic relative bioavailability in rice using a mouse arsenic urinary excretion bioassay and its application to assess human health Risk. Environ. Sci. Technol. 2017, 51, 4689–4696. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.X.; Van de Wiele, T.; Alava, P.; Tack, F.; Du Laing, G. Arsenic in cooked rice: Effect of chemical, enzymatic and microbial processes on bioaccessibility and speciation in the human gastrointestinal tract. Environ. Pollut. 2012, 162, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Sharafi, K.; Nodehi, R.N.; Mahvi, A.H.; Pirsaheb, M.; Nazmara, S.; Mahmoudi, B.; Yunesian, M. Bioaccessibility analysis of toxic metals in consumed rice through an in vitro human digestion model-Comparison of calculated human health risk from raw, cooked and digested rice. Food Chem. 2019, 299, 125126. [Google Scholar] [CrossRef]
- Chen, J.Y.; Zeng, J.Y.; Ding, S.; Li, J.; Liu, X.; Guan, D.X.; Ma, L.Q. Arsenic contents, speciation and bioaccessibility in rice grains from China: Regional and variety differences. J. Hazard Mater. 2022, 437, 129431. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Shen, J.; Wu, J.; Tang, Z.; Shen, Q.; Zhao, F.J. Impact of agronomic practices on arsenic accumulation and speciation in rice grain. Environ. Pollut. 2014, 194, 217–223. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Exposure Factors Handbook; EPA/600/R-09/052F; U.S.EPA: Washington, DC, USA, 2011. [Google Scholar]
- Zhao, D.; Wang, J.Y.; Tang, N.; Yin, D.X.; Luo, J.; Xiang, P.; Juhasz, A.L.; Li, H.B.; Ma, L.Q. Coupling bioavailability and stable isotope ratio to discern dietary and non-dietary contribution of metal exposure to residents in mining-impacted areas. Environ. Int. 2018, 120, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Mu, T.; Wu, T.; Zhou, T.; Li, Z.; Ouyang, Y.; Jiang, J.; Zhu, D.; Hou, J.; Wang, Z.; Luo, Y.; et al. Geographical variation in arsenic, cadmium, and lead of soils and rice in the major rice producing regions of China. Sci. Total Environ. 2019, 677, 373–381. [Google Scholar] [CrossRef]
- Carey, M.; Meharg, C.; Williams, P.N.; Marwa, E.; Xiao, J.J.; Farias, J.G.; De Silva, P.M.C.S.; Signes-Pastor, A.; Lu, Y.; Nicoloso, F.T.; et al. Global sourcing of low-inorganic arsenic rice grain. Expo. Health 2019, 12, 711–719. [Google Scholar] [CrossRef]
- Islam, S.; Rahman, M.M.; Islam, M.R.; Naidu, R. Geographical variation and age-related dietary exposure to arsenic in rice from Bangladesh. Sci. Total Environ. 2017, 601–602, 122–131. [Google Scholar] [CrossRef]
- Grau-Perez, M.; Kuo, C.C.; Gribble, M.O.; Balakrishnan, P.; Jones Spratlen, M.; Vaidya, D.; Francesconi, K.A.; Goessler, W.; Guallar, E.; Silbergeld, E.K.; et al. Association of low-moderate arsenic exposure and arsenic metabolism with incident diabetes and insulin resistance in the Strong Heart Family Study. Environ. Health Perspect. 2017, 125, 127004. [Google Scholar] [CrossRef]
- Kuo, C.C.; Moon, K.A.; Wang, S.L.; Silbergeld, E.; Navas-Acien, A. The association of arsenic metabolism with cancer, cardiovascular disease, and diabetes: A systematic review of the epidemiological evidence. Environ. Health Perspect. 2017, 125, 087001. [Google Scholar] [CrossRef]
- He, Y.; Pedigo, C.E.; Lam, B.; Cheng, Z.; Zheng, Y. Bioaccessibility of arsenic in various types of rice in an in vitro gastrointestinal fluid system. J. Environ. Sci. Health B 2012, 47, 74–80. [Google Scholar] [CrossRef]
- Li, J.; Chen, S.; Li, H.; Liu, X.; Cheng, J.; Ma, L.Q. Arsenic bioaccessibility in rice grains via modified physiologically-based extraction test (MPBET): Correlation with mineral elements and comparison with As relative bioavailability. Environ. Res. 2021, 198, 111198. [Google Scholar] [CrossRef]
- Islam, S.; Rahman, M.M.; Duan, L.; Islam, M.R.; Kuchel, T.; Naidu, R. Variation in arsenic bioavailability in rice genotypes using swine model: An animal study. Sci. Total Environ. 2017, 599–600, 324–331. [Google Scholar] [CrossRef]
- Zhou, Z.; Kang, Y.; Li, H.; Cao, S.; Xu, J.; Duan, X.; Yang, G.; Shao, K. Estimating inorganic arsenic exposure from rice intake in Chinese urban population. Environ. Pollut. 2020, 263, 114397. [Google Scholar] [CrossRef]
- Mondal, D.; Banerjee, M.; Kundu, M.; Banerjee, N.; Bhattacharya, U.; Giri, A.K.; Ganguli, B.; Sen Roy, S.; Polya, D.A. Comparison of drinking water, raw rice and cooking of rice as arsenic exposure routes in three contrasting areas of West Bengal, India. Environ. Geochem. Health 2010, 32, 463–477. [Google Scholar] [CrossRef]
- Rose, M.; Baxter, M.; Brereton, N.; Baskaran, C. Dietary exposure to metals and other elements in the 2006 UK Total Diet Study and some trends over the last 30 years. Food Addit. Contam. Part A 2010, 27, 1380–1404. [Google Scholar] [CrossRef]
- Cubadda, F.; D’Amato, M.; Aureli, F.; Raggi, A.; Mantovani, A. Dietary exposure of the Italian population to inorganic arsenic: The 2012–2014 Total Diet Study. Food Chem. Toxicol. 2016, 98, 148–158. [Google Scholar] [CrossRef]
- GB 2762-2022; National Food Safety Standard—Maximum Levels of Contaminants in Foods. National Standard of the People’s Republic of China: Beijing, China, 2022.

| Region | Contribution of Rice to Overall Dietary iAs Intake (%) | Ingestion Rate (g/day) | Limits for iAs in Rice with Consideration of Bioaccessibility (mg/kg) | GB 2762-2022 [40] | |
|---|---|---|---|---|---|
| Based on Cancer Risk | Based on Non-Cancer Risk | ||||
| National | 57.8 | 238.3 | 0.01 | 0.05 | 0.2 |
| Urban | 52.4 | 217.8 | 0.01 | 0.05 | |
| Rural | 59.9 | 246.2 | 0.01 | 0.05 | |
| North | 41.7 | 123.82 | 0.02 | 0.07 | |
| South | 64.1 | 326.65 | 0.01 | 0.04 | |
| Coastal | 52.6 | 235.09 | 0.01 | 0.05 | |
| Inland | 59.6 | 244.63 | 0.01 | 0.05 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D. Arsenic Bioaccessibility in Rice and Its Application to Derive Health-Based Limits in China. Foods 2024, 13, 2741. https://doi.org/10.3390/foods13172741
Zhao D. Arsenic Bioaccessibility in Rice and Its Application to Derive Health-Based Limits in China. Foods. 2024; 13(17):2741. https://doi.org/10.3390/foods13172741
Chicago/Turabian StyleZhao, Di. 2024. "Arsenic Bioaccessibility in Rice and Its Application to Derive Health-Based Limits in China" Foods 13, no. 17: 2741. https://doi.org/10.3390/foods13172741






